Abstract
Rare-earth doped glasses have garnered interest due to their potential applications in light-emitting devices. Although the sol–gel technique is useful in preparing them at moderately low temperatures, developing silicate glasses with excellent photoluminescence performance remains a formidable challenge due to their low solubility in the glass matrix and the difficulty in controlling valence states of rare-earth ions (RE). Here, we investigated whether these RE ions are reduced by heating in a hydrogen gas atmosphere or by irradiating with X-rays. We have succeeded in synthesizing Sm3+ and Eu3+ ion-doped Al2O3–SiO2 glasses with exceptionally strong photoluminescence. When heated in hydrogen gas, the Sm3+ and Eu3+ ions were reduced to their divalent states. However, when irradiated with X-rays, only Sm3+ ions were reduced to Sm2+; no reduction occurred in the Eu3+ ions. This was because when irradiated with X-rays, the hole centers become trapped in the oxygen ions bound to the Al3+ ions, and the electrons released from the oxygen ions are consequently captured by the nearest Sm3+ ions, resulting in the formation of Sm2+. In contrast, such a reduction does not occur in the Eu3+-doped glasses. It was further found that the reduced Sm2+ ions are easily oxidized to Sm3+ ions by heating at 250 °C in air. Thus, the Sm3+-doped Al2O3–SiO2 glasses could be used for X-ray therapy and sensor applications due to their fast redox reactions.

The Sm3+ ions doped in Al2O3–SiO2 glasses are reduced by irradiating X-ray and the reduced Sm2+ ions are easily oxidized by heating in air. The fast redox reaction between Sm3+ and Sm2+ ions would be appropriate for X-ray therapy and sensor applications.
Highlights
-
Glasses showing X-ray response were prepared to dope Sm3+ ions by the sol–gel method.
-
Al2O3–SiO2 glasses were appropriate to dope rare-earth ions exhibiting highly intense photoluminescence, in which the doped-Sm3+ ions were reduced to the Sm2+ by irradiating with X-rays.
-
The reduction of Sm3+ ions proceeded by forming hole centers in oxygen ions bound to Al3+ ions and consequently capturing the emitted electrons in the Sm3+ ions.
-
The reduced Sm2+ ions were easily oxidized to the Sm3+ ions by heating in air
-
The fast redox reaction between Sm3+ and Sm2+ ions would be appropriate for X-ray therapy and sensor applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The sol–gel technique has been effective in preparing high-purity and homogeneous glasses at temperatures lower than those required by the melt-quenching technique. An author (MN) employed the sol–gel technique and successfully prepared the first-of-its-kind glass that cannot be obtained from the conventional melting method, for example, ZrO2–SiO2 [1] or P2O5–SiO2 [2] glasses with high mechanical toughness and fast proton conductivity, respectively. Recently, we prepared glasses doped with rare-earth (RE) ions that showed persistent spectrum hole-burning at room temperature [3] and strong photoluminescence (PL) [4]. These glasses have attracted considerable interest due to their possible applications in photonic fields, such as phosphors, optical amplifiers, lasers, and memories.
In melt-quenching glasses, RE ions with multiple valence states can usually be doped with only higher valence states. The Eu3+ and Sm3+ ions are particularly interesting among the RE ions because they can occupy the divalent states and exhibit completely different PL properties than their trivalent ions [5,6,7]. Controlling the valence states of the RE ions, if achievable, would offer numerous opportunities for glass applications. One technique for reducing the RE ions is melting the glasses together with the reductants [8,9,10,11]; however, it causes glass contamination due to residual reductant. The other technique is the secondary heat-treatment of glass in a reducing H2 gas atmosphere [12,13,14]. The reduction of the RE ions in glass occurs by the diffusion of H2 gas molecules in glass and their subsequent reaction with the RE ions. However, because of the low diffusion rate of H2 gas through glass, the reduction of silicate glasses is limited to near the surface [14]. We successfully prepared the Eu3+-doped Al2O3-containing silicate glasses exhibiting fast H2 diffusion rate and discovered that the Al–O− bonds promote the reaction between the Eu3+ ions and H2 molecules. Consequently, the Eu3+ ions located on the surface and in the center of the glass were reduced by heating in a short time [15,16,17]. Thus, the Al2O3-containing glasses were promising as a host for doping the RE ions. However, it is difficult to produce glasses containing a large amount of Al2O3 using the conventional melting method because Al2O3 raises the melting temperature of the glass. This is the reason for applying the sol–gel method to prepare glasses with high Al2O3 content [18, 19].
