Abstract
The main purpose of this study is to investigate the β-crystallization tendency in the β-nucleated iPP blends. The β-nucleated iPP/compatibilizers blends, β-nucleated iPP/PET blends and its compatibilized versions with four kinds of compatibilizers (PP-g-MA, PP-g-GMA, POE-g-MA, and EVA-g-MA) were prepared by different blending ways. The effect of compatibilizers and blending ways on the non-isothermal crystallization and melting characteristics and the β-crystallization tendency of β-nucleated iPP blends were studied by differential scanning calorimetry. The relative content of the β-phase were characterised by the k β values determined on the basis of the wide angle X-ray diffractogram. The results indicated that the β-crystallization tendency of β-nucleated iPP blends depends on the kinds of compatibilizer. Addition of PP-g-MA significantly reduced the β-crystallization tendency of β-nucleated iPP, while PP-g-GMA, POE-g-MA, and EVA-g-MA have little effect on it. In the compatibilized β-nucleated iPP/PET blends, the blending ways, which controlled the dispersion of β-nucleating agent, influences the β-crystallization tendency intensively. The high β-crystallization tendency and β-crystal content were obtained for compatibilized β-nucleated iPP/PET blends prepared firstly at high temperature and β-nucleating agent added into blends at low temperature; however, the type of compatibilizers has little effect on β-crystallization tendency and melting behavior of blends.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Compared to α-iPP, β-iPP exhibits a superior performance characteristic, including improved elongation at break, impact strength and higher heat distortion temperature [1–14]. However, the yield strength and elastic modulus of β-iPP are lower. In order to improve the yield strength and elastic modulus of β-iPP, β-iPP blending with other polymers with high yield strength and elastic modulus shall become an increasingly important method.
The β-iPP blends with other crystalline polymers had been investigated. Varga and coworkers [15–17] found that the iPP matrix consisting mainly of the α-modification was formed in the β-nucleated iPP/PVDF and iPP/PA6 blends. It is attributed to two impact factor. First, the second polymer PVDF and PA6 exert strong α-nucleating ability. Second, the β-nucleating agent is selectively encapsulated in the PA6 phase due to the polar interaction, suppressing the formation of the β-crystal of iPP. On the contrary, predominantly β-iPP matrix developed in β-nucleated iPP/PA6 blends compatibilized by PP-g-MA. Zhang et al. [18] obtained high β-crystal content in a compatibilized iPP/PA6 blend prepared by compression moulding.
Recently, β-nucleated iPP/PA6 blends compatibilized by PP-g-MA, PP-g-GMA, POE-g-MA and EVA-g-MA were prepared in our lab [19–22]. It was found that the β-crystallization tendency and β-crystal content in the β-nucleated iPP/PA6 blends obviously decreased as incorporating with PA6 and depended on the blending ways and the type of compatibilizers. The high β-crystallization tendency and β-crystal content were obtained for iPP/PA6 blends prepared at temperature of 240 °C, and the β-nucleating agent added into the blends at temperature of 180 °C. The additions of PP-g-MA, POE-g-MA, and EVA-g-MA increased the β-crystallization tendency and β-crystal content in the β-nucleated iPP/PA6 blends.
The iPP/PET blends have been widely investigated to improve performance of iPP [23–31]. Similarly, it is expected that β-iPP blended with PET can increase its yield strength and elastic modulus of β-iPP. So far, the β-nucleated iPP/PET blends have been little investigated [32, 33].In our lab, β-nucleated iPP/PET blends were prepared by using nano-CaCO3 supported β-nucleating agent (β-NA). Effects of blending ways and PET contents on crystallization behavior and melting characteristic, as well as β-crystallization tendency and polymorphic composition of iPP in the blends, were investigated in previous study [34]. The results indicated that the β-nucleation efficiency of β-NA significantly decreased with addition of PET and increasing PET content. Addition of 5 wt% PET decreased the β-crystal content from 0.92 to 0.46 in β-nucleated iPP/PET blends. With increasing the content of PET to 20 wt%, almost no β-crystal was formed in β-nucleated iPP/PET blend. The β-nucleating ability of β-NA in the blends was also dependent on the blending ways.
