Abstract
In order to lower the heating temperature, inorganic-organic hybrid films prepared by a sol-gel process are attractive candidates for glaze. Here, we prepared the inorganic-organic hybrid thick films on glass substrates by the sol-gel process at 100 °C. The precursor sol was prepared by hydrolysis and condensation of 3-glycidoxypropyltrimethoxysilane (GPTMS) and zirconium propoxide chemically modified with acetylacetone. The obtained GPTMS-ZrO2-based thick films had a transmittance higher than 80% in the visible light of 390–800 nm. Thick films with a pencil hardness of 5H and a thickness of more than 100 µm were obtained.

Graphical abstract
Highlights
-
3-glycidoxypropyltrimethoxysilane (GPTMS)-based inorganic-organic hybrid thick films were prepared by a casting process.
-
Transmittance of the films was higher than 80% in the visible light of 390–800 nm.
-
Pencil hardness of the films was increased by the introduction of Zr–O bonds in GPTMS-derived polysiloxane.
-
Thick films with a pencil hardness of 5H and a thickness of more than 100 µm were obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coating ceramics with thick films is important technology affording water resistance, strength, and glossy appearance. For example, a glaze is applied to the surface of ceramics, and it is fired to obtain a glassy, glossy, transparent thick film [1]. These thick films give water resistance, strength, and design characteristics to the ceramic. However, a glaze needs to be fired at temperatures higher than 1000 °C, consuming a large amount of energy. To fabricate coating films at lower temperatures, organic hard coating films have been proposed. Acrylic polymer is an example of organic hard coatings cured by UV light [2,3,4,5,6]. These films are highly transparent, light, and flexible, but they have low hardness. Properties of inorganic-organic hybrid coating films by a sol-gel process have also been investigated [7, 8]. By using this process, thin films with a pencil hardness of 9H or over 9H can be fabricated at 200 °C or even at room temperature [9, 10]. However, thick films tend to be cracked during heat treatment [9, 11,12,13,14,15,16].
In order to overcome this problem, there are several reports on the preparation of thick films by using a sol-gel process [17,18,19]. For example, Aparicio et al. have fabricated consolidated melting gel coatings with a thickness of around 1100 µm [18]. However, the hardness of these thick films is low (0.035 GPa). Furthermore, transparency is also important in fabricating thick films by a sol-gel process. For instance Katagiri et al. have reported transparent films with a thickness up to 3 μm by electrophoretic deposition and subsequent thermal softening of phenylsilsesquioxane [19]. Thus, it is still challenging to fabricate hard, thick films with a thickness larger than 100 µm and high transparency.
In order to obtain transparent thick films with high hardness, precursors for inorganic-organic hybrids such as 3-glycidoxypropyltrimethoxysilane (GPTMS) have been proposed as a component of the precursor solution. GPTMS is a trifunctional alkoxide with an epoxy ring at the end of a long organic chain, and it is widely used in the preparation of films [20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The limited number of hydrolyzable alkoxy groups of GPTMS suppresses the shrinkage of the films [20]. In addition to that, such a long organic chain can promote the relaxation of the stress generated during drying. These may be the reason why self-standing membranes with a thickness of 200–300 µm could be prepared and have been reported [21]. In addition, the epoxy ring opens to form a diol in the sol, which forms an organic network by polymerization of the organic chains during film deposition [26, 27].
In this study, transparent, mechanically hard, inorganic-organic hybrid films were prepared from GPTMS and zirconium propoxide at 100 °C. Hereafter we call the films “GPTMS-ZrO2 thick films”. The inorganic network based on Zr–O bonds may be incorporated into the Si–O inorganic network by hydrolysis and condensation of zirconium propoxide, which forms Si–O–Zr network [26,27,28]. Furthermore, it has been reported that zirconium propoxide promotes the ring-opening of the epoxy ring of GPTMS, promoting the formation of a crosslinked organic-inorganic network [29, 34]. Thus, the incorporation of Zr-alkoxide is expected to improve the hardness of the film. The refractive index of the films can be increased by imparting Zr–O network to the film, which leads to an increase in gloss. In addition, improvement in chemical resistance is also anticipated.
Hardness and transmittance spectra were measured for the GPTMS-ZrO2 thick films prepared by the sol-gel method using GPTMS and zirconium propoxide at a low temperature, and the relationship between the thickness and the pencil hardness was also investigated.
