Abstract
This study validates and improves the Cm radiochronometry method developed to determine the model discharge date of nuclear spent fuels based on a three isotopes system, specifically the 244Cm/246Cm and 244Cm/245Cm ratios change since discharge time. Six spent fuel samples with continuous irradiation histories were used. After Cm separation from the matrix, the Cm isotopes were measured by accelerator mass spectrometry using one of the samples as internal standard for normalization. The mean average deviation from the known fuel discharge date improved from 3.3 ± 2.8 to 2.7 ± 2.6 yr when using spent fuel with continuous irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nuclear forensics aims to support national and international law enforcement investigations into the illicit and malicious use of nuclear materials [1]. A wide range of nuclear forensics methods are applied to determine the origin and intended use of either a stolen or found radioactive material [2,3,4]. Developing and improving nuclear forensic techniques is important to deter against the harmful use of nuclear materials and be better prepared to support investigations when nuclear materials are found outside of regulatory control.
Radiochronometry is an important technique in nuclear forensics to determine model ages of nuclear and radioactive materials. Model ages are usually determined by measuring the amounts of a parent isotope and its progeny in a sample of the material and then, by calculating the time since purification and analysis using the Bateman equation. A number of progeny and parent isotope pairs have been used as radiochronometers such as 230Th/234U and 231Pa/235U for uranium materials, 234U/238Pu, 235U/239Pu, 236U/240Pu, and 241Am/241Pu for Pu materials [2, 3], as well as 60Ni/60Co and 137Ba/137Cs for Co [5] and Cs [6] radioactive sources, respectively. However, when estimating model ages, two key assumptions are made: the progeny isotope was completely removed during purification and both elements were contained in a closed system following processing [7].
Along with the mentioned assumptions, the measurement of both isotopes should be as precise and accurate as possible to correctly determine a material's model separation or discharge date [8, 9]. A precise and accurate determination of the parent isotope is usually easier to achieve than for the progeny isotope because the progeny isotope is typically present at trace level in the material. Furthermore, a chemical yield tracer is needed for most radiochronometers to correct for recovery losses during radiochemical separations. The recovery tracer can contain the progeny and render the measurement less accurate. Consequently, complex radiochemical separations, very pure or well characterized tracers, representative method blanks, ultra-sensitive measurement techniques, and specialized installations, where highly radioactive materials can be handled and a low laboratory background is maintained to reduce the risk of contamination, are needed. Measurements are usually made with radiometric and mass spectrometry techniques such as gamma spectrometry, alpha spectrometry, and inductively coupled plasma mass spectrometry [10], or a combination of the above techniques. Finally, the use of multiple radiochronometers is recommended when possible to add confidence in the determination of the model age [3].
The model discharge date of a spent nuclear fuel is particularly challenging to determine with high accuracy because the parent/progeny ratio used for the radiochronometry model can be affected by fission reactions, neutron captures, and radioactive decay of other isotopes. For this reason, most of forensics investigations on nuclear fuels focus on determining the 235U enrichment and type of reactor used based on selected isotopic ratios (e.g. U and Pu) [2, 10, 11]. Only a few studies have investigated the use of isotopic radiochronometers such as 241Pu/241Am, 242Pu/240Pu vs 241Pu/239Pu, 152Eu/154Eu, and 88Sr/90Sr to determine the model discharge date of a spent nuclear fuel [12,13,14,15]. We recently studied the use of Cm isotopic ratios as a new method to determine the discharge age of spent nuclear fuels [16]. This was a new type of application for Cm isotopes because Cm isotopes in spent nuclear fuels are mainly studied for their toxicity in the context of nuclear wastes [17,18,19].
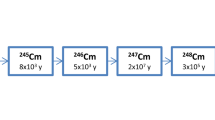
The new Cm radiochronometry technique we developed to determine the model discharge date of a spent nuclear fuel is based on three Cm isotopes (244Cm, 245Cm, and 246Cm) [16]. In a nuclear reactor, 244Cm is mainly formed by neutron capture from 243Cm and 243Am (243Cm(n, γ)244Cm and 243Am(n, γ)244Cm). Then, the higher mass Cm isotopes (245Cm to 250Cm) are also mainly produced by neutron capture reactions from a lower mass Cm isotope (24xCm(n, γ)24(x+1)Cm) and the total fluence determines the proportion of Cm isotopes formed [20]. Only a very small proportion of the Cm isotopes is formed by the decay of some Bk and Cf isotopes. A nuclear fuel irradiated for a longer time will contain a greater proportion of higher-mass Cm isotopes. The ratio 245Cm/246Cm does not change much after irradiation as these two isotopes of Cm have relatively long half-lives (t1/2 = 8,250 ± 70 yr and 4,723 ± 27 yr, respectively [21]). On the other hand, the 244Cm/246Cm ratio decreases significantly with time due to the shorter 244Cm half-life of 18.11 ± 0.03 yr [21]. The relation between 244Cm/246Cm and 245Cm/246Cm ratios at end of irradiation (time since discharge from the reactor) seemed to be universal based on previous results [16]. The relation can be used as a reference curve to calculate the 244Cm/246Cm ratio at end of irradiation. To determine the model discharge date of a spent nuclear fuel sample using the Bateman equation, the current ratio of 244Cm/246Cm is determined by mass spectrometry and the 244Cm/246Cm ratio at end of irradiation is calculated using the reference curve of 244Cm/246Cm ratio as a function of 245Cm/246Cm ratio [16]. Like other single element radiochronometers, the main advantages of this method are that no tracer is needed, the Cm isotopes have the same behavior in the environment (the system does not have to be closed), and the method can work on a nuclear material where the progeny did not have to be separated from the parent at end of irradiation. In the case of reprocessed fuels, where Cm removal could have been incomplete following the first irradiation, the model age would be a composite of both times since irradiation.