In the β-nucleated iPP/PA6 blends, it is found that addition of compatibilizer can increase the β-crystallization tendency and β-crystal content in compatibilized β-nucleated iPP/PA6 blends [15–22]. However, the crystallization and melting behavior and the β-crystallization tendency of compatibilized β-nucleated iPP/PET blends are lack of investigation. In this paper, the β-nucleated iPP/compatibilizer blends, β-nucleated iPP/PET blends, and its compatibilized versions with four kinds of compatibilizers (PP-g-MA, PP-g-GMA, POE-g-MA, and EVA-g-MA) were prepared with an internal mixer. The different blending ways for preparing compatibilized β-nucleated iPP/PET blends were used to control the dispersion of β-nucleating agent in different components in the blends. Effects of compatibilizers and blending ways on the crystallization behavior and melting characteristic, as well as β-crystallization tendency and polymorphic composition of iPP in compatibilized β-nucleated iPP/PET blends, were investigated.
Experiment
Materials
iPP (N-T30S) with MFR = 2.5 g/10 min at 200 °C, was supplied by Maoming Petroleum Chemical Industry Limited Company, Sinopec Group, China. PET (PET105) was purchased from Groups of Foshan Plastics. PP-g-MA, EVA-g-MA, POE-g-MA, and PP-g-GMA were of commercial grade supplied by Guangzhou Lushan Chemical Materials Co., Ltd., and the grafted degrees of maleic anhydride (MA) and glycidyl methacrylate (GMA) were 1.0 and 1.1 wt%, respectively. A nano-CaCO3 supported β-nucleating agent (abbreviated to β-NA) was prepared in our lab [35–37]. The mass ratio of nano-CaCO3/pimelic acid is 100/1 in this article.
Specimen preparation
Before blending, all the materials were adequately dried in a vacuum oven at appropriate temperatures for 12 h. All β-nucleated iPP/PET80/20 blends containing 5 phr β-NA and 5 phr compatibilizer were prepared at different temperatures at 50 rpm using an internal mixer HL-200 from Jilin University science instrument factory, China.
In order to control the dispersion of β-nucleating agent in different components, the β-nucleated iPP/PET blends compatibilized by PP-g-MA were prepared by different blending ways. In way A, β-NA was added to PET phase first at the temperature of 265 °C for 2 min, and then iPP and PP-g-MA were added with mixing for 5 min. In way B, all the components iPP, β-NA, PET and PP-g-MA were simultaneously mixed at the temperature of 265 °C for 7 min. In way C, iPP, PET and PP-g-MA were melted at the temperature of 265 °C for 2 min, and then β-NA was added to the homogenized melt with mixing for 5 min. In way D, β-iPP was first prepared by adding 5 wt% β-NA into iPP matrix on a twin-screw extruder at temperature of 220 °C, and then extrudates were mixed with PET and PP-g-MA at the temperature of 265 °C for 5 min. In way E, iPP, PET, and PP-g-MA were melted at the temperature of 265 °C for 5 min, and then the blend was cooled to 180 °C, and β-NA was added at this temperature with mixing for 5 min. It is needed to notice that β-nucleated iPP/PET blends modified by PP-g-GMA, POE-g-MA and EVA-g-MA were prepared only in way E.
Apparatus and characterization procedures
The non-isothermal crystallization and melting curves were recorded by a Perkin-Elmer DSC 7 apparatus. The temperature was calibrated by indium and zinc as reference materials. The mass of the samples was between 3 and 5 mg. The heating and cooling rates were 10 °C/min during all DSC scans. The samples were melted at 280 °C for 5 min to erase the thermal and mechanical prehistory, and then cooled to 50 °C for the X-ray diffraction (XRD) measures, followed by reheating to 280 °C for the second DSC heating run.
Wide-angle XRD experiment was conducted with a Rigaku Geigerflex Model D/Max-IIIA rotating anode X-ray diffractometer. Graphite monochromatic Cu-Ka radiation was employed as a radiation source. The samples were prepared on Perkin-Elmer DSC-7 thermal system under primary crystallization condition. The operating conditions of the X-ray source were set at a voltage of 40 kV and a current of 30 mA in a range of 2θ = 5°–35°. The samples were scanned with a speed of 4°/min and step length of 0.02°. The k β value representing the β-crystal content in blends was calculated from X-ray diffractograms according to Turner-Jones et al. [38, 39].
where H α(110), H α(040), and H α(130) are the intensities of α-diffraction peaks corresponding to angles 2θ equals 14.2°, 17.0°, and 18.8°, respectively, and H β is the intensity of β-diffraction peak at 2θ equals 16.2°.