More recently, sources such as X-rays and lasers have been reportedly used for controlling the valence states of RE ions [20,21,22,23,24]. The reduction of RE ions was dependent on glass compositions and energy of the beams, and high-energy beams often caused defects in the glass structure. Nevertheless, this method would be an effective technique because of enabling the reduction of the RE ions at the focusing point of beams. Herein, we studied the possibility of X-rays as a tool for reducing the Sm3+ and Eu3+ ions doped in the sol–gel-derived glasses. The glasses containing various oxides were prepared to dope the Sm3+ and Eu3+ ions by the sol–gel method and tested for whether the RE ions were reduced by irradiating with X-rays. Only the Sm3+ ions were reduced by irradiating with X-rays, and their reduction process was completely different from that of the glasses heated in an H2 gas atmosphere. We discussed the reduction mechanism of irradiating with X-rays and elucidated the role of Al3+ ions in reducing the RE ions. The reduced Sm2+ ions were reversibly oxidized to Sm3+ by heating in an air atmosphere or irradiating with an Ar+ laser. Thus, the Sm3+-doped Al2O3–SiO2 glasses would be appropriate for practical applications, such as X-ray therapy and dosimetry [25,26,27,28,29,30], because the Sm2+ and Sm3+ ions can be clearly distinguished by the difference in the PL color.
2 Experiments
100SiO2, 5B2O3·95SiO2, 10Na2O·90SiO2, 10(or 20)Al2O3·90(or 80)SiO2, 10ZrO2·90SiO2, and 10TiO2·90SiO2 in mol% glasses were prepared to dope 10 wt% Sm2O3 and Eu2O3 using the sol–gel method. The materials were commercially available and were used as received. Due to the varying hydrolysis rates of the metal alkoxides used in the sol–gel process, there was a risk of not achieving a homogeneous glass structure. To avoid the heterogeneous hydrolysis of metal alkoxides, we developed a multistep hydrolysis process. Si(OC2H5)4 was first hydrolyzed using a mixed solution of C2H5OH and H2O (including 0.15 mol/L HCl) at room temperature for 1 h, followed by the addition of the complementary compounds Al(OC4H9)3, Ti(OC3H7)4, Zr(OC4H9)4, B(OC2H5)3, or CH3COONa. Alkoxides of RE atoms were commercially available but not used because of their insolubility in alcohol. SmCl3·6H2O or EuCl3·6H2O was dissolved in alcohol and then added to the solution while stirring. Finally, a mixed solution of C2H5OH and H2O was added to hydrolyze the metal alkoxides further. The resultant transparent and homogeneous solutions were placed in a sealed container to form stiff gels with a thickness of about 1 mm. The dried gel plates were completely hydrolyzed by exposing them to water vapor in a sealed vessel at 150 °C for 15 h. Using this process, homogeneous gels were prepared with no residual unhydrolyzed alkoxides. The obtained gels were heated in air at a rate of about 50 °C/h to a predetermined temperature of 600 °C for B2O3– and Na2O–SiO2 glasses and 800 °C–900 °C for Al2O3–, TiO2–, and ZrO2–SiO2 glasses, respectively. The obtained glasses were transparent with no crystalline precipitates.
X-ray irradiation was performed using the Cu-Kα line of a Rigaku, Rad-B with 40 kV and 20 mA (corresponding to ~150 mGy/min) for various periods at room temperature. Heating of the glasses in H2 gas was performed at 600 °C in a fused silica glass tube furnace with a flow rate of ~5 ml/min of 100% H2. The glasses’ optical absorption and PL spectra were measured with Shimazu UV-3600 Plus and Horiba Jobin Yvon FL3-22 spectrometers. 27Al MAS solid-state nuclear magnetic resonance (NMR) data were collected using a JEOL ECX 800-MHz spectrometer equipped with an 18.79 T magnet at the National Institute for Materials Science. The electron spin resonance (ESR) measurement was performed using a Jasco, JES-FE ME3X spectrometer at room temperature. The g-values were calibrated using diphenyl-picryl-hydrazal.
