Abstract
β-nucleated PP/PET blends were prepared using nano-CaCO3 supported β-nucleating agent (β-NA), PP as matrix, and PET as dispersion phase. The effects of preparation methods, PET content, and melting temperature on the non-isothermal crystallization behavior and the melting characteristic and polymorphic composition of PP in the blends were investigated by differential scanning calorimeter (DSC) and wide angle X-ray diffraction (WAXD). The results indicated that the PP crystallized predominantly in β-modification in the presence of β-NA. However, efficiency of β-NA for PP crystallization decreased with addition of PET and increasing PET contents. The β-nucleation of β-NA for PP crystallization in the blends was dependant on the preparation methods. The high β-nucleation and high β-PP content were obtained for PP/PET blend prepared at the temperature of 265 °C and added the β-NA into the blend at the temperature of 180 °C. However, the addition of β-PP or β-NA into blends at 265 °C decreased the β-nucleation, and no β-PP was formed because the β-NA mainly dispersed on the PET dispersion phase or at the interface between PP and PET.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been widely reported that β-nucleated PP exhibits a superior performance characteristic, including improved elongation at break, impact strength, and higher heat distortion temperature as compared to their non-nucleated or α-nucleated homologues [1–14]. However, the yield strength and elastic modulus of β-PP are lower than those of α-PP. In order to improve the yield strength and elastic modulus of β-PP, β-PP blending with other polymers with high yield strength and elastic modulus shall become an increasingly important method.
In the past few decades, many researchers investigated the effect of the second polymer on the β-nucleation for β-nucleated PP. It is found that β-PP/elastomer and β-PP/LDPE blends can be prepared without any difficulty in a wide composition range. However, PP in the β-nucleated PP/HDPE blends crystallizes in the mixed polymorphic composition, suggesting the effect of the a-nucleation semicrystalline HDPE [15–18]. Recently, Tang et al. [19, 20] also indicated that the addition of β-nucleating agent induced effectively the formation of β-PP in PP/EPDM blends. The presence of β-PP could effectively enhance the fracture toughness of PP/EPDM blends. Bai et al. [21–24] found that PP/EPDM β-nucleated blends showed not only a significant enhancement in toughness, but also a promotion of brittle–ductile transition (BDT). Meanwhile, the synergistic toughening effect of β-nucleating agent and POE on PP was found. When the β-nucleating agent and POE (ethylene–octene copolymer) are simultaneously added into PP, β-nucleated PP/POE blends show great improvement of toughness even at low POE content. Guo et al. [25] and Cao et al. [26] found that β-nucleated PP/POE sample exhibited a greater notched Izod impact strength. The addition of β-nucleating agent increased the toughness of PP and PP/POE blends but decreased their tensile strength. The BDT occurred in PP/POE blends, but no obvious BDT point could be seen in β-nucleated PP/POE blends. Qu et al. [27] studied the influence of β-nucleating agent on the mechanical properties of homo-polymerized polypropylene (PPH), random-copolymerized polypropylene (PPR), block-copolymerized polypropylene (PPB), and PPH/PPR/PPB blends and found that the toughnesses of β-nucleated PP and its blends were enhanced without losing the tensile strength and flexural strength.
It is found that the addition of the second component suppressed the formation of β-PP because of the effect of the second component. Varga et al. [28–30] indicated that the most important factor for the formation of a blend with β-crystalline phase is the α-nucleating effect of the second polymer. β-nucleated PP/PVDF and PP/PA6 blends cannot form even in the presence of a highly effective β-nucleating agent because of the strong α-nucleating ability and higher crystallization temperature range of PVDF and PA6.
The addition of the third component compatibilizer might be conducive to raising the content of β-PP in the blend. Later, Menyhárd et al. [30] studied the melting and crystallization characteristics, as well as the polymorphic composition of PP/PA6 blends and their blends compatibilized using PP-g-MA. In the non-compatibilized β-nucleated blends, the PP matrix consisting mainly of the α-modification was formed already at low PA6 content. On the contrary, predominantly, β-PP matrix developed in the presence of PP-g-MA compatibilizers. The formation of α-PP matrix in the absence of compatibilizer is related to the selective encapsulation of the β-nucleating agent in the polar PA6 phase. The influence of the blending technique on the polymorphic composition of the matrix supports the hypothesis of selective encapsulation. The addition of the compatibilizer not only assists the distribution of the β-nucleating agent between both phases of the blends, but also promotes the formation of a matrix rich in β-PP. In the presence of β-nucleating agent, PP-g-MA with low anhydride content and blends of PP containing PP-g-MA crystallize predominantly in the β-form. Zhang et al. [31] obtained high β-crystal content in compatibilized PP/PA6 blends prepared by compression molding.
