Abstract
Mesoporous silica microspheres were synthesised through a sol–emulsion–gel process using Span 80 as the surfactant in silica sol/n-hexane water in oil emulsion system. Surface modification of the microspheres was done with trimethylchlorosilane to obtain hydrophobic silica microspheres. Various parameters related to the synthesis of microspheres, including concentration of surfactant and viscosities of sol were studied. The hydrophobicity (wettability), thermal stability, porosity, and morphological features were also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The synthesis of mesoporous materials having regular geometries has been of considerable interest in recent years owing to their great potential in catalysis, adsorption, separation, sensing, medical use, ecology, and nanotechnology [1–6]. Microspheres are widely employed as a feed material for plasma sprayed coatings, as thermal insulators, light weight filler in composites, internal confinement fusion of nuclear material, substrates for controlled drug release formulations, and gas storage materials [7–10]. Microspheres are generally synthesized by the dual nozzle hollow drop generation [11], spray pyrolysis [12], emulsion–evaporation [13], melting in dc plasma [14], or sol–emulsion–gel techniques [15–18]. The sol–emulsion–gel method is widely practiced for the preparation of ceramic microspheres. Mesoporous silica microspheres find application in diverse fields including clinical, pharmaceutical, electronic packaging, selective adsorption, chemical sensing, controlled drug delivery, and also in the preparation of silica glass, structural ceramics, and chromatographic columns [19, 20]. Artificial opals are SiO2 microspheres synthesized by self assembly methods to form a close packed structure [21]. Silica microspheres have been used as an immobilization matrix for several molecules [9, 10, 22]. Spherical silica particles with modified surface properties are important in composite materials, adsorbents, pigments, detergents, cosmetics, and pharmaceuticals [23–25]. Functionalization of the silica particle surface can be used to enhance or control the overall properties [26–28]. In the present work, a modified sol–emulsion–gel process was developed for the synthesis of hydrophobic silica microspheres. Hydrophobicity has been induced by surface modification using trimethylchlorosilane. The sol–emulsion–gel process involves silica sol generated from TEOS as aqueous phase, n-hexane as the oil phase and sorbitan monooleate (Span 80) as the surfactant to enhance the emulsion formation. The effect of surfactant concentration, aging time, and viscosity of the silica sol on microsphere formation is presented.
2 Experimental
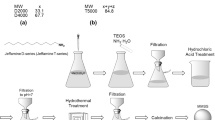
The water in oil emulsion was prepared using silica sol as the aqueous phase and n-hexane (S.D. Fine Chemicals, Mumbai, India) as the oil phase. Silica sol was obtained by hydrolyzing tetraethylorthosilicate (Sigma Aldrich Chemie GmbH, Steinheim, Germany) with 10−3 M hydrochloric acid (S.D. Fine Chemicals, Mumbai, India) using isopropanol (S.D. Fine Chemicals, Mumbai, India) as the homogenizing solvent. The tetraethylorthosilicate:isopropanol:water ratio was kept as 1:4:16 [29]. In a typical synthesis of silica microspheres, 20 mL hexane was mixed with 5 mL silica sol and sorbitan monooleate (Span 80, S.D. fine chemicals, Mumbai, India) was added with stirring (500 rpm). The effect of surfactant concentration on microsphere properties was studied by varying the concentration of surfactant as 0.5, 1, 1.4, 1.8, and 2 vol%. Constant stirring for 20 min resulted in silica microspheres. The spheres were decanted, the solvent exchanged (five times) with n-hexane and the product divided into two parts. One part was made hydrophobic by treating with trimethylchlorosilane (TMCS) (Sigma Aldrich Chemie GmbH, Steinheim, Germany) for a period of 24 h and the other part was dried at 70 °C. The hydrophobic spheres were solvent exchanged with n-hexane (five times) after functionalization and dried at 70 °C. The flow chart for microsphere synthesis is provided in Fig. 1.
In order to study the effect of viscosity, silica sols aged for different time periods (2, 5, 8, 11, and 17 h) were used for the preparation of the microspheres. Rheological behavior of the precursor sol was monitored using a Rheoviscometer (Anton Paar GmbH, Graz, Austria) employing the cylinder and bob method.