2 Experimental
GPTMS was dissolved in ethanol, and 1.44 wt% HClaq was added to the solution to hydrolyze the silane. After the solution was stirred for 1 h, the precursor Sol 1 was obtained. Zirconium tetra-n-propoxide, Zr(O-n-Pr)4, was dissolved in ethanol, and acetylacetone was added to stabilize the Zr-alkoxide. After the solution was stirred for 1 h, the solution was added dropwise to the precursor Sol 1. The obtained sol was stirred overnight to obtain the precursor Sol 2. The molar ratio of GPTMS:Zr(O-n-Pr)4:EtOH:H2O:HCl = was 1:x:y:2:1.4 × 10−2, where x and y were varied from 0 to 0.8 and from 2 to 8, respectively. The detailed molar ratios of the starting materials are given in Table 1, where the sample names, G1-G6, are also given. The coating was carried out on soda-lime silicate glass plates by the casting process. In the casting process, a predetermined amount of Sol 2 was deposited on the substrate. After the coating, the films were dried at 100 °C for 20 h in an oven to obtain GPTMS-ZrO2 films.
Transmittance spectra of the films coated on the glass substrates were measured by ultraviolet–visible-light (UV-VIS) spectrophotometry (V-750, JASCO, Tokyo, Japan), and the reference side was the air atmosphere. IR absorption spectra of the films were measured using Fourier transform IR spectrometer (FT/IR-4700, JASCO, Tokyo, Japan). In this measurement, films were coated on the silicon wafer by the dipping-withdrawing process at a withdrawal speed of 2.5 mm/sec, and the bare silicon wafer was used as the reference. Scanning electron microscopy (SEM) (Miniscope TM3030Plus, Hitachi, Tokyo, Japan) was used to observe the cross-section and surface of the films. The thickness of the films was measured by digital micrometer (395-271, Kanagawa, Japan), and the pencil hardness was determined using pencils (Uni, Mitsubishi Pencil, Tokyo, Japan) and a portable tester (Yoshimitsu Seiki, Tokyo, Japan), according to JIS K-5600.
3 Results and Discussion
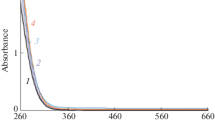
Figure 1 shows the transmittance spectra of the thick films formed on the glass substrates. All samples except for G4 had a transmittance higher than 80% around 390–800 nm in the visible region. G4 showed lower transmittance than the other samples, resulting from the light scattering by the cracks in the film. G1 showed almost the same transmittance as the glass substrate. However, G2, G3, G5, and G6 showed absorption in the UV region, which suggests that the Zr acetylacetonate complexes remain in the films even though the heat-treatment is at 100 °C [28].
Figure 2(a) shows the FT IR spectra of the precursor solution and the Sol 1 for sample G6 with HClaq. Before adding HClaq, GPTMS in ethanol shows several absorption bands at 1700–700 cm−1. The bands at around 1254 cm−1 and 855 cm−1 are assigned to epoxy ring [26, 35]. The strong bands at around 1194 cm−1 and 822 cm−1 are assigned to Si–OCH3 [28, 36, 37]. The strong broad bands at around 1152–1005 cm−1 are assigned to Si–O–Si bonds [27, 37]. The band at around 909 cm−1 is assigned to Si–OH and unopened epoxy rings [26, 27]. After adding HClaq, the intensity of the bands at around 1194 cm−1 and 822 cm−1 decreased and the intensity of the band at around 909 cm−1 increased, which indicates that the hydrolysis and condensation reaction of GPTMS are partially undergoing. At the same time, the decrease in the intensity of the bands at around 1254 cm−1, 909 cm−1, and 855 cm−1 indicate that the ring-opening reaction of the epoxy ring is also occurring. Figure 2(b) shows the FT-IR spectra of GPTMS-ZrO2 films G1-G6 on the silicon wafer. Bands at 1590 cm−1, 1528 cm−1, and 1377 cm−1 are assigned to Zr acetylacetonate complexes [28, 38]. The band at around 957 cm−1 is assigned to Si–O–Zr bonds [26,27,28]. The strong broad band at around 1152–1005 cm−1 indicates the formation of Si–O–Si network, and the band at around 957 cm−1 indicates the formation of Si–O–Zr network. G3 and G4 show the strong bands of Zr acetylacetone complexes, which is because the concentration of ZrO2 in the films becomes higher. Thus, ZrO2 is considered to be partially incorporated into the Si–O–Si network making the Si–O–Zr network.
Figure 3(a) shows a cross-sectional SEM image of sample G6. The GPTMS-ZrO2 film with a thickness of 120 µm adhered to the glass substrate. The cracks in the image are assumed to be formed during the cross-section fabrication by mechanical fracture of the films. Figure 3(b) shows the SEM surface and cross-sectional image. It is also confirmed that the surface of the thick film is smooth.