Recently, β-nucleated PP/PA6 blends and its blends compatibilized by PP-g-MA, PP-g-GMA, POE-g-MA, and EVA-g-MA were prepared in our lab [32, 33]. The effects of different compatibilizer and preparation methods on the formation of β-PP were investigated. It is found that the β-PP content in the β-nucleated PP blends depends not only on the preparation conditions, but also on the compatibilizer type. A high β-PP content (>95%) in the β-nucleated PP/PA6 blends was obtained. The addition of PP-g-MA, POE-g-MA, and EVA-g-MA to the β-nucleated iPP/PA6 blends increased the content of β-PP but decreased the crystallization temperature of PP in the β-nucleated iPP/PA6 blends.
In order to increase the yield strength and elastic modulus of β-PP, the second polymers with high yield strength and elastic modulus shall be employed to prepare the β-PP blends. The PP/PET blends have been widely investigated to improve the performance of PP [34–42]. Varga et al. [18, 43, 44] found that in the PP/PET blend melt-shearing, caused by fiber pulling, would form α-row-nuclei in situ, the surface of which may induce the growth of the β-modification of iPP resulting in a cylindrite of polymorphous composition. However, the effect of PET on β-nucleation for β-nucleated PP was not been reported. In order to develop the high performance β-PP blends and investigate the effect of second polymers with high yield strength and elastic modulus on the formation of β-PP in the blends, β-nucleated PP/PET blends were prepared with a highly efficient nano-CaCO3 supported β-nucleating agent in our lab. In this article, effects of the PET content, preparation method and melting temperatures on the non-isothermal crystallization behavior, and the melting characteristics and the β-PP content of the β-nucleated PP/PET blends were investigated by differential scanning calorimeter (DSC) and wide angle X-ray diffraction (WAXD).
Experiment
Materials
The PP (N-T30S) with MFR = 2.5 g 10 min−1 at 200 °C, was supplied by Maoming Petroleum Chemical Industry Limited Company, Sinopec Group, China. PET (PET105) was purchased from Groups of Foshan Plastics. A nano-CaCO3 supported β-nucleating agent, called as β-NA, was prepared in our lab [45–47].
Specimen preparation
Before blending, PET was dried in a vacuum oven at 110 °C for 12 h. The content of β-NA was 5 wt% of PP, in which the β-nucleating agent is 0.05%. In order to investigate the preparation conditions on efficiency of β-NA in the PP/PET blends, β-nucleated PP/PET blends were prepared at different temperatures and 50 rpm with an internal mixer HL-200 was made by Jilin University science instrument factory, China. The preparation conditions for PP/PET blends were as follows:
Method A The β-PP was first prepared with PP and β-NA for 5 min at the temperature of 220 °C, and then mixed with PET for 5 min at 265 °C.
Method B The PP, β-NA, and PET were mixed at the temperature of 265 °C for 7 min.
Method C The PP and PET were first mixed at the temperature of 265 °C for 2 min, and then mixed with β-NA for 5 min at 265 °C.
Method D The PET and β-NA were first mixed at the temperature of 265 °C for 5 min, and then mixed with PP for 5 min at 265 °C.
Method E The PET and PP were first mixed at the temperature of 265 °C for 2 min, then the blend was cooled to 180 °C, and the β-NA was added at this temperature with mixing for 5 min.
Characterization of specimen
The non-isothermal crystallization and the melting behavior of β-nucleated PP/PET blends were studied by differential scanning calorimetry (DSC). The DSC experiment was carried out by a Perkin-Elmer DSC-7 in a nitrogen atmosphere at heating and cooling rate of 10 °C min−1. About 5–7 mg of specimen was weighted accurately. The specimens were heated to 280 °C at heating rate of 200 °C min−1 and hold on for 5 min to erase their thermal and mechanical history. Then, In order to remain the thermal history as same as the XRD measures, the specimen was cooled to 50 °C at cooling rate of 10 °C min−1 for the crystallization behavior investigation, and subsequently heated to 280 °C at heating rate of 10 °C min−1 for the melting characteristic and polymorphic composition of PP investigation. In this article, it should be pointed out that β-PP has a theoretical upper [T (βα) = 140–141 °C] and a lower limit temperature [T (βα) = 105 °C] [2], so that the values of fusion enthalpy of the β-phase are lower than the real values because of βα growth transition.