3 Results and discussion
3.1 Role of the glass compositions on the PL properties
Some borate and phosphate glasses have been used to dope RE ions with divalent states [8, 10, 31]; however, they are not suitable for practical applications because they lack chemical and mechanical durability. We chose silicate glasses as the host of RE ions because they have no such drawbacks. All the glasses prepared by heating in air had their optical absorption and PL spectra analyzed. Figure 1 shows the optical absorption spectra of Sm2O3-doped 100SiO2 glass and Eu2O3-doped 10Al2O3·90SiO2 glass; similar spectra were observed for glasses having other compositions. The absorption bands are all assigned to the f–f transitions of Sm3+ and Eu3+ ions, respectively, as shown in Fig. 1. Few Eu2+ ions are detected in as-prepared Eu2O3-doped glasses, which will be discussed later. The Eu2+ ions exhibit optical absorption in the ultraviolet region, but it may be hidden behind the large absorption bands of Eu3+ and host glass. However, in the Sm2O3-doped glasses, only the absorption bands are observed due to the Sm3+ ions, indicating that the Sm ions are incorporated only as a trivalent state.
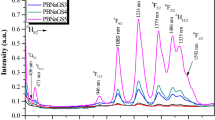
Unlike the absorption spectra, the PL spectra varied greatly depending on the glass compositions, which were grouped into three types based on the PL intensities: group-a: SiO2 and B2O3–SiO2 glasses for no PL, group-b: ZrO2–, TiO2– and Na2O–SiO2 glasses for weak PL, and group-c: Al2O3–SiO2 glass for highly intense PL. Among the glasses in the three groups, the PL spectra of 10B2O3·90SiO2, 10ZrO2·90SiO2, and 10Al2O3·90SiO2 glasses doped with Sm2O3 and that of 10Al2O3·90SiO2 glass doped with Eu2O3 are shown in Fig. 2. The PL bands at 565, 605, and 650 nm in the Sm2O3-doped glasses are assigned to 4G5/2 → 6HJ (J = 5/2, 7/2, 9/2) transitions of Sm3+ ions and the bands at 580, 600, 620, 650, and 710 nm in the Eu2O3-doped glass to 5D0 → 7FJ (J = 0, 1, 2, 3, 4) transitions of Eu3+ ions, respectively.
Si4+ and B3+ ions form a highly rigid network structure connecting SiO4 and BO3 by bridging oxygen. When a large number of RE ions are introduced into a rigid network structure, a limited number of SiO4 and BO3 ions surround the RE ions. Consequently, the RE ions cannot be dispersed homogeneously in the matrix and instead cluster, resulting in the quenching of the PL intensity. However, Na+, Ti4+, Zr4+, and Al3+ ions do not form the glass network structure by themselves but create nonbridging oxygen to open the network structure, where the RE ions are well dispersed to increase the PL intensities. Among these metal ions, the Al3+ ions play a completely different role in modulating PL intensity as the PL intensities increase by a factor of three or more compared with the glasses in group-b. It is reported that the Al3+ ions work to increase the solubility of the RE ions, resulting in intense PL [27].
Further, Al3+ ions form AlO6 octahedra and AlO4 tetrahedra in silicate glasses. The AlO4 tetrahedron creates a network structure by bridging oxygen atoms with the SiO4 tetrahedron, and the negative charge in AlO4 is compensated by the alkali ions such as Li+ and Na+ [32]. The tetrahedral AlO4 groups allow the RE ions to be homogeneously doped into the glass structure, contributing to the increase in the PL intensities. Thus, the Al3+ ions act to increase the PL intensities of the RE ions coexisting with alkali ions. In the present glasses, however, no alkali ion is added for compensating the negative charge in AlO4 tetrahedron, so that the role of Al3+ ions in the glass structure may be different from the glasses containing alkali ions. The effect of Al3+ ions was studied using 27Al MAS-NMR spectra; the results are shown in Fig. 3. In this figure, the MAS-NMR spectrum of 12.5Na2O·12.5Al2O3·75SiO2 glass, prepared using the melt-quenching process, is shown for reference, where only one 27Al signal is detected at around 60 ppm that is assigned to Al species in AlO4 tetrahedra [33]. This result agrees with the structural model accepted for alkali-alumino-silicate glasses, i.e., all the Al3+ ions form AlO4 and all the oxygen atoms are bridging, accompanied by Na+ ions as charge compensators [32].