Structural characteristics of the microspheres were investigated by a Fourier Transform Infrared spectrophotometer (Magna 560, Nicolet, Madison, Wisconsin) in the range 400–4,000 cm−1 by the KBr pellet method and thermal decomposition curves of the dried microspheres obtained using a Differential Thermal Analyzer (DTA 50, Shimadzu, Kyoto, Japan) in air at a heating rate of 10 °C min−1. Specific surface area and adsorption isotherm of the samples were obtained using a BET surface area analyzer (Gemini 2360, Micromeritics, Norcross, USA). Contact angles were measured using a Tensiometer (DCAT11, Data Physics GmbH, Filderstadt, Germany). The increase in square of sample weight, when in contact with water was calculated using the same instrument. Microsphere morphology was observed using an Optical Microscope (MZ16 A, Leica, Wetzlar, Germany) in transmission mode and by Scanning Electron Microscopy (JSM 5600LV, JEOL, Tokyo, Japan) in the secondary electron image (SEI) mode.
3 Results and discussion
3.1 Effect of surfactant concentration
In a sol–emulsion–gel process, an aqueous sol phase is dispersed in a nonpolar oil phase to form the emulsion. The high interfacial tension between the aqueous phase and the oil phase is reduced by the addition of an amphiphilic surfactant making the emulsion kinetically stable [30, 31]. Mechanical agitation provides the energy to overcome the interfacial tension and disperse the aqueous phase to form the emulsion. Surfactant concentration and viscosity of the sol droplets control sphere formation in a sol–emulsion–gel process [15, 16], and although these influences have been observed in alumina and other systems there are few reports on their effect for silica [31]. Hence the present paper investigated the effect of surfactant concentration (Span 80), effect of aging time, and sol viscosity on the synthesis outcome. Altering the concentration of Span 80 modified the morphology, diameter, and uniformity of the resultant silica microspheres (Fig. 2). For this study, unaged silica sol was used. When 1 vol% surfactant was used, mostly irregular particles were obtained, as sphere formation was incomplete and the concentration of surfactant inadequate to form a monodispersed emulsion. On increasing the Span 80 concentration to 1.4 vol% smaller and more uniform particles (50 μm) resulted (Fig. 3). For a surfactant concentration of 1.8 vol% clustering and aggregation was observed while a further increase to 2 vol% yielded larger spheres and many spheres collapsed. The critical micelle concentration (CMC) of the surfactant in the continuous liquid phase controls emulsion stability and hence sphere formation. Figure 4 shows the variation of surface tension of n-hexane with Span 80, where the discontinuity gives the CMC as between 1 and 1.5 vol% [30, 31]. In a surfactant-hydrophobic solvent system the surfactant molecules tend to reduce unfavorable interactions by minimizing exposure of the hydrophilic end to the hydrophobic solvent molecules by locating at the interface at low concentrations and forming assembled structures at higher concentrations. The minimum concentration required to form assembled structures coincides with the CMC. In an emulsion when the surfactant concentration is above CMC destabilization of the emulsion, flocculation will ultimately occur, a phenomenon exploited in the fractionation technique of Bibette [32]. Aggregated and deformed structures result when flocculated emulsion droplets undergo gelation.
The surface area of the microspheres decreases when the concentration of Span 80 increases (Table 1). For 1 vol% a surface area of 363 m2/g was obtained and decreases to 296 m2/g for 1.8 vol%. Sol–gel condensation of silica results in a highly porous gel network which collapse partially during drying due to stress. The high surface area values are a clear indication of the porous nature of the spheres. The slight increase in average pore diameter with surfactant concentration arises from agglomeration observed for high surfactant concentrations. In the adsorption isotherms (Fig. 5) spheres prepared at lower surfactant concentrations (1–1.4 vol% Span 80) show a plateau at high pressures that is absent at higher concentrations. This indicates a greater contribution from larger pores (>50 nm) in latter material due to agglomeration [33]. When the contribution to the pore volume from larger pores increases the surface area decreases.