Wide-angle X-ray diffraction (WAXD) experiment was conducted with a Rigaku Geigerflex Model D/Max-IIIA rotating anode X-ray diffractometer. Graphite monochromatic Cu-K α radiation was employed as a radiation source. The scanning range was 5–35° at the rate of 4° min−1 and a step length of 0.02°. The content of β-crystal modification was determined according to the standard procedures described in the literature [48, 49], employing the formula:
where I300, I110, I040, and I130 are the diffraction intensities of the β (300), α (110), α (040), and α (130) planes, respectively.
Results and discussion
Effect of PET on non-isothermal crystallization and melting behavior of β-PP
In order to study the effect of PET on the crystallization behavior of PP, the peak temperature of the crystallization (T cp ) of PP, β-PP, and PP/PET (80/20) was investigated. Figure 1 is the DSC crystallization curves of PP, β-PP, and PP/PET blends. It can be observed that the T cp of β-PP is the highest and the T cp in pure PP and PP/PET blends are the same. However, the half-peak width of PP/PET blend is smaller than that of PP, indicating that PET is a weak α-nucleating agent for PP crystallization.
The non-isothermal crystallization and the melting DSC curves of β-PP blends with different PET content prepared by method A are shown in Fig. 2 and the data is listed in Table 1. It can be seen that T cp of PP decreases from 119.6 to 115.4 °C with addition of 10 wt% PET. However, the increase of PET content had slightly influenced the T cp of PP. The T cp of PET in the presence of PP is higher than that of the neat PET.
It can be clearly seen from the DSC melting curves of β-PP that there are four endothermic peaks for β-PP. The double low-temperature melting peaks are attributed to the β-crystal, and two endothermic peaks at high-temperature belong to α-crystal [16, 49–51]. The highest intensity of lower-temperature melting peak of β 1-crystal indicates that β-PP is obtained for PP nucleated by β-NA. The lower-temperature melting peak of β-crystal is considered to reflect the melting range of the more or less disordered β 1-phase, the second one is attributed either to the melting of the original β 1-crystal into a more stable β 2-crystal during scanning or to a perfection and thickening of existing β-lamellae without any change in the geometry and order of the β-crystals.
The DSC melting curves of blends are different from that of β-PP. Although the melting peaks of β 1-and β 2-crystals are observed for β-PP blends containing 5 and 10 wt% PET, respectively, the intensity of the melting peak of β-crystal is significantly decreased. With increasing the PET content, the melting peaks of β-crystal disappears, and only one melting peak of α-crystal is observed.
Figure 3 shows the X-ray diffraction diagrams of β-PP blends with different PET contents prepared by method A. Based on the formula of Turner Jones, the K β values representing β-crystal content are listed in Table 1. It is apparent that the addition of 5 wt% PET decreases the K β value from 0.92 to 0.46. With increasing content of PET to 20 wt%, almost no β-crystal is formed in β-PP/PET blends.
The results of DSC and XRD indicated that the addition of PET decreased the β-nucleating ability of β-NA in β-nucleated PP and the T cp of β-PP. This behavior is attributed to the fact that PET have both the encapsulation role for β-NA and the weak α-nucleating ability for PP. A reasonable interpretation for this observation is that the addition of PET resulted in the β-NA diversion from PP phase to PET phase in blends because of the interaction between PET and β-NA. In the β-nucleated PP blends containing lower content of PET, the addition of PET could not encapsulate the β-NA completely. β-nucleation of β-NA led to the formation of low content of β-crystal and the decreased T cp . However, the addition of high content of PET encapsulated the β-NA completely. The heterogeneous α-nucleation of PET resulted in the formation of α-PP for PP crystallization and increased the T cp of PP with increasing PET content. In the β-nucleated blends with high content of PET, no β-crystal was formed. The above results indicated that it is difficult to obtain the β-PP/PET blends containing high content of PET prepared by method A.