Conversely, Al2O3–SiO2 glasses prepared in this study exhibit two additional signals peaking at 35 and 5 ppm in addition to the 60 ppm signal. These NMR signals at 35 and 5 ppm are assigned to AlO5 and AlO6 units, respectively [34,35,36]. When doped with Eu3+ ions, the spectrum became broader, making signal identification more difficult. These broad spectra were decomposed into three components using Gaussian distribution curves; the figure shows the result. Comparing the area of decomposed bands, it is evident that the ratio of the AlO5 group increases by doping Eu3+ ions. Nevertheless, the effect of Al–O polyhedral on the glass structure and doping RE ions is still uncertain; further studies are required.
3.2 The reduction of RE ions by irradiating with X-rays
The prepared glasses were tested for whether the RE ions were reduced by heating in the H2 gas atmosphere or by X-ray irradiation. The reduction of the Sm3+ and Eu3+ ions was examined by measuring the PL spectra to show completely different spectra between trivalent and divalent states. No change was observed in the PL spectra of group-a and group-b glasses, with only group-c Al2O3–SiO2 glasses showing changes in their PL spectra when heated in H2 gas atmosphere or irradiated with X-rays. Figure 4 shows the PL spectra of 20Al2O3·80SiO2 glasses doped with Sm3+ and Eu3+ ions (hereafter abbreviated as Sm: AS and Eu: AS glass) after heating in H2 gas and irradiating with X-rays. When heated in H2 gas atmosphere, both the Eu: AS and Sm: AS glasses exhibited the reduction of the Eu3+ and Sm3+ ions into Eu2+ and Sm2+, respectively (see Fig. 4a). A broad PL band in the wavelength region of 400–550 nm appeared in the Eu: AS glass. This broad PL band can be assigned to the 4f65d → 4f7 (8S7/2) transition of Eu2+ ions. The other, Sm: AS glass, exhibited new PL bands ranging from 690 to 730 nm in addition to the FL bands due to the Sm3+ ions. These new PL bands are assigned to 5D0 → 7FJ (J = 0, 1, 2) transitions of the Sm2+ ions. We previously studied the reduction of Eu3+ and Sm3+ ions under heating in H2 gas atmosphere and found that the reducing reaction was completely different between Eu: AS and Sm: AS glasses [37]. When the Eu: AS glass was heated in H2 gas, the diffusing H2 gas molecules reacted to form Al–OH bonds while reducing the Eu3+ ions to Eu2+. The Al–O– bonds surrounding the Eu3+ ions were oxidized to form Al–OH bonds, in which the released electrons were captured by the Eu3+ ions forming Eu2+. However, Sm3+ ions were reduced without forming the Al–OH bond.
The difference in the reduction between Eu3+ and Sm3+ ions was more prominent in the X-ray irradiation experiment. Figure 4b shows the PL spectra of the Eu: AS and Sm: AS glasses after irradiating with an X-ray beam. X-ray irradiation allows only the Sm3+ ions to reduce and not the Eu3+ ions, even though they are doped in the same Al2O3–SiO2 glass. These findings strongly suggest that the reduction process between Sm: AS and Eu: AS glasses is completely different. The Sm2+ ion has the same electron configuration as the Eu3+ ion and shows the PL bands due to the 5D0 → 7FJ transitions in the visible wavelength region. Among the 5D0 → 7FJ transitions, the 5D0 → 7F0 transition is a forced electric dipole transition and varies with chemical bonds in the vicinity of RE ions. Conversely, the 5D0 → 7F1 transition is a magnetic dipole transition, which is unaffected by chemical bonding around the RE ions. Therefore, the intensity ratio of the 5D0 → 7F0 transition (578 nm (Eu3+), 688 nm (Sm2+)) to the 5D0 → 7F1 transition (590 nm (Eu3+), 705 nm (Sm2+)) can be used to explore the chemical bonding surrounding the RE ions [38]. As shown in Figs. 2 and 4, the Sm2+ ions in AS glass exhibited a much higher intensity ratio than the Eu3+ ions in AS glass. A large value for the intensity ratio indicates that the Sm3+ ions are more covalently bonded with the surrounding oxygen. Therefore, the Al–O– bonds surrounding the Sm3+ ions hardly form the Al–OH bonds.