It is known that viscosity of polymeric silica sol varies with time [34]. Kim et al. observed the influence of viscosity of silica sol on sphere formation in a hollow drop generation method for preparing hollow silica spheres [11]. Silica sol aged for different times of 5–17 h were used at a surfactant concentration of 1.4 vol%. Optical microscopy showed that 8 h aging give more uniform spheres of size ~50 μm (Fig. 6), with longer times (11 and 17 h) resulting in aggregated and deformed spheres. During aging, condensation reactions proceed in the silica sol and networked clusters start to form. After gelation has proceeded considerably, it will be difficult to form spherical droplets of uniform size and shape from the sol. The optimum viscosity to obtain uniform spheres represents the balance between the extent of gelation and the droplet sizes achievable for the particular surfactant concentration, volume ratio (discontinuous phase/continuous phase), and shear rate. The viscosity of silica sol aged for different times shows a direct correlation between increasing viscosity and the degree of polymerization between silica particles (Fig. 7). Under mechanical agitation this polymer network breaks down at higher viscosities.
Figure 8 shows the FTIR spectra of silica microspheres with and without surface modification using trimethylchlorosilane. A broad peak at 3,449 cm−1 observed for the unmodified and modified microspheres can be ascribed to isolated hydrogen bonded Si–OH groups [35]. Peak intensity is lower in the latter case due to the replacement of Si–OH groups by the organic group leading to reduced hydrophilicity. Silanol hydroxyls undergo silanization with chlorosilane thereby replacing the hydroxyl groups by methyl groups. The peak at 1,639 cm−1 corresponds to the deformation mode of adsorbed water molecules in the unmodified sample, and is very weak in the modified sample. The features at 840 and 1,275 cm−1 correspond to the Si–C bond present in the hydrophobic silica microspheres [36], while the CH2 stretching vibration is located at 2,959 cm−1 in the hydrophobic silica microspheres [36]. A schematic representation of the formation of hydrophobic silica microspheres is provided in Fig. 9.
Contact angles of different silica microspheres heated at 70 and 350 °C are provided in Table 2. The increase in square of weight of the sample when in contact with water is also given. Surface modification with trimethylchlorosilane resulted in a contact angle of 90° and a very low increase in weight per second (0.00003 g2/s). Spheres without modification showed a contact angle of 84° with an increase in weight per second of 0.00256 g2/s and shows that surface modification with trimethylchlorosilane is effective in making the microspheres hydrophobic. Contact angle of unmodified silica decreases to 37° after calcination at 350 °C where as contact angle of hydrophobic silica microspheres remains the same. The decrease in contact angle can be attributed to the removal of some Span 80 that were not washed off by the solvent exchange.
Hydrophilic microspheres show strong endothermic peak at ~120 °C due to the removal of adsorbed water, while an exothermic peak is due to removal of residual surfactant at 345 °C (Fig. 10) [30]. The endothermic peak at 120 °C is absent in the case of hydrophobic microspheres as expected. A sharp exothermic peak at 442 °C reflects the decomposition of the methyl groups and so hydrophobicity is retained to that temperature.
Silica microspheres (~50 μm) have been synthesized through a sol–emulsion–gel technique using Span 80 as the surfactant in silica sol/n-hexane water in oil emulsion system. Surface modification by trimethylchlorosilane resulted in hydrophobic microspheres with a contact angle of ~90°. Differential thermal analysis shows that the decomposition of organic moieties responsible for hydrophobicity occurs around 440 °C and hence it can be concluded that hydrophobicity is retained to that temperature. It was also found that the concentration of surfactant and viscosity of silica sol are important factors in the synthesis of SiO2 microspheres. A surfactant concentration of 1.4 vol% yielded highly regular spheres with a surface area of 319 m2/g and a pore size of 8.5 nm. Silica sol aged for 8 h gave the most uniform spheres when the aging time was varied in the preparation of microspheres. It can be concluded that there is an optimum surfactant concentration and aging time for the silica sol to obtain uniform spheres, below which clustering, aggregation, and structural collapse occurs.