Effects of preparation methods on non-isothermal crystallization and melting behavior of β-nucleated PP/PET blends
Figure 4 shows the non-isothermal crystallization and the melting DSC curves of β-nucleated PP/PET (80/20) blends prepared by different methods and the data is listed in Table 2. It can be clearly observed that the non-isothermal crystallization and the melting DSC curves of the β-nucleated PP/PET (80/20) blends prepared by method E are the same as those of the β-nucleated PP, in which four melting peaks are observed. However, the temperature of melting peak of β 1-crystal shifts to lower temperature because of the effect of PET on the perfection and thickening of β 1-crystal lamellae. The T cp of β-nucleated PP/PET blends prepared by other methods is lower than that of β-PP, and no melting peak of β-crystal is observed. Figure 5 shows the X-ray diffraction diagrams of β-nucleated PP/PET (80/20) blends prepared by different methods. Based on the formula of Turner Jones, the K β values of β-crystal content are listed in Table 2. It is observed that the K β values of β-nucleated PP significantly decreases by addition of the second component (PET). The β-nucleated PP/PET (80/20) blends prepared by different methods have different K β values. The order of the content of β-PP is β-PP > PET20E > PET20C > PET20A > PET20B > PET20D. Speaking concretely, the K β value of β-nucleated PP is 0.92, and the K β value of PP/PET blends (PET20E) prepared at the temperature of 265 °C and added with the β-NA into PP/PET blends at the temperature of 180 °C is still 0.63. No β-PP is obtained for the PP/PET blends prepared by adding the β-NA at the temperature of 265 °C.
The above results indicated that the formation of β-PP in β-nucleated PP/PET blends depends on the preparation methods. It is difficult to obtain the β-PP for β-nucleated PP/PET blends prepared at the mixing temperature of 265 °C. However, high content of β-PP was obtained by adding β-NA at the temperature of 180 °C after mixing PP and PET at 265 °C. It is suggested whether or not the blends were rich in β-form rests with the dispersion of β-NA which was up to the preparation methods. For the PET20E, the β-NA mainly dispersed in the PP matrix or the interface between PP and PET because the PET has crystallized at the temperature of 180 °C at which the β-NA is added. Therefore, high β-nucleating ability and high content of β-PP is obtained in the PET20E blend. For the PP/PET blends prepared by adding the β-NA at the temperature of 265 °C, the β-NA should diffuse into the dispersed phase of PET because of the polar interaction between β-NA and PET. It resulted in the reduced β-nucleating ability of β-NA and no formation of β-PP. Under some research studies, it would be foreseen that the addition of the compatilizer can increase the content of β-PP in the blend further, as the compatilizer assists the distribution of the β-nucleating agent between both phases of the blends.
In order to confirm the β-NA dispersing in the PP matrix or the interface between PP and PET, the PET20E sample was etched with concentrated sulfuric acid to remove PET. Figure 6 shows the non-isothermal crystallization and the melting DSC curves of PET20E and etched PET20E. It can be seen that there are no crystallization and melting peaks of PET. Therefore, it is suggested that PET is completely etched. For the unetched PET20E, four melting peaks are observed. However, there is only one melting peak of α-crystal of PP in the etched PET20E. It is indicated that β-nucleation is absent in the etched PET20E. Therefore, it is suggested that the β-NA mainly dispersed in the interface between PP and PET or dispersed phase of PET. The β-NA dispersed in the interface between PP and PET or PET phase was etched along with PET, resulting in no β-nucleation for PP crystallization. According to the above analysis, it is concluded that the β-NA mainly dispersed in the dispersed phase of PET or the interface between PP and PET. Therefore, the β-NA cannot include the β-nucleation for PP crystallization.
The influence of the final temperature of the melt (T f )
The above results indicated that the β-nucleation in the β-nucleated PP/PET blends depends on the mixing temperature in the blend preparation. In order to investigate the stability of β-nucleation in β-nucleated PP/PET blends, the multiplied heating and cooling DSC of PET20A and PET20E was carried out in different T f .
