Abstract
This paper presents a facile and rapid synthesis route of metallic Ni and Co nanocrystallites at ~150 °C in the mixture composed of the corresponding metal nitrates and 1,3-propanediol, as reducing agent. The metal oxides NiO, CoO, Co3O4 nanocrystallites were, also, successfully synthesized by thermal decomposition at 300 °C of the hydroxycarboxylate coordination products, obtained in the redox reaction between 1,3-propanediol and Ni(II) and Co(II) nitrates. The formation of the Ni(II) and Co(II) hydroxycarboxylate complexes depends on the diol which generates the carboxylate anion, the transition metal and the process parameters. Ni(II) and Co(II) nanocomposites were also synthesized by thermal decomposition of the complex combinations formed within the pores of the hybrid silica gels. One of the purposes of the present study was to investigate the phase constitution of the composites obtained in similar synthesis conditions, from Ni(II) and Co(II) complex combinations embedded in silica gels. These gels were submitted to various thermal treatments and the changes occurring during these treatments were described by X-ray diffraction. Thermal analysis is an excellent tool for the study of the processes implied in the formation and decomposition of the Co(II) and Ni(II) carboxylate complexes. X-ray diffraction evidenced the nanometer sized metal and/or metal oxide phases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, Ni and Co nanosized materials have become one of the most interesting materials in research communities due to the diverse promising applications in the field of catalysis, magnetism, magnetic recording media, and electrodes [1–3].
Transition metal oxides exhibit acidic or base properties, which make them appropriate as supports for highly dispersed metal catalysts or as precursors for metal phases. As with most materials, many of the properties of metal oxides (NiO, CoO) or of transition metals (Ni, Co) depend strongly on the preparation procedure employed in their synthesis [4–9].
Because of the volume effect, the quantum size effect and the surface effect nickel oxide (NiO) is one of the most important transition metal oxides and has applications in diverse fields [10–13]. Most of these applications require particles with small and narrow size distribution. Through many years of investigation, the interest has been focused on synthesis, characterization, and properties of cobalt oxides [14]. Cobalt oxides comprise two readily accessible cation oxidation states: Co2+ and Co3+, which are thermodynamically competitive under common ambient conditions, and two species of cobalt oxide (CoO, Co3O4) which are stable in the natural environment [15, 16]. The properties of these oxides depend on their morphology and particle size.
The researcher’s attention is mainly oriented toward a more efficient control of the purity, homogeneity, grain size, porosity, morphology, phase composition, and texture of the product. The transition metal oxides undispersed and dispersed in different matrices can be obtained by conventional and unconventional synthesis methods: microwave-assisted synthesis [17], sol–gel methods [18], thermal decomposition method [19], and reduction in aqueous media [20].
Our previous studies have shown that the thermal decomposition of homopolynuclear complex combinations generates a reducing environment (CO, C) which implies gas–solid surface phenomena leading to species such as metal oxides [21]. The embedding of these complex combinations within the pores of organic–inorganic hybrid gels (TEOS-diol) allows the obtaining of oxide nanosystems uniformly distributed at molecular level. The thermal decomposition, at low temperatures, of these complex combinations embedded in silica gels followed by appropriate thermal treatments leads to nanocomposites with directed properties [22, 23].
This study is dedicated to the original, facile and rapid synthesis of nanosized metallic Ni and Co at ~150 °C, in the mixture composed of the corresponding metal nitrates and 1,3-propanediol, as reducing agent. By thermal decomposition of the Ni(II) and Co(II) hydroxycarboxylate type complexes at 300 °C, NiO and CoO are obtained. Another original synthesis method presented in this study is the “modified sol–gel method,” which consists in the formation and decomposition of the Ni(II) and Co(II) hydroxycarboxylates inside the silica gel. This method allows the obtaining of Ni(II)- and Co(II)-based nanocomposites. This work also may contribute to further understanding and optimization of these synthesis techniques, which will act as novel routes in developing Ni and Co nanoscale materials as advanced materials.
Experimental
Materials and preparations
Method 1
The reagents used for the synthesis of the coordination products were the metal nitrates Ni(NO3)2·6H2O, Co(NO3)2·6H2O, and 1,3-propanediol (1,3PG), of analytical grade purity, purchased from Merck.