Results and discussion
Effect of compatibilizers on β-crystallization tendency of β-nucleated iPP
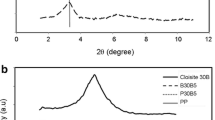
A compatibilizer (i.e., PP-g-MA) is generally used to improve the compatibility and performance of an incompatible iPP/PET polymer blend [29–31]. In this paper, four compatibilizers (PP-g-MA, POE-g-MA, EVA-g-MA, and PP-g-GMA) were used to prepare the compatibilized β-nucleated iPP/PET blends. First, the effect of compatibilizers on β-crystallization tendency of β-iPP was investigated. Figure 1 shows the DSC curves of β-nucleated iPP/compatibilizer 95/5 blends. It can be seen that different compatibilizers have different influences on the crystallization behavior and melting characteristic. Compared with β-nucleated iPP, the peak temperature of the crystallization (T cp) of iPP increases in the β-nucleated iPP/PP-g-MA blend, which varies hardly in the β-nucleated iPP/PP-g-GMA blend and then decreases in the β-nucleated iPP/POE-g-MA and iPP/EVA-g-MA blends. The melting curve of β-nucleated iPP presents four melting peaks, attributed to the melting of β 1-, β 2-, α 1-, and α 2-crystal, respectively. [39] Additions of compatibilizers, POE-g-MA, EVA-g-MA, and PP-g-GMA, have little influence on the melting characteristic of β-nucleated iPP. The four melting peaks of β 1-, β 2-, α 1- and α 2-crystal are observed in β-nucleated iPP/compatibilizer blends. It is needed to mention that the melting characteristic of β-nucleated iPP/PP-g-MA blend is different from those of other β-nucleated iPP blends. Addition of PP-g-MA obviously reduces the intensity of melting peak of β 1-crystal and increases that of α 1-crystal, and no melting peak of β 2-crystal and α 2-crystal are observed, which could be connected with its melt compatibility with iPP [40]. It is needed to mention that the melting behaviour depend strongly on the post-crystallization thermal history of samples [41, 42]. The β-iPP samples cooled below the critical temperature (TR = 100–105 °C) recrystallises into the alpha form (β to α recrystallization) during partial melting of the β-phase. Therefore, the fusion enthalpy cannot be determined exactly and hence it is not presented in the Tables.
The relative content of β-crystal noted as k β calculated based on Fig. 2 is listed in Table 1. It can be observed that EVA-g-MA, POE-g-MA, and PP-g-GMA have little influence on the β-crystal content of β-nucleated iPP. However, the intensity of α(110), α(040), and α(130) strengthen dramatically, and the k β drops down to 0.43 in the β-nucleated iPP/PP-g-MA blend. Above results indicated that addition of PP-g-MA increased the crystallization of iPP and decreased obviously the β-nucleating ability of β-NA for iPP.
These results indicate that the three compatibilizers with the same polar groups (MA) but different backbones have different effect on β-crystallization tendency in β-nucleated iPP. We suggested that there exist two kinds of interaction between compatibilizers and β-NA in β-nucleated iPP/compatibilizer blends: (1) interaction between the MA group of compatibilizers and β-NA, and (2) interaction between the MA group of compatibilizers and the support of β-NA. If the interaction between the MA group of compatibilizers and β-NA takes place, the β-NA can interact with the polar group MA by polarity and chemical reaction to reduce the β-nucleating ability of β-NA and the β-nucleated iPP blends with EVA-g-MA, POE-g-MA and PP-g-MA should show the same crystallization behavior and melting characteristics. In fact, there still exists higher β-crystallization tendency for β-nucleated iPP blends with EVA-g-MA and POE-g-MA, and the content of β-crystal is as high as 95 %, while the β-crystallization tendency significantly decreases and the content of β-crystal drops down to 0.43 in β-nucleated iPP blends with PP-g-MA. Therefore, the interaction between the MA group of compatibilizers and β-NA is not the factor for the reduction of β-crystallization tendency of β-nucleated iPP/compatibilizer blends.