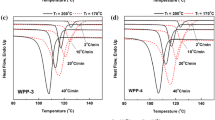
The DSC thermographs of PET20A melted at different T f are presented in Fig. 7 and the data is listed in Table 3. It can be seen that the crystallization and melting characteristics are almost the same as the T f decreased from 280 to 180 °C. The T f has little influence on PP crystallization and melting temperatures. It is suggest that although β-nucleated PP/PET is prepared with β-PP and PET, the β-NA in the β-PP should diffuse into the PET phase during the preparation process because of the strong polar interaction between PET and β-NA. Therefore, it is difficult to obtain β-PP in the PET20A blend by changing the T f . The higher T cp and T m of PP in the PET20A blend melted at T f of 160 °C was attributed to the effect of the self-nucleation of unmolten PP crystals and re-crystallization of PP.
Figure 8 shows DSC crystallization and melting curves of PET20E melted at different T f and the data is listed in Table 4. It can be seen that the T f had no influence on the T cp of PP as the T f decreased from 280 to 180 °C. The higher T cp of PP in the blend melted at T f of 160 °C is attributed to the effect of the self-nucleation of unmolten PP crystals.
It can be seen from DSC melting curves that there are three or four melting peaks for PP in the blends melted at T f between 280 and 200 °C. However, the intensity of melting peak of β 2-crystal weakens and finally disappears, while that of α 1-crystal increases with decreasing the T f . At T f of 160 °C, the self-nucleation of unmolten PP crystals was higher than that of the β-nucleation of β-NA resulting in the formation of α-crystal with high melting temperature.
Conclusions
-
(1)
The PP crystallized predominantly in β-modification in the presence of nano-CaCO3 supported β-nucleating agent. However, the β-nucleation of nano-CaCO3 supported β-nucleating agent for PP crystallization decreased with the addition of PET and increasing the PET content.
-
(2)
The β-nucleation of nano-CaCO3 supported β-nucleating agent for PP crystallization in the blends was dependant on the blending methods. The high β-nucleation and high β-PP contents were obtained for PP/PET blends prepared at the temperature of 265 °C and added with the β-nucleating agent into a blend at the temperature of 180 °C. However, the addition of β-PP or β-nucleating agent into blends at 265 °C decreased the β-nucleation and no β-PP was formed because of the β-nucleating agent mainly dispersed on the dispersed phase of PET or the interface between PP and PET.
References
Grein C. Toughness of neat, rubber modified and filled β-nucleated polypropylene: from fundamentals to applications. Adv Polym Sci. 2005;188:43–104.
Varga J. β-modification of isotactic polypropylene: preparation, structure, processing, properties, and application. J Macromol Sci Phys. 2002;41B:1121–71.
Uchiyama Y, Iwasaki S, Ueoka C, Fukui T, Okamoto K, Yamacuchi M. Molecular orientation and mechanical anisotropy of polypropylene sheet containing N′-dicyclohexyl-2, 6-naphthalene dicarboxamide. J Polym Sci Part B Polym Phys. 2009;47:424–33.
Wang SW, Yang W, Gong G, Xie BH, Liu ZY, Yang MB. Effect of α and β-nucleating agents on the fracture behavior of polypropylene-co-ethylene. J Appl Polym Sci. 2008;108:591–7.
Jacoby P. Beta nucleating masterbatch offers enhanced properties in polypropylene products. Plast Add Compd. 2007;9:32–5.
Zhang P, Liu X, Li Y. Influence of β-nucleating agent on the mechanics and crystallization characteristics of polypropylene. Mater Sci Eng A. 2006;434:310–3.
Cermák R, Obadal M, Ponízil P, Polášková M, Stoklasa K, Hecková J. Injection-moulded α- and β-polypropylenes: II. Tensile properties vs. processing parameters. Eur Polym J. 2006;42:2185–91.
Raab M, Šcudla J, Kolarík J. The effect of specific nucleation on tensile mechanical behavior of isotactic polypropylene. Eur Polym J. 2004;40:1317–23.
Kotek J, Kelnar I, Baldrian J, Raab M. Tensile behavior of isotactic polypropylene modified by specific nucleation and active fillers. Eur Polym J. 2004;40:679–84.
Trongtorsak K, Supaphol P, Tantayanon S. Effect of calcium stearate and pimelic acid addition on mechanical properties of heterophasic isotactic polypropylene/ethylene-propylene rubber blend. Polym Test. 2004;23:533–9.
Kotek J, Raab M, Baldrian J, Grellmann W. The effect of specific β-nucleation on morphology and mechanical behavior of isotactic polypropylene. J Appl Polym Sci. 2002;85:1174–84.