References
Vinu A, Mori T, Ariga K et al (2006) Sci Technol Adv Mater 7:753
Park SS, Ha CS (2006) Chem Rec 6:32
Vinu A, Hossain KZ, Ariga K et al (2005) J Nanosci Nanotechnol 5:347
Stein A (2001) Microporous Mesoporous Mater 44:227
Kosuge K, Singh PS et al (2001) Microporous Mesoporous Mater 44:139
Pol VG, Gedanken A, Calderon-Moreno J et al (2003) Chem Mater 15:1111
Duret B, Saudin A et al (1994) Int J Hydrogen Energy 19:757
Zhanwen Z, Yongjian T, Chaoyang W, Bo L, Xiaobo Q et al (2006) J Chem Ind Eng 57:1677
Zeng XM, Martin GP, Marriott C et al (1995) Eur J Pharm Sci 3:87
Kortesuo P, Ahola M, Kangas M, Kangasniemi I, Yli-Urpo A, Kiesvaara J et al (2000) Int J Pharm 200:223
Kim KK, Jang KY, Upadhye RS et al (1991) J Am Ceram Soc 74:1987
Gadalla AM, Yu HF et al (1990) J Mater Res 5:2923
Sarikaya Y, Akinc M et al (1988) Ceram Int 14:239
Pravdic G, Gani MSJ et al (1996) J Mater Sci 31:3487
Chatterjee M, Naskar MK, Ganguli D et al (1999) J Sol-Gel Sci Technol 16:143
Chatterjee M, Naskar MK, Ganguli D et al (2003) J Sol-Gel Sci Technol 28:217
Naskar MK, Chatterjee M, Lakshmi NS et al (2002) J Mater Sci 37:343
Miller CR, Vogel R, Surawski PPT, Jack KS, Corrie SR, Trau M et al (2005) Langmuir 21:9733
Ma Y, Qi L, Ma J, Wu Y, Liu O, Cheng H et al (2003) Colloids Surf A 229:1
Roque-Malherbe R, Marquez F et al (2005) Surf Interface Anal 37:393
Roque-Malherbe R, Marquez F et al (2004) Mater Sci Semicond Process 7:467
Ramesh S, Minti H, Reisfeld R, Gedanken A et al (1999) Opt Mater 13:67
Wu Z, Han H, Han W, Kim B, Ahn KH, Lee K et al (2007) Langmuir 23:7799
Wu Z, Xiang H, Kim T, Chun MS, Lee K et al (2006) J Colloid Interface Sci 304:124
Marquez M, Grady BP, Robb I et al (2005) Colloids Surf A 266:18
Nozawa K, Gailhanou H, Raison L, Panizza P, Ushiki H, Sellier E, Delville JP, Delville MH et al (2005) Langmuir 21:1516
Matsumoto A, Tsutsumi K, Schumacher K, Unger KK et al (2002) Langmuir 18:4014
Guise MT, Hosticka B, Earp BC, Norris PM et al (2001) J Non-Cryst Solids 285:317
Smitha S, Shajesh P, Aravind PR, Kumar SR, Pillai PK, Warrier KGK et al (2006) Microporous Mesoporous Mater 91:286
Chatterjee M, Patra A et al (2001) J Am Ceram Soc 84:1444
Siladitya B, Chatterjee M, Ganguli D et al (1999) J Sol-Gel Sci Technol 15:271
Bibette J (1991) J Colloid Interface Sci 147:474
Gregg SJ, Sing KSW (1995) Adsorption, surface area and porosity. Academic Press, London
Iler RK (1979) The chemistry of silica: solubility, polymerization, colloid and surface properties and biochemistry of silica. Wiley and Sons, New York
Brinker CJ, Scherer GW (1990) The physics and chemistry of sol–gel processing. Academic Press, San Diego
Bhagat SD, Kim YH, Ahn YS et al (2006) Appl Surf Sci 253:2217
Acknowledgments
The authors, S. S. and P. S. acknowledge Council of Scientific and Industrial Research (CSIR, India) for their fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smitha, S., Shajesh, P., Mukundan, P. et al. Synthesis of mesoporous hydrophobic silica microspheres through a modified sol–emulsion–gel process. J Sol-Gel Sci Technol 48, 356–361 (2008). https://doi.org/10.1007/s10971-008-1803-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-008-1803-3