Two solutions were prepared: SNi (Ni(NO3)2·6H2O–1,3PG) and SCo (Co(NO3)2·6H2O–1,3PG) in the molar ratio 1,3PG:NO −3 = 3:8 according to the equation presented below:
In the synthesis, there was used an excess of 50 % 1,3-propanediol calculated to the nitrate anion (1,3PG:NO −3 = 2.25:4).
According to the synthesis method [21, 24, 25], the oxidation of 1,3PG by metal nitrates (Ni(NO3)2·6H2O, Co(NO3)2·6H2O) takes place with formation of the complex combinations, of malonate type, in the reaction system. For the initiation of the redox reaction, the solutions were heated at a controlled temperature. Depending on temperature, the redox reaction occurs differently:
-
(a)
The redox reaction takes place at a controlled temperature (~140 °C), when the complex combination can be synthesized and isolated;
-
(b)
The redox reaction takes place simultaneous with the combustion of the complex combination (the ligands burning) at temperatures higher than 150 °C.
Method 2
For the synthesis of the coordination products embedded in silica gels were used metal nitrates: Ni(NO3)2·6H2O, Co(NO3)2·6H2O, 1,3-propanediol (1,3-PG) and tetraethyl orthosilicate (TEOS), ethanol (EtOH), water, and nitric acid. All reagents were of analytical purity and purchased from Merck.
The preparation of the gels for composites with 30 wt% metal oxide/70 wt% SiO2 was achieved by dissolving the metal nitrates in the corresponding 1,3-PG, water and TEOS amounts. The reagents' amounts were calculated for 3 g of nanocomposite (30 %NiO/70 %SiO2 and 30 %CoO/70 %SiO2, respectively) (Table 1).
The ethanolic TEOS solution was added dropwise, under intense magnetic stirring, to the metal nitrates-diol solution. After 30-min stirring, the solutions were left for gelation at room temperature, for ~116 h. The obtained gels were dried at 40 °C for 6 h and then thermally treated at 140 °C, for 5 h, at ambient temperature and pressure, with a heating rate of 5 °C min−1, when the redox reaction between the metal nitrates and diol took place with formation of hydroxycarboxylate type compounds within the pores of the hybrid gel [26]. The obtained gels where thermally treated at different temperatures in the range 500–1,000 °C, in air and atmospheric pressure, with a heating rate of 5 °C min−1.
Characterization techniques
The thermal analysis, for description of the redox reaction evolution, was performed on a 1500D MOM Budapest derivatograph by deposition of a reactants solution thin layer on plate like Pt crucibles. The experiment was carried out, in air, under identical conditions maintaining the following instrumental parameters: heating rate 5 °C min−1, sample mass 100 mg and reference material for DTA was α-Al2O3. The thermal curves, of the hydroxycarboxylates synthesized at 140 °C, were recorded, in air, on a Diamond Perkin Elmer thermobalance. Both experiments were carried out under identical conditions maintaining the following instrumental parameters: heating rate 10 °C min−1, mass of the sample ~20 mg, and reference material for DTA was α-Al2O3. FT-IR spectra of the complex combinations were recorded in the range 400–4,000 cm−1 on a Shimadzu Prestige-21 FT-IR spectrophotometer using KBr pellets. The powder XRD patterns of the decomposition products were recorded at room temperature with an Advanced-Bruker AXS diffractometer by the MoKalpha radiation (λ = 0.7093 Å). Magnetic measurements were performed with a laboratory installation equipped with a data acquisition system [27].
Results and discussion
Method 1a
Synthesis of the complex combinations
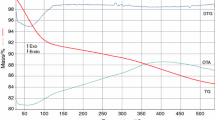
The thermal analysis on the solutions SNi (Ni(NO3)2·6H2O–1,3PG) and SCo (Co(NO3)2·6H2O–1,3PG), deposed as thin layers on platinum crucibles, was achieved in the temperature range 20–400 °C. Figure 1 presents the evolution of the thermal curves corresponding to the solution SNi.