Tjong S, Shen J, Li R. Impact fracture toughness of beta-form polypropylene. Scripta Metallurgica et Materalia. 1995;33:503–8.
Karger-Kocsis J, Varga J, Ehrenstein G. Comparison of the fracture and failure behavior of injection moulded alpha- and beta-polypropylene in high-speed three-point bending tests. J Appl Polym Sci. 1997;64:2059–66.
Karger-Kocsis J, Varga J. Effects of beta-alpha transformation on the static and dynamic tensile behaviour of isotactic polypropylene. J Appl Polym Sci. 1996;62:291–300.
Varga J, Garzó G. The properties of polymer blends of β-modification of polypropylene and an elastomer. Angew Makromol Chem. 1990;180:15–33.
Varga J. β-Modification of polypropylene and its two component systems. Thermal Anal. 1989;35:1891–912.
Grein C, Plummer CJG, Kausch HH, Germain Y, Béguelin Ph. Influence of β-nucleation on the mechanical properties of isotactic polypropylene and rubber modified isotactic polypropylene. Polymer. 2002;43:3279–93.
Varga J, Schulek-Tóth F, Mudra I. Blends of the β-modification of polypropylene. Macromol Symp. 1994;78:229–41.
Tang X, Bao R, Yang W, Xie B, Yang M. Effect of β-phase on the fracture behavior of dynamically vulcanized PP/EPDM blends studied by the essential work of fracture approach. Eur Polym J. 2009;45:1448–53.
Tang X, Yang W, Shan G, Xie B, Yang M, Fu Q. The β phase of isotactic polypropylene in TPVs based on PP/EPDM. J Macromol Sci Part B Phys. 2007;46:841–52.
Bai H, Wang Y, Song B, Huang T, Han L. Effects of nucleating agents on microstructure and fracture toughness of poly(propylene)/ethylene-propylene-diene terpolymer blends. J Polym Sci Part B Polym Phys. 2009;47:46–59.
Bai H, Wang Y, Song B, Fan X, Zhou Z, Li Y. Nucleating agent induced impact fracture behavior change in PP/POE blend. Polym Bull. 2009;62:405–19.
Bai H, Wang Y, Song B, Han L. Synergistic toughening effects of nucleating agent and ethylene-octene copolymer on polypropylene. J Appl Polym Sci. 2008;108:3270–80.
Bai H, Wang Y, Song B, Li Y, Liu L. Effect of nucleating agent on the brittle-ductile transition behavior of polypropylene/ethylene-octene copolymer blends. J Polym Sci Part B Polym Phys. 2008;46:577–88.
Guo M, Yu L, Xiong Z, Zheng D, Wang W, Chen M. Mechanical and melting crystallization properties of β-form polypropylene/POE blends. Gaofenzi Cailiao Kexue Yu Gongcheng/Polym Mater Sci Eng. 2006;22:80–3.
Cao J, Zhao Z, Du R, Zhang Q, Fu Q. Effect of β nucleating agent on mechanical properties of PP/POE blends. Plast Rub Compos. 2007;36:320–5.
Qu N, Fu Y, An F, Qu J, Wang K, Li B. Study on mechanical properties and crystallization behavior of polypropylene and its blends modified by β crystalline form nucleating agent. Polym Plast Technol Eng. 2006;45:637–40.
Varga J, Menyhárd A. Crystallization, melting and structure of polypropylene/poly(vinylidene-fluoride) blends. J Therm Anal Calorim. 2003;73:735–43.
Menyhárd A, Varga J, Liber A, Belina G. Polymer blends based on the β-modification of polypropylene. Eur Polym J. 2005;41:669–77.
Menyhárd A, Varga J. The effect of compatibilizers on the crystallisation, melting and polymorphic composition of β-nucleated isotactic polypropylene and polyamide 6 blends. Eur Polym J. 2006;42:3257–68.
Zhang RH, Shi D, Tjong SC, Li RKY. Study on the β to α transformation of polypropylene crystals in compatibilized blend of polypropylene/polyamide-6. J Polym Sci Part B Polym Phys. 2007;45:2674–81.
Yang Z, Zhang Z, Tao Y, Mai K. Effects of polyamide 6 on the crystallization and melting behavior of β-nucleated polypropylene. Eur Polym J. 2008;44:3754–63.