The effect of the introduction of Zr–O bonds in GPTMS-derived polysiloxane films was investigated. Table 2 shows the pencil hardness and thickness of sample G1-G4. Because the thickness was different between the edges and central area, the thickness is represented in Table 2 with a range for each sample obtained with several measurements. Sample G2 containing ZrO2 at x = 0.2 with a thickness of around 50 µm exhibited the pencil hardness of 9H, which was higher than that of sample G1 (HB) without ZrO2. It suggests that the addition of Zr–O bonds in Si–O inorganic network contributes to the increase of the pencil hardness [26,27,28]. Sample G3 with a larger amount of ZrO2 at x = 0.4 and a thickness of around 50 µm showed the pencil hardness of 5H. Sample G4 was cracked, and the pencil hardness could not be measured. There are some reports about not interacting with the Si–O network or decreasing the hardness when more amount of ZrO2 was introduced to the films [27, 28]. The decrease in the pencil hardness in the samples larger than x = 0.4 indicates that the ZrO2 larger than x = 0.4 was not incorporated into the inorganic network, and the extra ZrO2 can affect the degree of condensation of the inorganic and organic network. Based on these results, ZrO2 content of x = 0.2 was used for the following studies.
When preparing thick films by the casting process, the viscosity of the sol with G2 was very low and the sol flows or leaks from the substrate. Thus, films thicker than 100 µm were not obtained with this composition. To get the sol with higher viscosity, the concentration of the mixed sol was increased by decreasing the amount of the solvent (G5 and G6). The thickness of the films increased with a decrease in the solvent ratio (G5-G6 > G1-G4) and the thickness of G6 achieved more than 100 µm. However, the pencil hardness of G6 was 5H, which is smaller than those of G2 and G5 with smaller thickness. This indicates that the glass substrate affects the pencil hardness of GPTMS-ZrO2 films when the thickness of GPTMS-ZrO2 films is rather small. Therefore, the pencil hardness of GPTMS-ZrO2 thick films itself is supposed to be 5H at the heating temperature of 100 °C.
In addition to G2, G5, and G6 samples, films with various thickness were prepared by changing the amount of solvent in the sol and casting the different amount of sol. Figure 4 shows the relationship between the film thickness and the pencil hardness of GPTMS-ZrO2 films. The thick films with a thickness of up to 60 µm showed the highest pencil hardness of 9H. With an increase in the thickness of over 60 µm, the pencil hardness decreased. This must be because the pencil hardness of the films is affected by the substrate when the film thickness is small, as mentioned above. With an increase in the film thickness, the pencil hardness of the films is hardly affected by the substrate, and the films with smaller pencil hardness were obtained with a thickness of 100 µm or more.
From these results, we succeeded in fabricating transparent films with a pencil hardness of 5H and a thickness of over 100 µm. In addition, these films were prepared with a heat-treatment temperature as low as 100 °C. Thus, these films can be used for alternative films to glaze on ceramics that is prepared with a low-energy process.
4 Conclusion
GPTMS-ZrO2 thick films were prepared by the sol-gel method by adding zirconium propoxide stabilized with acetylacetone to GPTMS solution. The hardness of the thick films was greatly improved by the addition of zirconium propoxide. The transmittance of the thick films was more than 80% at around 390–800 nm in the visible region. By casting a sol with higher inorganic concentration and drying at 100 °C, transparent and hard thick films with a thickness of over 100 µm and a pencil hardness of 5H were obtained.