The DTA curve presents an exothermic effect at 135 °C attributed to the oxidation of 1,3PG by nickel nitrate with generation of the Ni(II) complex combination. The second exothermic effect in the temperature range 250–300 °C, accompanied by mass loss, is attributed to the oxidative decomposition of the Ni(II) complex combination. The evolution of the redox reaction in the solution SCo was similar to the one presented for the solution SNi.
Based on the thermal analysis data, the coordination compounds were synthesized at the controlled temperature of ~140 °C. The samples were grinded and washed with an acetone–water mixture. Figure 2 presents the evolution of the thermal curves of Ni (II) hydroxycarboxylate synthesized at 140 °C. Up to ~200 °C a mass loss corresponding to the coordination water takes place, followed by the oxidative decomposition of the complex combination (the ligands burning), process which takes place with a high rate. Up to 500 °C, the mass remains constant and corresponds to the metal oxide NiO. The decomposition of Co (II) hydroxycarboxylate takes place similarly, leading to CoO.
The formation of the coordination compounds between the metal ions and the carboxylate ligand was evidenced by FT-IR spectroscopy (Fig. 3).
The bands at ~1,560 and 1,420 cm−1 are attributed to the coordinated carboxylate group ν as (COO−) and ν s (COO−), respectively, [28, 29] evidencing the existence of the carboxylate groups which are coordinated to the metal cations for both compounds.
Method 1b
Redox reaction and combustion of the complex combination
The mixture Ni(NO3)2·6H2O–1,3PG was continuously heated, when at ~150 °C started the redox reaction simultaneous with the burning of the organic ligand (massive gas release (NO x , CO, CO2, H2O)) and formation of an expanded material (Fig. 4).
In these conditions, the redox reaction (NO −3 –1,3PG) evolves energically and the reaction products (hydroxycarboxylate) are difficult to isolate (combustion), leading to the compounds denoted CNi and CCo.
The thermal behavior of the resulted products was studied in a 100 mL min−1 synthetic air flow, on alumina crucibles, up to 750 °C. Figure 5 presents the thermal curves of the compounds CNi and CCo resulted from the combustion. The TG curves present a mass increase, indicating the oxidation of the metals (Ni and Co, respectively) present in the compounds CNi and CCo. In the case of compound CNi (Fig. 5a), the mass variation Δm = 20.0 % takes place in the range 400–800 °C accompanied on DTA by an exothermic effect at 550 °C. The mass increase corresponds to the oxidation of metallic Ni to NiO with a percent of 74.0 % Ni.
For compound CCo (Fig. 5b). the mass increase Δm = 22.0 % takes place in the temperature range 350–650 °C, accompanied on DTA by an exothermic effect at 450 °C. The mass increase corresponds to the oxidation of metallic Co to CoO with a percent of 81.5 % Co.
The XRD analysis on the compound CNi (Fig. 6a) resulted from the combustion, presents the peaks corresponding to metallic Ni, as single phase [30]. From thermal analysis, a purity of 74.0 % metallic Ni resulted. This can be explained by the fact that there is also some organic residue formed during combustion. By annealing of the compound CNi at 500 °C, for 3 h (Fig. 6b), the XRD pattern shows not only the peaks of NiO [30] but also the peaks of metallic Ni, showing that at this temperature, metallic Ni was not completely oxidized.
The XRD analysis on the compound CCo (Fig. 7a) resulted from the combustion, presents the peaks corresponding to metallic Co together with the less intense peaks of CoO [30]. This fact justifies the purity of metallic Co of 81.5 %. By annealing, in air, of the compound CCo at 500 °C, for 3 h (Fig. 7b), only the peaks of the single phase Co3O4 were evidenced in the XRD pattern [30].
Figure 8 presents the magnetization curves of the samples CNi (Fig. 8a) and CCo (Fig. 8b) annealed at different temperatures. The samples obtained by combustion have a tendency to superparamagnetic behavior characteristic for the metallic Ni and Co nanocrystallites, but the nanocrystallites size is very small (3–4 nm). The released heat during the combustion helps the crystallization and formation of the desired phases such as well-crystallized Ni nanocrystallites or Co and CoO nanocrystallites. This phenomenon influences the crystallite size and magnetic properties.