The ESR spectra of Eu: AS glass treated under various conditions are shown in Fig. 5A. We observe that the as-prepared Eu: AS glass has a weak ESR signal between 1300 and 2500 Gauss, which can be attributed to the Eu2+ ions (spectrum (a)), suggesting that a part of the Eu dopant has already been reduced in the glass prepared by heating in air. This small amount of Eu2+ ions does not contribute to the PL spectrum, as shown in Fig. 2. After X-ray irradiation, a new ESR signal was observed at 3300 Gauss (spectrum (b)). This ESR signal at 3300 Gauss can be assigned to the Al-oxygen hole trap centers (AlOHC) [39]. The ESR signal caused by AlOHC defects was also detected in the AS glass doped without RE ions and irradiated with X-rays (spectrum (d)). These results indicate that the AlOHC is formed by irradiating with X-rays but is independent of the presence of RE ions. Further, the ESR intensity of Eu2+ ions did not change after irradiating with X-rays, indicating that Eu3+ ion is not reduced by irradiating with X-rays.
Figure 5B shows the ESR spectra of Sm: AS glass. Like the as-prepared glass, the Sm: AS glass heated in H2 gas did not show any ESR signal (spectra (a) and (c), respectively). Sm2+ ions do not contribute to the ESR signal because of their nonmagnetic property. However, the Eu: AS glass heated in H2 gas exhibited greatly increased signals of the Eu2+ ions and obscured another signal. Alternately, when irradiated with X-rays, the ESR signal of AlOHC was measured to be around 3250 Gauss.
Table 1 summarized the phenomena caused by irradiating with X-rays or heating in H2 gas. Mackey and Nahum studied the X-ray irradiation of Eu3+-doped silicate glasses and concluded that the electrons released from the oxygen ions are transferred to the Eu3+ ions, forming the activated [Eu3+]− state different from the Eu2+ ions [40]. In a series of experiments, we noticed that the formation of AlOHC was related to the reduction of Sm3+ ions, so we collected the PL and ESR data of glasses treated under various conditions. The results are shown in Fig. 6, where the intensities of the AlOHC signal and PL of Sm2+ ions are plotted. The linear relation between these two values indicates that the reduction of Sm3+ ions proceeds through the formation of AlOHC, which differs greatly from the reduction in Eu: AS glasses.
The reduction of Sm3 + ions by X-ray irradiation, however, was considered as follows: the hole centers were trapped in the oxygen ions bound to Al3+ ions, and the electrons released from the oxygen ions were consequently captured by the adjacent Sm3+ ions, resulting in the formation of the Sm2+. In contrast, such a reaction did not occur in the Eu: AS glass on irradiating with X-rays. The X-rays causes the defects (see Fig. 5B), but the Eu3+ ions cannot capture the released electrons. The Eu3+ ions are only reduced, accompanied by the formation of Al–OH bonds in the H2 gas atmosphere.
We further investigated the PL properties of Sm2+ ions reduced using two different methods. Figure 7 shows the comparison between the PL spectra in the region of 5D0 → 7FJ transitions and the PL decay curves. The X-ray-irradiated glass had the PL bands at a lower energy side than the H2-heated glass. A shift to low energy indicates strong covalent bonding between Sm2+ and O2- ions. The X-ray-irradiated glass had a shorter lifetime of emission than the H2 gas-heated glass.
3.3 Sm2+ ions reduced by irradiating with X-rays or heating in H2 gas
The intended application determines whether X-rays or H2 gas-treated glass is used. When heated in an H2 gas atmosphere, the reduction proceeds from the surface to the inside, as measured by the diffusion rate of H2 molecules in the glass structure. The Al2O3–SiO2 glass was discovered to be effective at accelerating the diffusion rate of H2 molecules. Nevertheless, it was necessary to heat for more than 10 h at 500 °C to reduce the 1 mm thick glass. In contrast, X-rays demonstrate excellent capacity to reduce the Sm3+ ions in a limited area due to its ability to focus on a single point.
Figure 8A shows the relationship between the PL intensities of the Sm2+ ions and the X-ray irradiation time at room temperature. The Sm3+ ions are reduced quickly at room temperature, the rate of which is comparable with that for the glass heated at 600 °C in an H2 gas atmosphere. We further noticed that the reduced Sm2+ ions are reversibly oxidized to Sm3+ ions by heating at moderately low temperatures in the air atmosphere. Those heated at 160 °C and 260 °C are shown in Fig. 8B. It is evident that the oxidation to Sm3+ occurs by heating at low temperatures such as 160 °C and is completed within 100 min at 260 °C. In this figure, the Sm: AS glass reduced in H2 gas is also compared, indicating that the oxidation is so slow that heating at 600 °C or higher is needed for oxidizing to Sm3+. Thus, X-ray irradiation has an advantage over the heat treatment in H2 gas because Sm3+ ions can be reduced at room temperature and back to their original state by heating at low temperatures in air. The oxidation of the Sm2+ ions also occurred by laser beam irradiation. Figure 9 shows the PL spectra after irradiating with X-rays (a), followed by heating in air at 400 °C (b), or irradiating with Ar+ (488 nm wavelength) gas laser for 30 min at room temperature (c). The Ar+ laser can be used to change Sm2+ to Sm3+ ions.