Some research results have shown that the polar group MA of compatibilizers can react with CaCO3 to form calcium salt with a strong α-nucleating ability for iPP crystallization [43], which is affected by the different backbones of compatibilizers [44–46]. In the β-nucleated iPP blends with EVA-g-MA, POE-g-MA and PP-g-MA, the calcium salt with strong α-nucleating ability can be formed in β-nucleated iPP/compatibilzer blends due to the chemical reaction between the polar group MA of compatibilizers and the CaCO3 used as support in β-NA. However, the strong α-nucleating ability of calcium salt is affected by compatibility between iPP matrix and macromolecular chains of compatibilizers [44–46]. Because the macromolecular chain of PP-g-MA is compatible with iPP matrix and can crystallize, the α-nucleation of calcium salt can transfer across the crystallization of its macromolecular chain into iPP matrix to induce α-nucleation of iPP matrix, which would decrease the β-nucleating ability of β-NA. The compatibilization between the macromolecular chains of EVA-g-MA or POE-g-MA and iPP matrix is weak and the EVA or POE of compatibilizers hardly crystallize. Hence, the formed calcium salt with α-nucleating ability cannot transfer through the interface between compatibilizers and iPP to effect the iPP crystallization and decreases the β-nucleating ability of β-NA. Therefore, we suggest that the interaction between the polar groups MA of compatibilizers and the CaCO3 as support in β-NA is the most impact factor to decrease the β-crystallization tendency of β-nucleated iPP/compatibilizer blends.
Effect of blending ways on β-crystallization tendency of β-nucleated iPP/PET blends compatibilized by PP-g-MA
It has been found that β-nucleation is obviously decreased and even cannot induce the formation of β-crystal when the β-nucleated iPP is blended with PET, PA, PVDF, etc. It is ascribed to transfer the β-nucleating agent from iPP phase to the polar polymer phase due to the role of polar effect. In order to investigate the effect of dispersion of β-NA in different phases on β-crystallization tendency of the blends, various blending ways was carried out to prepare compatibilized β-nucleated iPP/PET blends with different compatibilizers in this article.
Figures 3 and 4 show the DSC and XRD curves of PP-g-MA compatibilized β-nucleated iPP/PET blends prepared by different ways, respectively, and the relative data are listed in Table 2. The β-nucleated iPP/PET blends exhibit the same crystallization behavior despite being prepared under different ways. Only a single crystallization peak of iPP can be observed, supposing that the simultaneous crystallization of β-crystal and α-crystal took place during the cooling process. As shown in Table 2, the blending ways have no distinct influence on T cp’s of iPP in the blends. However, the melting characteristics and the β-crystal contents are largely dependent on the blending ways. The order of k β is way E>D>C>B>A.
In order to prepare the blend in which the β-NA mainly dispersed in PET phase, β-NA was mixed with PET first at the temperature of 265 °C and then blended with iPP and PP-g-MA in way A. The absence of melting peak of β-crystal and β-diffraction peak at 16.2° for the blend proved that there is no β-crystallization tendency for the blend prepared by way A due to the β-NA mainly dispersed in PET phase. Our previous study of β-nucleated iPP/PET blends [34] has shown that addition of PET made β-nucleation fail and consequently the iPP matrix only formed α-crystal.
In order to investigate the selective distribution of the β-NA between iPP and PET, all the components iPP, β-NA, PET, and PP-g-MA were simultaneously mixed at the temperature of 265 °C in way B. In our previous work of β-nucleated iPP/PET blends without compatibilizer [34], it was found that addition of PET significantly decreased the β-nucleating ability of the β-NA. However, after adding PP-g-MA, a melting peak of β-crystal at 148.3 °C in DSC and β-diffraction peak at 16.2° in XRD were observed, and the blend with k β value of 0.2 was obtained. Consequently the presence of compatibilizer is enough for influencing the distribution of the β-NA between the PET and iPP phases and a small amount of the β-NA, which is enough for providing the formation of minor β-crystal, remains in the iPP phase.
In way C, all the PP-g-MA, iPP, and PET were melted at 265 °C, and then blended with β-NA. It can be seen that the melting peak of β-crystal at 147.3 °C and β-diffraction peak at 16.2° is obvious, and the k β value is 0.63. Compared to way B, in which the β-NA was added at the beginning of the blending, the β-NA was added 2 min later in way C. Consequently in way B the compatibilization procedure and the selective encapsulation occur simultaneously, and so the β-NA can migrate into the PET phase easily at the beginning. After 2 min the PET phase is compatibilized, so the PET droplets were covered by PP-g-MA layer and the selective encapsulation of β-NA is suppressed by the different interfacial conditions. It is suggested that the compatibilization of PP-g-MA would be favorable for the increased β-nucleating ability of β-NA in β-nucleated iPP/PET blends.