Change history
28 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10971-022-05883-0
References
Pradell T, Molera J (2020) Ceramic technology. How to characterise ceramic glazes. Archaeol Anthropol Sci 12:189. https://doi.org/10.1007/s12520-020-01136-9
Xu DM, Zhang KDA, Zhu XL (2004) A novel dendritic acrylate oligomer: Synthesis and UV curable properties. J Appl Polym Sci 92:1018–1022. https://doi.org/10.1002/app.20069
Charitidis C, Gioti M, Logothetidis S et al. (2004) Comparison of the nanomechanical and nanoscratch performance of antiscratch layers on organic lenses. Surf Coat Technol 180–181:357–361. https://doi.org/10.1016/j.surfcoat.2003.10.088
Pomázi Á, Toldy A (2021) Development of fire retardant epoxy-based gelcoats for carbon fibre reinforced epoxy resin composites. Prog Org Coat 151:106015. https://doi.org/10.1016/j.porgcoat.2020.106015
Fieberg A, Reis O (2002) UV curable electrodeposition systems. Prog Org Coat 45:239–247. https://doi.org/10.1016/S0300-9440(02)00065-6
Lin D, Kou H, Shi WF et al. (2001) Photopolymerizaton of hyperbranched aliphatic acrylated poly(amide ester). II. Photopolymerization kinetics. J Appl Polym Sci 82:1637–1641. https://doi.org/10.1002/app.2003
Mammeri F, Le Bourhis E, Rozes L, Sanchez C (2005) Mechanical properties of hybrid organic-inorganic materials. J Mater Chem 15:3787–3811. https://doi.org/10.1039/b507309j
Que W, Zhang QY, Chan YC, Kam CH (2003) Sol-gel derived hard optical coating via organic/inorganic composites. Compos Sci Technol 63:347–351. https://doi.org/10.1016/S0266-3538(02)00227-0
Sasaki T, Kamitani K (2008) Preparation of thick and hard coating films via sol-gel process with a low temperature treatment. J Sol-Gel Sci Technol 46:180–189. https://doi.org/10.1007/s10971-008-1685-4
Kozuka H, Nakajima K, Uchiyama H (2013) Superior properties of silica thin films prepared from perhydropolysilazane solutions at room temperature in comparison with conventional alkoxide-derived silica gel films. ACS Appl Mater Interfaces 5:8329–8336. https://doi.org/10.1021/am400845y
Rosero-Navarro NC, Curioni M, Castro Y et al. (2011) Glass-like CexOy sol-gel coatings for corrosion protection of aluminium and magnesium alloys. Surf Coat Technol 206:257–264. https://doi.org/10.1016/j.surfcoat.2011.07.006
Zaharescu M, Predoana L, Barau A et al. (2009) SiO2 based hybrid inorganic-organic films doped with TiO2-CeO2 nanoparticles for corrosion protection of AA2024 and Mg-AZ31B alloys. Corros Sci 51:1998–2005. https://doi.org/10.1016/j.corsci.2009.05.022
Correa PS, Malfatti CF, Azambuja DS (2011) Corrosion behavior study of AZ91 magnesium alloy coated with methyltriethoxysilane doped with cerium ions. Prog Org Coat 72:739–747. https://doi.org/10.1016/j.porgcoat.2011.08.005
Hu RG, Zhang S, Bu JF et al. (2012) Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog Org Coat 73:129–141. https://doi.org/10.1016/j.porgcoat.2011.10.011
Zomorodian A, Brusciotti F, Fernandes A et al. (2012) Anti-corrosion performance of a new silane coating for corrosion protection of AZ31 magnesium alloy in Hank’s solution. Surf Coat Technol 206:4368–4375. https://doi.org/10.1016/j.surfcoat.2012.04.061
Lamaka SV, Montemor MF, Galio AF et al. (2008) Novel hybrid sol-gel coatings for corrosion protection of AZ31B magnesium alloy. Electrochim Acta 53:4773–4783. https://doi.org/10.1016/j.electacta.2008.02.015
Hasegawa K, Kunugi S, Tatsumisago M, Minami T (1999) Preparation of thick films by electrophoretic deposition using surface modified silica particles derived from sol-gel method. J Sol-Gel Sci Technol 15:243–249. https://doi.org/10.1023/A:1008789025826
Aparicio M, Mosa J, Rodriguez G et al. (2019) Consolidated melting gel coatings on AZ31 magnesium alloy with excellent corrosion resistance in nacl solutions: an interface study. ACS Appl Mater Interfaces 11:3493–3505. https://doi.org/10.1021/acsami.8b20199
Katagiri K, Hasegawa K, Matsuda A et al. (1998) Preparation of transparent thick films by electrophoretic sol-gel deposition using phenyltriethoxysilane-derived particles. J Am Ceram Soc 81:2501–2503. https://doi.org/10.1111/j.1151-2916.1998.tb02653.x
Feng Z, Liu Y, Thompson GE, Skeldon P (2010) Crack-free sol-gel coatings for protection of AA1050 aluminium alloy. Surf Interface Anal 42:306–310. https://doi.org/10.1002/sia.3162