From magnetic measurements, the saturation magnetization (σ sat/emu g−1), remanence magnetization (σ r/emu g−1), and coercivity field (H c/kOe) are derived and listed in Table 2.
This combustion method generates metallic Ni and Co nanocrystallites with high saturation magnetization values.
Method 2: the modified sol–gel method
The method envisages the obtaining of hybrid gels TEOS-1,3PG-M(NO3)2 (M = Co, Ni), which were dried at 40 °C. Figure 9 presents the thermal curves of the hybrid gel GNi dried at 40 °C with two exothermic effects on DTA with mass loss on TG. The exothermic effect at ~110 °C corresponds to the redox reaction between the ion NO −3 and 1,3PG with the formation of the complex combination within the pores of the hybrid silica gel. The corresponding mass loss on the TG curve (~60 %) corresponds to elimination of nitrogen oxides, water, and unreacted diol.
The exothermic effect at 250 °C is attributed to the oxidative decomposition of the complex combination when the metal oxide is obtained within the silica matrix. A similar behavior was also found in the case of the gel GCo.
The FT-IR spectra (Fig. 10) corresponding to the gels dried at 40 °C shows that the redox reaction did not take place at this temperature, by the presence of the intense band from 1,380 cm−1 corresponding to the NO −3 ion, free in the gel pores.
The temperature for the synthesis of the hydoxycarboxylates was chosen 140 °C, when the redox reaction was finished and the Co(II) and Ni(II) hydroxycarboxylate complexes were formed within the pores of the hybrid gel.
Figure 11 presents the thermal curves of the gels GNi and GCo heated at 140 °C. The evolution of the thermal curves is similar. The decomposition of the hydroxycarboxylates is accompanied by the exothermic effects at 290 °C for Ni(II) hydroxycarboxylate and 250 °C for Co(II) hydroxycarboxylate. The large exothermic effects are due to the high dispersion (low concentration of 30 wt% metal oxide/SiO2) of the hydroxycarboxylates within the pores of the silica matrix. The decomposition products up to 500 °C are NiO and CoO.
Figure 12 presents the FT-IR spectra of the gel GNi (a) and GCo (b) heated at 140 °C when the hydroxycarboxylate type complexes are formed in the pores of the silica gels. Both spectra present the bands characteristic for the carboxylate group ν as(COO−) and ν s(COO−) in the range 1,500–1,700 and 1,300–1,400 cm−1 [28], which confirms the formation of the coordinative compounds.
In the FT-IR spectrum, the bands characteristic to silica gels at: 580 cm−1 (Si–O–Si rings), 800 cm−1 (SiO4-tetrahedra), 1060 cm−1 (Si–O–Si), and 3,400 cm−1 (–OH) are also recorded [31]. The absorption bands in the range 2,900–3,000 cm−1 are characteristic for the stretching vibrations of C–H bonds [32].
Figure 13 presents the XRD patterns of the gel GNi annealed at 500, 700, and 1,000 °C with the single phase NiO/amorphous SiO2. The crystallites' size was estimated by the Scherrer [33] equation to give diameters of 3 nm at 700 °C and 5 nm at 1,000 °C.
The reducing atmosphere generated during decomposition of Co(II) hydroxycarboxylate, at ~300 °C, inside the silica gel, stabilizes the low oxidation state (II) for cobalt (CoO). At this low temperature, CoO exists in an amorphous, very reactive state, which facilitates the interaction with the amorphous silica gel, leading to Co2SiO4 nuclei. With temperature increase, Co2SiO4 (one of olivine mineral group) crystallizes as single phase starting with 700 °C (Fig. 14). The crystallites size is 16 nm at 700 °C and 18 nm at 1,000 °C.
Conclusions
The first method allows the obtaining of metal oxides nanocrystallites (NiO, CoO, Co3O4) by thermal decomposition of some Ni(II)- and Co(II) hydroxycarboxylate-type precursors, formed in the redox reaction between the corresponding metal nitrates and 1,3-propanediol.