In order to study the migration of β-NA, iPP was first mixed with β-NA at the temperature of 180 °C, and then the prepared β-iPP was homogenized with PET and PP-g-MA at 265 °C in way D. The β-NA is mainly dispersed in the iPP phase, and so there exists stronger β-crystallization tendency. However, the intensity of melting peak of β-crystal is lower than that of α-crystal as shown in Fig. 3, and the β-crystal content is lower than that of neat β-nucleated iPP. We suggested that the β-NA dispersed in the iPP phase would migrate into the PET phase during the β-nucleated iPP mixing with PET and PP-g-MA at high temperature due to the polar interaction and result in the decrease of β-nucleation and β-crystal content in the blend.
In way E, iPP, PET, and PP-g-MA were first melted at the temperature of 265 °C, and then the β-NA was added to the blend as it was cooled to 180 °C. Besides the interaction between PET and PP-g-MA would decrease the effects of PET and PP-g-MA on the β-nucleating ability of β-NA. It is difficult to make the β-NA migrate into the PET phase due to high melting viscosity of PET in low temperature, which leads the β-NA to mainly dispersed in the iPP phase because of its molten state at 180 °C. The β-NA dispersed mainly in the iPP phase plays a heterogeneous nucleating role in iPP crystallization and induces the formation of high β-crystal content in the blend. The highly attainable β-content by this way E had been proved earlier by Menyhárd [17] and Yang [22] in the PA6/β-nucleated iPP blends. Therefore, the intensity of melting peak of β-crystal is the most intensive and a blend with the k β of 0.97 is obtained in the blend prepared by way E.
The above mentioned results indicated that the β-nucleating ability of the β-NA and the content of β-crystal in the blends are dependent on the dispersion of the β-NA in the components. The dispersion of the β-NA in the different components results in different interactions among components and different β-crystallization tendencies. These results suggest that the dispersion of β-NA in the β-nucleated iPP/PET blends can be controlled by blending ways.
To confirm the dispersion of the β-NA in different phases, the blends were etched with concentrated sulfuric acid to remove PET phase and were characterized by DSC, as shown in Fig. 5. In order to completely dissolve the PET phase, the samples were pressed into thin film, and immersed in the concentrated sulfuric acid for 24 h. It can be seen that the etched blend prepared by way A did not show β-crystallization tendency. It is ascribed to the dispersion of nearly all the β-NAs in the PET phase. In the etched blend prepared by way B in which all the components were added simultaneously, the β-NA easily dispersed into the PET phase due to polar effect. Therefore, the β-NA dispersed in the PET phase was etched with PET, and subsequently, the mostly α-PP was formed. Compared to the unetched blends, the intensities of melting peak of β-crystal were significantly increased in the etched blends prepared by ways C and D. It is proved that partial β-NA is dispersed into the iPP phase and the β-nucleating ability of β-NA is increased after the removal of PET. The higher β-crystallization tendency in the etched blends prepared by way C and D also prove the effect of PET on β-nucleation of β-NA. Although the blend prepared by way E has as high as 97% content of β-crystal, no melting curve of β-crystal in etched sample is observed. We suggest that the β-NA mainly disperses in the interface between PET and iPP phase because it is difficult to enter the high viscosity of PET and iPP phases at low temperature. The β-NA dispersed in the interface between PET and iPP is easily etched. Therefore, β-nucleation of β-NA is absent, and no β-crystal is formed in the etched blend prepared by way E.
These results suggest that the presence of PET plays the most important role for β-nucleation of β-NA. Once the β-NA disperses in the PET phase, its β-nucleation of β-NA significantly decreases. On the contrary, the β-NA exhibits high β-nucleation as the β-NA disperses in the PET/iPP interface.
Effect of compatibilizer type on β-crystallization tendency of β-nucleated iPP/PET blends
The DSC curves of β-nucleated iPP/PET blends with different compatibilizers prepared by way E are plotted in Fig. 6, and the relative data are shown in Table 3. It can be seen that the T cp’s of iPP increase in the blend compatibilized by PP-g-MA and decrease by the others. It is basically the same as β-nucleated iPP/compatibilizer blends. It also proves that various compatibilizers show different effect on the crystallization behavior of iPP in the blends due to the different compatibility between iPP and compatibilizer with different backbones [44–46].