Tadanaga K, Yoshida H, Matsuda A et al. (2003) Preparation of proton-conductive inorganic-organic hybrid films from 3-glycidoxypropyltrimethoxysilane and orthophosphoric acid. Chem Mater 15:1910–1912. https://doi.org/10.1021/cm020692l
Tezuka T, Tadanaga K, Matsuda A et al. (2005) Utilization of glass papers as a support for proton conducting inorganic-organic hybrid membranes from 3-glycidoxypropyltrimethoxysilane, tetraalkoxysilane and orthophosphoric acid. Solid State Ion 176:3001–3004. https://doi.org/10.1016/j.ssi.2005.09.039
Daniels MW, Francis LF (1998) Silane adsorption behavior, microstructure, and properties of glycidoxypropyltrimethoxysilane-modified colloidal silica coatings. J Colloid Interface Sci 205:191–200. https://doi.org/10.1006/jcis.1998.5671
Wu LYL, Chwa E, Chen Z, Zeng XT (2008) A study towards improving mechanical properties of sol-gel coatings for polycarbonate. Thin Solid Films 516:1056–1062. https://doi.org/10.1016/j.tsf.2007.06.149
Hwang DK, Moon JH, Shul YG et al. (2003) Scratch resistant and transparent UV-protective coating on polycarbonate. J Sol-Gel Sci Technol 26:783–787. https://doi.org/10.1023/A:1020774927773
Milošev I, Kapun B, Rodič P, Iskra J (2015) Hybrid sol-gel coating agents based on zirconium(IV) propoxide and epoxysilane. J Sol-Gel Sci Technol 74:447–459. https://doi.org/10.1007/s10971-015-3620-9
Oliver MS, Blohowiak KY, Dauskardt RH (2010) Molecular structure and fracture properties of ZrOX/Epoxysilane hybrid films. J Sol-Gel Sci Technol 55:360–368. https://doi.org/10.1007/s10971-010-2262-1
Ak A, Çiçek B (2021) Synthesis and characterization of hydrophobic glass-ceramic thin film derived from colloidal silica, zirconium(IV) propoxide and methyltrimethoxysilane via sol-gel method. J Sol-Gel Sci Technol 98:252–263. https://doi.org/10.1007/s10971-021-05482-5
Hoebbel D, Nacken M, Schmidt H (2000) The effect of nanoscaled metal oxide sols on the structure and properties of glycidoxypropyltrimethoxysilane derived sols and gels. J Sol-Gel Sci Technol 19:305–309. https://doi.org/10.1023/A:1008777414416
Abdollahi H, Ershad-Langroudi A, Salimi A, Rahimi A (2014) Anticorrosive coatings prepared using epoxy-silica hybrid nanocomposite materials. Ind Eng Chem Res 53:10858–10869. https://doi.org/10.1021/ie501289g
Rüdiger Nass, Ertugrul Arpac, Walther Glaubitt, Helmut Schmidt (1990) Modelling of ORMOCER coatings by processing. J Non Cryst Solids 121:370–374. https://doi.org/10.1016/0022-3093(90)90160-N
Pirhady Tavandashti N, Sanjabi S, Shahrabi T (2009) Corrosion protection evaluation of silica/epoxy hybrid nanocomposite coatings to AA2024. Prog Org Coat 65:182–186. https://doi.org/10.1016/j.porgcoat.2008.10.010
Aoki M, Tadanaga K, Tatsumisago M (2010) All-solid-state electric double-layer capacitor using ion conductive inorganic-organic hybrid membrane based on 3-glycidoxypropyltrimethoxysilane. Electrochem Solid-State Lett 13:52–55. https://doi.org/10.1149/1.3305323
Philipp G, Schmidt H (1986) The reactivity of TiO2 and ZrO2 in organically modified silicates. J Non Cryst Solids 82:31–36. https://doi.org/10.1016/0022-3093(86)90107-9
Innocenzi P, Brusatin G, Guglielmi M, Bertani R (1999) New synthetic route to (3-Glycidoxypropyl)trimethoxysilane-based hybrid organic−inorganic materials. Chem Mater 11:1672–1679. https://doi.org/10.1021/cm980734z
Sousa RPCL, Figueira RB, Gomes BR et al. (2021) Organic-inorganic hybrid sol-gel materials doped with a fluorescent triarylimidazole derivative. RSC Adv 11:24613–24623. https://doi.org/10.1039/d1ra03997k
Wei L, Chen Q, Gu Y (2010) Preparation and characterization of transparent PANI-SiO2 hybrid conducting films. Polym Eng Sci 50:986–990. https://doi.org/10.1002/pen.21552
Zhang Y, Wang MC, He H, Li H (2016) Effect of heat treatment on the crystalline structure and hydrophilic properties of TiO2 porous thin films. J Sol-Gel Sci Technol 80:881–892. https://doi.org/10.1007/s10971-016-4173-2
Acknowledgements
The present study was partly supported by JST. A-STEP Grant Number JPMJTM19AH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toide, T., Rosero-Navarro, N.C., Miura, A. et al. Preparation of transparent and mechanically hard inorganic-organic hybrid thick films from 3-glycidoxypropyltrimethoxysilane and zirconium propoxide. J Sol-Gel Sci Technol 104, 478–483 (2022). https://doi.org/10.1007/s10971-022-05836-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05836-7