The proposed original synthesis procedure, which implies the simultaneous formation and combustion of the hydroxycarboxylates represents an excellent tool for the obtaining of metallic Co and Ni nanocrystallites with diameters of 3–4 nm. This process is influenced by the reducing atmosphere generated during the burning of the ligand as well as by the nature of the metallic ion. This atmosphere has a significant effect on the crystallite size, homogeneity, and magnetic properties. By controlled thermal treatments, one could obtain the desired metal/metal oxide ratios with directed properties. Depending on the process parameters: temperature, atmosphere, heating rate, and reagents ratio, the synthesis yields high metal amounts, which are potential catalysts.
The modified sol–gel method is an original method for the obtaining of Ni(II) and Co(II) hydroxycarboxylates embedded in hybrid gels TEOS-1,3PG. Appropriate thermal treatments of Ni(II) hydroxycarboxylates embedded in hybrid gels results in NiO nanocrystallites (<10 nm) which are stable up to 1,000 °C within the silica matrix. In the case of the same thermal treatment on Co(II) hydroxycarboxylates embedded in hybrid gels, the resulted CoO is stable up to 700 °C, when it reacts with amorphous SiO2 with the formation of an olivine group member (Co2SiO4).
References
Chang H, Su HT. Synthesis and magnetic properties of Ni nanoparticles. Rev Adv Mater Sci. 2008;18:667–75.
Saxena A, Kumar A, Mozumdar S. Ni-nanoparticles: an efficient green catalyst for chemo-selective oxidative coupling of thiols. J Mol Catal A Chem. 2007;269:35–40.
Donaldson JD. Cobalt and cobalt compounds. In: Bailey JE, editor. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed. Weinheim: Wiley-VCH Verlag GmbH; 2003. p. 759–793.
Gomes DJC, Caires FJ, Lima LS, Gigante AC, Ionashiro M. Synthesis, characterization and thermal study of solid mandelate of some bivalent transition metal ions in CO2 and N2 atmospheres. J Therm Anal Calorim. 2012;. doi:10.1007/s10973-011-2189-y.
Wen W, Wu JM, Tu JP. A novel solution combustion synthesis of cobalt oxide nanoparticles as negative-electrode materials for lithium ion batteries. J Alloys Compd. 2012;513:592–6.
Ahmed J, Ahmad T, Ramanujachary KV, Lofland SE, Ganguli AK. Development of a microemulsion-based process for synthesis of cobalt (Co) and cobalt oxide (Co3O4) nanoparticles from submicrometer rods of cobalt oxalate. J Colloid Interface Sci. 2008;321:434–41.
Wang X, Wan L, Yu T, Zhou Y, Guan J, Yu Z, Li Z, Zou Z. Non-basic solution eco-routes to nano-scale NiO with different shapes: synthesis and application. Mater Chem Phys. 2011;126:494–9.
Seong G, Takami S, Arita T, Minami K, Hojo D, Yavari AR, Adschiri T. Supercritical hydrothermal synthesis of metallic cobalt nanoparticles and its thermodynamic analysis. J Supercrit Fluids. 2011;60:113–20.
Kalam A, Al-Sehemi AG, Du Al-Shihri G, Ahmad T. Synthesis and characterization of NiO nanoparticles by thermal decomposition of nickel linoleate and their optical properties. Mat Char. 2012;68:77–81.
El-Shobaky GA, Petro NS, Ghazy TM, Dessouky AM. Effects of γ radiation on the surface and catalytic properties of NiO, Co3O4 and Fe2O3-Cr2O3 solids. Surf Technol. 1983;19:17–26.
Nuli YN, Zhao SL, Qin QZ. Nanocrystalline tin oxides and nichel oxide film anodes for Li-ion batteries. J Power Sources. 2003;114:113–20.
Nagai J, Morisaki S. Molecular properties of partially substituted nickel oxide clusters. Solid State Ion. 2003;165:149–53.
Ichiyanagi Y, Wakabayashi N, Yamazaki J, Yamada S, Kimishima Y, Komatsu E, Tajima H. Magnetic properties of NiO nanoparicles. Phys B Condens Mater. 2003;329:862–3.
Laureti S, Agostinelli E, Scavia G, Varvaro G, Rossi Albertini V, Generosi A, Paci B, Mezzi A, Kaciulis S. Effect of oxygen partial pressure on PLD cobalt oxide films. Appl Surf Sci. 2008;254:5111–5.