Yang Z, Zhang Z, Tao Y, Mai K. Preparation, crystallization behavior, and melting characteristics of β-nucleated isotactic polypropylene blends with polyamide 6. J Appl Polym Sci. 2009;112:1–8.
Tao Y, Mai K. Non-isothermal crystallization and melting behavior of compatibilized polypropylene/recycled poly(ethylene terephthalate) blends. Eur Polym J. 2007;43:3538–49.
Shi W, Li Y, Xu J, Ma G, Sheng J. Morphology development in multi-component polymer blends: I. composition effect on phase morphology in PP/PET polymer blends. J Macromol Sci Part B Phys. 2007;46:1115–26.
Akbari M, Zadhoush A, Haghighat M. PET/PP blending by using PP-g-MA synthesized by solid phase. J Appl Polym Sci. 2007;104:3986–93.
Chiu H, Hsiao Y. Compatibilization of poly(ethylene terephthalate)/polypropylene blends with maleic anhydride grafted polyethylene-octene elastomer. J Polym Res. 2006;13:153–60.
Zhong G, Li Z, Li L, Shen K. Crystallization of oriented isotactic polypropylene (iPP) in the presence of in situ poly(ethylene terephthalate) (PET) microfibrils. Polymer. 2008;49:4271–8.
Zhong G, Li Z, Li L, Mendes E. Crystalline morphology of isotactic polypropylene (iPP) in injection molded poly(ethylene terephthalate) (PET)/iPP microfibrillar blends. Polymer. 2007;48:1729–40.
Bae TY, Park KY, Kim DH, Suh KD. Poly(ethylene terephthalate)/polypropylene reactive blends through isocyanate functional group. J Appl Polym Sci. 2001;81:1056–62.
Pang YX, Jia DM, Hu HJ, Hourston DJ, Song M. Effects of a compatibilizing agent on the morphology, interface and mechanical behavior of polypropylene/poly(ethylene terephthalate) blends. Polymer. 2000;41:357–65.
Papadopoulou CP, Kalfoglou NK. Comparison of compatibilizer effectiveness for PET/PP blends: their mechanical, thermal and morphology characterization. Polymer. 2000;41:2543–55.
Varga J, Karger Kocsis J. The difference between transcrystallization and shear induced cylindritic crystallization in fibre reinforced polypropylene composites. J Mater Sci Lett. 1994;13:1069–71.
Varga J, Karger-Kocsis J. Rules of supermolecular structure formation in sheared isotactic polypropylene melts. J Polym Sci Part B Polym Phys. 1996;34:657–70.
Zhang Z, Tao Y, Yang Z, Mai K. Preparation and characteristics of nano-CaCO3 supported β-nucleating agent of polypropylene. Eur Polym J. 2008;44:1955–61.
Zhang Z, Wang C, Yang Z, Mai K. Crystallization behaviors and melting characteristics of PP nucleated by a novel supported β-nucleating agent. Polymer. 2008;49:5137–45.
Zhang Z, Chen C, Wang C, Zhang J, Mai K. A novel highly efficient β-nucleating agent for polypropylene using nano-CaCO3 as support. Polym Int. 2010;59:1199–204.
Turner Jones A, Aizlewood J, Beckett D. Crystalline forms of isotactic polypropylene. Makromol Chem. 1964;75:134–58.
Yi Q, Wen X, Dong J, Han CC. A novel effective way of comprising a β-nucleating agent in isotactic polypropylene (i-PP): polymerized dispersion and polymer characterization. Polymer. 2008;49:5053–63.
Varga J. Melting memory effect of the beta-modification of polypropylene. J Therm Anal. 1986;31:165–72.
Varga J, Garzó G, Ille A. Umkristallisation und Schmelzen der β Modifikation des Polypropylenes. Angew Makromol Chem. 1986;142:171–81.
Acknowledgements
The project for this research was supported by the Natural Science Foundation of China (Grant No. 50573094), Science and Technology Planning Project of Guangdong Province, China (Grant No. 0711020600002), and the Doctoral Fund of Ministry of Education of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Zhang, Z. & Mai, K. Preparation, non-isothermal crystallization, and melting behavior of β-nucleated isotactic polypropylene/poly (ethylene terephthalate) blends. J Therm Anal Calorim 106, 895–903 (2011). https://doi.org/10.1007/s10973-011-1614-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1614-6