It can be seen from the melting curves that there exist four distinct melting peaks. The type of compatibilizers has little effect on melting behavior of the blends. Compared to uncompatibilized blends, addition of EVA-g-MA and POE-g-MA increased the intensity of β 2-crystal melting peak in the blends, and the intensity α 1-crystal melting peak was higher than that of α 2-crystal in the blend by addition of PP-g-GMA. Researches have shown that low-temperature melting peak of β-crystal corresponds to melting of less perfect β-crystal, and the peak at high-temperature melting peak corresponding to recrystallization of the original β 1-crystal, or more perfect crystals of β-crystal melting [39, 41, 42, 47]. The presence of double β-crystal melting peak suggests that the less-perfect β-crystal is formed in β-nucleated iPP/PET blends.
The k β values calculated from Fig. 7 are listed in Table 3. The addition of compatibilizers increases the content of β-crystal in the blends. The k β values in the blends compatibilized by PP-g-MA and PP-g-GMA reach even ≥0.95 and higher than those of blends compatibilized by EVA-g-MA and POE-g-MA. The order of the contents of β-crystal is: PP-g-GMA>PP-g-MA>POE-g-MA>EVA-g-MA. The higher k β values in the blends compatibilized by PP-g-MA and PP-g-GMA indicated that the compatibility between iPP and compatibilizer has an influence on the β-nucleating ability of β-NA.
Figure 8 presents the melting curves of the etched blends. It is obviously seen that both the incompatibilized and compatibilized etched blends only exert a strong α-crystal melting peak. It is completely different in case of the unetched blends, all of which show four fusion peaks. It is also proved that the β-NA mostly disperses in iPP/PET interface in the blend prepared by way E, and addition of compatibilizers did not affect its distribution. In case of β-NA etched with the PET, no β-crystallization tendency is shown in the etched blends.
Conclusions
-
(1)
The β-crystallization tendency in β-nucleated iPP/compatibilizer blends is dependent on the type of compatibilizers. Addition of PP-g-MA significantly reduces the β-crystallization tendency and rises the crystallization temperature of β-nucleated iPP. However, PP-g-GMA, POE-g-MA and EVA-g-MA have little effect on it. It is attributed to the effect of different compatibilities between iPP and macromolecular chain of compatibilizers.
-
(2)
The β-NA easily migrates into PET phase due to the polar interaction between PET and β-NA, resulting in the disappearance of β-crystallization tendency, but the dispersion of β-nucleating agent in compatibilized β-nucleated iPP/PET blends can be controlled by blending ways. Once the β-NA disperses in the iPP or interface between iPP and PET, the high β-crystallization tendency and high β-iPP content were obtained, such as the compatibilized iPP/PET blend prepared at the temperature of 265 °C and added the β-NA into blend at the temperature of 180 °C.
-
(3)
The type of compatibilizer has little effect on β-nucleating ability of β-NA in the blends. The blends with high β-crystallization tendency and β-iPP content can be obtained for compatibilized iPP/PET blend prepared at high temperature and β-NA added into blend at low temperature.
References
Grein C. Toughness of neat, rubber modified and filled β-nucleated polypropylene: from fundamentals to applications. Adv Polym Sci. 2005;188:43–104.
Varga J. β-modification of isotactic polypropylene: preparation, structure, processing, properties, and application. J Macromol Sci Phys. 2002;41:1121–71.
Uchiyama Y, Iwasaki S, Ueoka C, Fukui T, Okamoto K, Yamacuchi M. Molecular orientation and mechanical anisotropy of polypropylene sheet containing N’-dicyclohexyl-2,6-naphthalene dicarboxamide. J Polym Sci B. 2009;47:424–33.
Wang SW, Yang W, Gong G, Xie BH, Liu ZY, Yang MB. Effect of α and β-nucleating agents on the fracture behavior of polypropylene-co-ethylene. J Appl Polym Sci. 2008;108:591–7.
Jacoby P. Beta nucleating masterbatch offers enhanced properties in polypropylene products. Plast Addit Compd. 2007;9:32–5.
Zhang P, Liu X, Li Y. Influence of β-nucleating agent on the mechanics and crystallization characteristics of polypropylene. Mater Sci Eng A. 2006;434:310–3.
Cermák R, Obadal M, Ponízil P, Polášková M, Stoklasa K, Hecková J. Injection-moulded α- and β-polypropylenes: II. Tensile properties vs. processing parameters. Eur Polym J. 2006;42:2185–91.