Verelst M, Ould Ely T, Amiens C, Snoeck E, Lecante P, Mosset A, Respaud M, Broto JM, Chaudret B.,Synthesis and characterization of CoO, Co3O4, and mixed Co/CoO nanoparticules. Chem Mater. 1999;11:2702–8.
Wegner D, Inglot Z, Lieb KP. The CoO/Co3O4 phase transition studied by PAC. Hyperf Interact. 1990;59:313–6.
Lagashetty A, Havanoor V, Basavaraja S, Balaji SD, Venkataraman A. Microwave-assisted route for synthesis of nanosized metal oxides. Sci Technol Adv Mater. 2007;8:484–93.
Li Q, Wang LS, Hu BY, Yang C, Zhou L, Zhang L. Preparation and characterization of NiO nanoparticles through calcination of malate gel. Mater Lett. 2007;61:1615–8.
Chen Z, Xu A, Zhang Y, Gu N. Preparation of NiO and CoO nanoparticles using M2+-oleate (M = Ni, Co) as precursor. Curr Appl Phys. 2010;10:967–70.
Montiel MG, Jacinto PS, Diaz Gongora JAI, Reguera E, Rodriguez-Gottorno G. Synthesis and thermal behavior of metallic cobalt micro and nanostructures. Nanomicro Lett. 2011; 3:12–9.
Stefanescu M, Stefanescu O, Stoia M, Lazau C. Thermal decomposition of some metal-organic precursor: Fe2O3 nanoparticles. J Therm Anal Calorim. 2007;88(1):27–32.
Stefanescu M, Dippong T, Stoia M, Stefanescu O. Study on the obtaining of cobalt oxides by thermal decomposition of some complex combinations, undispersed and dispersed in SiO2 matrix. J Therm Anal Calorim. 2008;94:389–93.
Stefanescu O, Davidescu C, Stefanescu M, Stoia M. Preparation of Fe x O y nanocomposites by thermal decomposition of some carboxylate precursors formed inside the silica matrix. J Therm Anal Calorim. 2009;97:203–8.
Niculescu M, Vaszilcsin N, Barzescu M, Budrugeac P, Segal E. Thermal and structural investigation of the reaction between 1,2-propanediol and Co(NO3)2·6H2O. J Therm Anal Calorim. 2001;65:881–9.
Barzescu M, Cristea M, Stefanescu M, Constantin G. Rom Pat 102501, 27 sept. 1990.
Stefanescu M, Stoia M, Caizer C, Stefanescu O. Preparation of x(Ni0.65Zn0.35Fe2O4)/(100−x)SiO2 nanocomposite powders by a modified sol-gel method. Mater Chem Phys. 2009;113:342–8.
Mihalca I, Ercuta A, Ionascu C. The Villari effect in Fe–Cr–B amorphous ribbons. Sens Actuators A. 2003;106:61–4.
Nakamoto K. Infrared spectra of inorganic and coordination compounds. New York: Wiley; 1970.
Prasad R, Sulaxna, Kumar A. Kinetics of thermal decomposition of iron(III) dicarboxylate complexes. J Therm Anal Calorim. 2005; 81:441–450.
Joint Committee on Powder Diffraction Standards International Center for Diffraction Data, Swarthmore, PA, 1993.
Lenza RFS, Vasconcelos WL. Study of the influence of some DCCAs on the structure of sol–gel silica membranes. J Non Cryst Solids. 2003;330:216–25.
Mondragon M, Castano VM, Garcia GM, Tellez CAS. Vibrational analysis of Si(OC2H5)4 and spectroscopic studies on the formation of glasses via silica gels. Vib Spectrosc. 1995;9:293–304.
Cheetham AK, Day P. Solid state chemistry. Oxford: Clarendon Press; 1987.
Acknowledgements
Special thanks to PhD Aurel Ercuta, from the West University of Timisoara, for the magnetic measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stefanescu, O., Vlase, T., Sorescu, S. et al. Thermal behavior of Co(II) and Ni(II) hydroxycarboxylate complexes obtained by two original synthesis methods. J Therm Anal Calorim 113, 1345–1354 (2013). https://doi.org/10.1007/s10973-013-2951-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-2951-4