A Dependence of PL intensities of Sm2+ ions on irradiating with X-rays (open circles) at room temperature or heating in H2 gas at 600 °C (closed triangles). B Change of PL intensities of Sm2+ ions with heating in air at given temperatures. Open and closed circles are for glasses irradiated with X-rays and closed triangles are for glasses heated in H2 gas
4 Conclusion
X-ray irradiation was used to study the reduction of RE ions doped in sol–gel-derived glasses. Only the Sm3+ ions doped in Al2O3–SiO2 glasses were converted to Sm2+ by X-ray irradiation. We discovered that Sm3+ ion reduction occurs by creating hole centers in oxygen ions linked to Al3+ ions and then trapping the released electrons in the Sm3+ ions. Sm2+ ions reduced by X-ray irradiation were easily oxidized to Sm3+ ions by heating in air at 250 °C. Consequently, the differing PL characteristics of Sm2+ and Sm3+ ions generated by X-ray irradiation can be used for radio-photoluminescent materials.
References
Nogami M, Tomozawa M (1986) ZrO2‐transformation‐toughened glass‐ceramics prepared by the sol‐gel process from metal alkoxides. J Am Ceram Soc 69:99–102. https://doi.org/10.1111/j.1151-2916.1986.tb04709.x
Nogami M, Nagao R, Wong C, Kasuga T, Hayakawa T (1999) High proton conductivity in porous P2O5−SiO2 glasses. J Phys Chem B 103:9468–9477. https://doi.org/10.1021/jp991277s
Nogami M, Abe Y, Hirao K, Cho DH (1995) Room temperature persistent spectra hole burning in Sm2+‐doped silicate glasses prepared by the sol‐gel process. Appl Phys Lett 66:2952–2954. https://doi.org/10.1063/1.114240
Nogami M, Enomoto T, Hayakawa T (2002) Enhanced fluorescence of Eu3+ induced by energy transfer from nanosized SnO2 crystals in glass. J Lumin 97:147–152. https://doi.org/10.1016/S0022-2313(02)00217-X
Hewes RA, Hoffman AV (1971) 4f7-4f7 emission from Eu2+ in the system MF2-AlF3. J Lumin 3:261–280. https://doi.org/10.1016/0022-2313(71)90064-0
Lubio OJ (1991) Doubly-valent rare-earth ions in halide crystals. J Phys Chem Solids 52:101–174. https://doi.org/10.1016/0022-3697(91)90062-5
Verwey JWM, Dirksen GJ, Blasse G (1992) The luminescence of divalent and trivalent rare earth ions in the crystalline and glass modifications of SrB4O7. J Phys Chem Solids 53:367–375. https://doi.org/10.1016/0022-3697(92)90170-I
Zhu C, Yang Y, Liang X, Yuan S, Chen G (2007) Composition induced reducing effects on Eu ions in borophosphate glasses. J Am Ceram Soc 90:2984–2986. https://doi.org/10.1111/j.1551-2916.2007.01775.x
Cao T, Chen G, Lu W, Zhou H, Li J, Zhu Z, You Z, Wang Y, Tu C (2009) Intense red and cyan luminescence in europium doped silicate glasses. J Non Cryst Solids 355:2361–2364. https://doi.org/10.1016/j.jnoncrysol.2009.08.008
Herrmann A, Fibikar S, Hert D (2009) Time-resolved fluorescence measurements on Eu3+- and Eu2+-doped glasses. J Non Cryst Solids 355:2093–2101. https://doi.org/10.1016/j.jnoncrysol.2009.06.033
Lin Z, Zeng H, Yang Y, Liang X, Chen G, Sun J (2010) The effect of fluorine anions on the luminescent properties of Eu-doped oxyfluoride and aluminosilicate glasses. J Am Ceram Soc 93:3095–3098. https://doi.org/10.1111/j.1551-2916.2010.04067.x
Johnston WD, Chelko AJ (1970) Reduction of ions in glass by hydrogen. J Am Ceram Soc 53:295–301. https://doi.org/10.1111/j.1151-2916.1970.tb12111.x