Raab M, Šcudla J, Kolarík J. The effect of specific nucleation on tensile mechanical behavior of isotactic polypropylene. Eur Polym J. 2004;40:1317–23.
Kotek J, Kelnar I, Baldrian J, Raab M. Tensile behavior of isotactic polypropylene modified by specific nucleation and active fillers. Eur Polym J. 2004;40:679–84.
Trongtorsak K, Supaphol P, Tantayanon S. Effect of calcium stearate and pimelic acid addition on mechanical properties of heterophasic isotactic polypropylene/ethylene-propylene rubber blend. Polym Test. 2004;23:533–9.
Kotek J, Raab M, Baldrian J, Grellmann W. The effect of specific β-nucleation on morphology and mechanical behavior of isotactic polypropylene. J Appl Polym Sci. 2002;85:1174–84.
Tjong S, Shen J, Li R. Impact fracture toughness of beta-form polypropylene. Scripta Metall Mater. 1995;33:503–8.
Karger-Kocsis J, Varga J, Ehrenstein G. Comparison of the fracture and failure behavior of injection moulded alpha- and beta-polypropylene in high-speed three-point bending tests. J Appl Polym Sci. 1997;64:2059–66.
Karger-Kocsis J, Varga J. Effects of beta-alpha transformation on the static and dynamic tensile behaviour of isotactic polypropylene. J Appl Polym Sci. 1996;62:291–300.
Varga J, Menyhárd A. Crystallization, melting and structure of polypropylene/poly(vinylidene-fluoride) blends. J Thermal Anal Calorim. 2003;73:735–43.
Menyhárd A, Varga J, Liber A, Belina G. Polymer blends based on the β-modification of polypropylene. Eur Polym J. 2005;41:669–77.
Menyhárd A, Varga J. The effect of compatibilizers on the crystallisation, melting and polymorphic composition of β-nucleated isotactic polypropylene and polyamide 6 blends. Eur Polym J. 2006;42:3257–68.
Zhang RH, Shi D, Tjong SC, Li RKY. Study on the β to α transformation of polypropylene crystals in compatibilized blend of polypropylene/polyamide-6. J Polym Sci B. 2007;45:2674–81.
Yang Z, Zhang Z, Tao Y, Mai K. Effects of polyamide 6 on the crystallization and melting behavior of β-nucleated polypropylene. Eur Polym J. 2008;44:3754–63.
Yang Z, Zhang Z, Tao Y, Mai K. Preparation, crystallization behavior, and melting characteristics of β-nucleated isotactic polypropylene blends with polyamide 6. J Appl Polym Sci. 2009;112:1–8.
Yang Z, Mai K. Crystallization and melting behavior of β-nucleated isotactic polypropylene/polyamide 6 blends with maleic anhydride grafted polyethylene- vinyl acetate as a compatibilizer. Thermochim Acta. 2010;511:152–8.
Yang Z, Chen C, Liang D, Zhang Z, Mai K. Melting characteristic and β-crystal content of β-nucleated polypropylene/polyamide 6 alloys prepared using different compounding methods. Polym Int. 2009;58:1366–72.
Tao Y, Mai K. Non-isothermal crystallization and melting behavior of compatibilized polypropylene/recycled poly(ethylene terephthalate) blends. Eur Polym J. 2007;43:3538–49.
Shi W, Li Y, Xu J, Ma G, Sheng J. Morphology development in multi-component polymer blends: I. Composition effect on phase morphology in PP/PET polymer blends. J Macromol Sci B. 2007;46:1115–26.
Akbari M, Zadhoush A, Haghighat M. PET/PP blending by using PP-g-MA synthesized by solid phase. J Appl Polym Sci. 2007;104:3986–93.
Chiu H, Hsiao Y. Compatibilization of poly(ethylene terephthalate)/polypropylene blends with maleic anhydride grafted polyethylene-octene elastomer. J Polym Res. 2006;13:153–60.
Zhong G, Li Z, Li L, Shen K. Crystallization of oriented isotactic polypropylene (iPP) in the presence of in situ poly(ethylene terephthalate) (PET) microfibrils. Polymer. 2008;49:4271–8.
Zhong G, Li Z, Li L, Mendes E. Crystalline morphology of isotactic polypropylene (iPP) in injection molded poly(ethylene terephthalate) (PET)/iPP microfibrillar blends. Polymer. 2007;48:1729–40.
Bae TY, Park KY, Kim DH, Suh KD. Poly(ethylene terephthalate)/polypropylene reactive blends through isocyanate functional group. J Appl Polym Sci. 2001;81:1056–62.
Pang YX, Jia DM, Hu HJ, Hourston DJ, Song M. Effects of a compatibilizing agent on the morphology, interface and mechanical behavior of polypropylene/poly(ethylene terephthalate) blends. Polymer. 2000;41:357–65.
Papadopoulou CP, Kalfoglou NK. Comparison of compatibilizer effectiveness for PET/PP blends: their mechanical, thermal and morphology characterization. Polymer. 2000;41:2543–55.
Varga J, Schulek Tóth F, Mudra I. Blends of the β-Modification of Polypropylene. Makromol Chem Macromol Symp. 1994;78:229–41.
Varga J, Karger-Kocsis J. The difference between transcrystallization and shear induced cylindritic crystallization in fiber reinforced Polypropylene composites. J Mater Sci Lett. 1994;13:1069–71.
Wang C, Zhang Z, Mai K. Preparation, non-isothermal crystallization and melting behavior of β-nucleated isotactic polypropylene/poly(ethylene terephthalate) blends. J Therm Anal Calorim. 2011;106:895–903.
Zhang Z, Tao Y, Yang Z, Mai K. Preparation and characteristics of nano-CaCO3 supported β-nucleating agent of polypropylene. Eur Polym J. 2008;44:1955–61.
Zhang Z, Wang C, Yang Z, Mai K. Crystallization behaviors and melting characteristics of PP nucleated by a novel supported β-nucleating agent. Polymer. 2008;49:5137–45.
Zhang Z, Chen C, Wang C, Zhang J, Mai K. A novel highly efficient β-nucleating agent for polypropylene using nano-CaCO3 as support. Polym Int. 2010;59:1199–204.
Turner-Jones A, Aizlewood J, Beckett D. Crystalline forms of isotactic polypropylene. Makromol Chem. 1964;75:134–58.
Yi Q, Wen X, Dong J, Han CC. A novel effective way of comprising a β-nucleating agent in isotactic polypropylene (i-PP): polymerized dispersion and polymer characterization. Polymer. 2008;49:5053–63.
Menyhárd A, Faludi G, Varga J. Beta-Crystallisation tendency and structure of polypropylene grafted by maleic anhydride and its blends with isotactic polypropylene. J Therm Anal Calorim. 2008;93:937–45.
Varga J. Melting memory effect of the beta-modification of polypropylene. J Thermal Anal Calorim. 1986;31:165–72.
Varga J, Garzó G, Ille A. Umkristallisation und schmelzen der β modifikation des polypropylenes. Angew Makromol Chem. 1986;142:171–81.
Tabtiang A, Venables R. The performance of selected unsaturated coatings for calcium carbonate filler in polypropylene. Eur Polym J. 2000;36:137–48.
Wang Y, Shen H, Li G, Mai K. Crystallization and melting behavior of PP/nano-CaCO3 composites with different interfacial interaction. J Therm Anal Calorim. 2010;99:399–407.
Wang Y, Shen H, Li G, Mai K. Effect of interfacial interaction on the crystallization and mechanical properties of PP/nano-CaCO3 composites modified by compatibilizers. J Appl Polym Sci. 2009;113:1584–92.
Wang Y, Shen H, Li G, Mai K. Role of interfacial interaction on the crystallization behavior and melting characteristics of PP/Nano-CaCO3 composites modified with different compatibilizers. e-Polymers. 2010; 053.
Varga J. β-Modification of polypropylene and its two component systems. J Thermal Anal Calorim. 1989;35:1891–912.
Acknowledgements
The project was supported by the Natural Science Foundation of China (Grant No. 50873115, 50573094), Natural Science Foundation of Guangdong (S2011020001212), and the Doctoral Fund of Ministry of Education of China (Grant No. 200805580011), and China Postdoctoral Science Foundation (Grant No. 20100480803).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Dai, W., Zhang, Z. et al. Effect of blending way on β-crystallization tendency of compatibilized β-nucleated isotactic polypropylene/poly(ethylene terephthalate) blends. J Therm Anal Calorim 111, 1585–1593 (2013). https://doi.org/10.1007/s10973-012-2425-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2425-0