Smedskjaer MM, Wang J, Yue Y (2011) Tunable photoluminescence induced by thermal reduction in rare earth doped glasses. J Mat Chem 21:6614–6620. https://doi.org/10.1039/C1JM10472A
Smedskjaer MM, Yue Y, Deubener J, Gunnlaugsson HP, Morup S (2010) Modifying glass surface via internal diffusion. J Non Cryst Solids 356:290–298. https://doi.org/10.1016/j.jnoncrysol.2009.12.004
Nogami M (2015) Reduction mechanism for Eu ions in Al2O3-containing glasses by heat treatment in H2 gas. J Phys Chem B 119:1778–1784. https://doi.org/10.1021/jp511513n
Nogami M, Koiwai A, Nonaka T (2016) Control of oxidation state of Eu ions in Na2O–Al2O3–SiO2 glasses. J Am Ceram Soc 99:1248–1254. https://doi.org/10.1111/jace.14111
Nogami M, Le XH, Vu XQ (2019) Novel silicate glasses in the acceleration of hydrogen diffusion for reducing dopant metal ions. J Non Cryst Solids 503−504:260–267. https://doi.org/10.1016/j.jnoncrysol.2018.10.003
Nogami M, Abe Y (1994) Sm2+‐doped silicate glasses prepared by a sol‐gel process. Appl Phys Lett 65:1227–1229. https://doi.org/10.1063/1.112078
Nogami M, Abe Y (1996) Enhanced emission from Eu2+ ions in sol‐gel derived Al2O3–SiO2 glasses. Appl Phys Lett 69:3776–3778. https://doi.org/10.1063/1.116995
Qiu J, Miura K, Suzuki T, Mitsuyu T, Hirao K (1999) Permanent photoreduction of Sm3+ to Sm2+ inside a sodium aluminoborate glass by an infrared femtosecond pulsed laser. Appl Phys Lett 74:10–12. https://doi.org/10.1063/1.123117
Qiu J, Nouchi K, Miura K, Mitsuyu T, Hirao K (2000) Room-temperature persistent spectral hole burning of x ray irradiated Sm3+-doped glass. J Phys Condens Matter 12:5061–5067. https://iopscience.iop.org/article/10.1088/0953-8984/12/23/314/pdf
Fujita K, Yasumoto C, Hirao K (2002) Photochemical reactions of samarium ions in sodium borate glasses irradiated with near-infrared femtosecond laser pulses. J Lumin 98:317–323. https://doi.org/10.1016/S0022-2313(02)00286-7
Nogami M, Suzuki K (2002) Fast spectral hole burning in Sm2+-Doped Al2O3–SiO2 glasses. Adv Mat 14:923–926. 10.1002/1521-4095(20020618)14:12%3C923::AID-ADMA923%3E3.0.CO;2-D
Ebendorff-Heidepriem H, Ehrt D (2020) Ultraviolet laser and x-ray induced valence changes and defect formation in europium and terbium doped glasses. Phys Chem Glasses 61:189–201. https://doi.org/10.13036/17533562.61.5.Ebendorff
Okada G, Morrell B, Koughia C, Edgar A, Varoy C, Belev G, Wysokinski T, Chapman D, Kasap S (2011) Spatially resolved measurement of high doses in microbeam radiation therapy using samarium doped fluorophosphate glasses. Appl Phys Lett 99:12110. https://doi.org/10.1063/1.3633102
Koughia C, Edgar A, Christopher E, Varo R, Okada G, Seggern H, Belev G, Kim CY, Ramaswami J, Sammynaiken R, Kasap S (2011) Samarium-doped fluorochlorozirconate glass–ceramics as red-emitting X-ray phosphors. J Am Ceram Soc 94:543–550. https://doi.org/10.1111/j.1551-2916.2010.04110.x
Vahedi S, Okada G, Morrell B, Muzar E, Koughia C, Edgar A, Varoy C, George B, Wysokinski T, Chapman D, Kasap S (2012) X-ray induced Sm3+ to Sm2+ conversion in fluorophosphate and fluoroaluminate glasses for the monitoring of high-doses in microbeam radiation therapy. J Appl Phys 112:073108. https://doi.org/10.1063/1.4754564
Martin V, Okada G, Tonchev D, Belev G, Wysokinski T, Chapman D, Kasap S (2013) Samarium-doped oxyfluoride borophosphate glasses for x-ray dosimetry in Microbeam Radiation Therapy. J Non Cryst Solids 377:137–141. https://doi.org/10.1016/j.jnoncrysol.2012.12.015
Edgar A, Varoy CR, Koughic C, Okada G, Belev G, Kasap S (2013) High-resolution X-ray imaging with samarium-doped fluoroaluminate and fluorophosphate glass. J Non Cryst Solids 377:124–128. https://doi.org/10.1016/j.jnoncrysol.2012.12.022
Okada G, Ueda J, Tanabe S, Belev G, Wysokinski T, Chapman D, Tonchev D, Kasap S (2014) Samarium-doped oxyfluoride glass-ceramic as a new fast erasable dosimetric detector material for microbeam radiation cancer therapy applications at the Canadian synchrotron. J Am Ceram Soc 97:2147–2153. https://doi.org/10.1111/jace.12938
Lian Z, Wang J, Lv Y, Wang S, Su Q (2007) The reduction of Eu3+ to Eu2+ in air and luminescence properties of Eu2+ activated ZnO–B2O3–P2O5 glasses. J Alloy Compd 430:257–261. https://doi.org/10.1016/j.jallcom.2006.05.002
Kreidl NJ (1983) Inorganic glass-forming systems. In: Uhlmann DR, Kreidl NJ (eds) Glass, science and technology, vol. 1, Academic Press NY, pp 105−299
Nogami M, Vu XQ, Nonaka T, Shimizu T, Ohki S, Deguchi K (2017) Diffusion and reaction of H2 gas for reducing Eu3+ ions in glasses. J Phys Chem Solids 105:54–60. http://www.sciencedirect.com/science/article/pii/S0022369716313142?via%3Dihub
Risbud S, Kirkpatrick RG, Taglialavore A, Montez B (1987) Solid-state NMR evidence of 4-, 5-, and 6-fold aluminium sites in roller-quenched SiO2−A12O3 glasses. J Am Ceram Soc 70:C10–C12. https://doi.org.ezproxy.ict.nitech.ac.jp/10.1111/j.1151-2916.1987.tb04859.x
Sato RK, McMillan PF, Dennison P, Dupree R (1991) High-resolution 27Al and 29Si MAS NMR investigation of SiO2-Al2O3 glasses. J Phys Chem 95:4483–4489. https://doi.org/10.1021/j100164a057
Schmucker M, MacKenzie KJD, Schneider H, Meinhold R (1997) NMR studies on rapidly solidified SiO2−A12O3 and SiO2−Al2O3−Na2O-glasses. J Non-Cryst Solids 217:99–105. https://doi.org/10.1016/S0022-3093(97)00127-0
Ho VT, Nogami M, Le XH (2020) Reduction of Sm3+ and Eu3+ ions-co-doped Al2O3–SiO2 glasses and photoluminescence properties. Opt Mat 100:109639. https://doi.org/10.1016/j.optmat.2019.109639
Capobianco JA, Proulx PP, Bettinelli M, Negrisolo F (1990) Absorption and emission spectroscopy of Eu3+ metaphosphate glasses. Phys Rev B 42:5936–5944. https://doi.org/10.1103/PhysRevB.42.5936
Friebele EJ, Griscom DL (1979) Radiation effects in glasses. In: Tomozawa M, Doremus RH (eds) Treatise on materials science and technology, vol. 17, Academic Press NY, pp 257-351
Mackey JH, Nahum J (1968) Spectral study of the interconversion of Eu2+ and Eu3+ in silicate glass. Phys Chem Glasses 9:52–63
Acknowledgements
Author (MN) is grateful to Drs. S. Ohki, K. Deguchi, and T. Shimizu of National Institute for Material Science for measurement of NMR.
Author information
Authors and Affiliations
Contributions
MN designed the study, collected all the data, and wrote the initial draft. VXQ critically reviewed the manuscript. HVT contributed to data collection of the fluorescence spectra and analyzed them. LXH contributed to data collection of the fluorescence spectra and discussed on data. The first draft of the manuscript was written by NM and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nogami, M., Quang, V.x., Tuyen, H.v. et al. X-ray responsiveness of sol–gel-derived glasses doped with rare-earth ions. J Sol-Gel Sci Technol 102, 504–512 (2022). https://doi.org/10.1007/s10971-022-05732-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05732-0