The 244Cm/246Cm ratio as a function of the 245Cm/246Cm ratio relation obtained in the initial work had a correlation that was good (R2 = 0.935) but that could be improved [16]. As a result, the maximum uncertainty using this radiochronometer for age-dating was ± 5 years [16]. The discontinuous irradiation of the fuel samples used for the initial study could explain the rather low accuracy. Indeed, the fuel samples available for the initial study were often removed and put back in the reactor over the years, resulting in a significant decay of 244Cm. The 244Cm/246Cm value at end of irradiation was potentially lower than it would have been for fuels with the same discharge date but continuous irradiation. This could be the source of a systematic deviation from the expected correlation and therefore lowered the precision of the model ages. A lower than expected 244Cm/246Cm ratio would result in an overestimated discharge age, thus the Cm model discharge date can be interpreted as the earliest possible discharge date.
For this current work we improved the 244Cm/246Cm vs 245Cm/246Cm ratios curve (reference curve) by adding six uranium fuel samples that had been continuously irradiated in CANDU power reactors. The fuel samples were dissolved and Cm was radiochemically purified and determined by accelerator mass spectrometry (AMS). The precision of the AMS measurement was improved using one sample as an external normalization standard. The fuel age was calculated using the Cm radiochronometry technique previously developed [16].
Experimental
Instruments
Accelerator mass spectrometry
Curium isotope measurements of six samples and two blanks (E series in Tables 1 and 2) were performed with the compact, low-energy AMS system MILEA at ETH Zurich, Switzerland. The MILEA system was installed at ETH Zurich in 2018, and since 2019 it has been routinely used for actinide analyses. A detailed description of the MILEA system and its setup for actinides is presented elsewhere [22], and here only a brief description of the instrumental setup for Cm-isotope measurements is presented. xCm isotopes (x = 244, 245, 246) were sequentially injected into the accelerator using the slow bouncing system. In total, the samples were analyzed for 12 passes, with one pass consisting of three repetitions of the following sequence: 244Cm (10 s), 245Cm (15 s), and 246Cm (30 s). Isotopic ratios of 244Cm/246Cm and 245Cm/246Cm were calculated from the method blanks corrected counting rates on each mass. To construct the reference curve, the isotopic ratios were decay-corrected using the known decay time between discharge and analysis of the samples (Table 1).
The fact that no certified standard material exists for 244Cm/246Cm and 245Cm/246Cm ratios complicates comparability with our previous AMS results [16]. For example, different machine tuning and ion beam transport may have a systematic effect on the measured isotopic ratios. Such effects are commonly known and generally eliminated by normalizing the measured ratios to an internal or external standard material with known isotopic ratios, however there is no available Cm isotopic standard. To circumvent this problem we decided to prepare two AMS targets using sample E-4 from this study as an external standard. In a separate AMS run, the samples from our previous study were measured again on the MILEA system together with the two newly prepared E-4 samples and with two blanks. By comparing the measured isotopic ratios for sample E-4 from both runs, the isotopic ratios of the initial samples were re-normalized relative to the new set of samples. The underlying isotopic ratios of all samples are reported relative to the measured isotopic ratios of sample E-4. Future analyses of spent nuclear fuel samples for Cm radiochronometry will thus also require the analysis of sample E-4 to allow the normalization of the measured isotopic ratios relative to the 244Cm/246Cm and 245Cm/246Cm ratios of sample E-4.
Gamma spectrometer
A coaxial high-purity germanium (HPGe) detector shielded with 10 cm of lead (AMETEK/ORTEC Inc., Oak Ridge, TN, USA) was used to confirm the removal of the lanthanides before the separation of Cm from Am.
Alpha spectrometer
An Octete Plus® alpha spectrometer with eight 450 mm2 ULTRA-AS ion-implanted silicon detectors (AMETEK/ORTEC Inc., Oak Ridge, TN, USA) was used to confirm the separation of Cm from Am and to estimate the amount of Cm isotopes in the samples prior to the preparation of the AMS targets.
Fuel description and irradiation history
Six natural UO2 fuel elements from CANDU commercial power reactors with continuous irradiation histories were selected for this work (see Table 1). The fuel samples had burnups between 190 and 230 MWh(kgU)−1 and were discharged from the reactor between 1992 and 2014. All samples were sectioned at the same distance from the reference end of the fuel element (fuel pin).
Curium purification
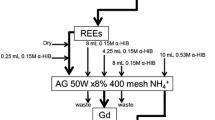
Curium isotopes were separated from the spent nuclear fuel samples as described in previous work [16]. In brief, the spent nuclear fuel element was cut open in a hot cell facility. A small amount of the spent nuclear fuel was dissolved in concentrated nitric acid. The solution was diluted based on the theoretical amount of Cm expected in the sample using the Monte-Carlo physics code SERPENT (version 2-2.1.31) [23] to reduce the radioactivity for handling during the separation. The solution radioactivity was tested to ensure staff safety before sending the samples from the hot cells to the laboratory. The sample solution was brought to 8 M HNO3 and passed through sequential UTEVA and DGA resins (Eichrom, Lisle, IL, USA). The UTEVA resin was discarded and Cm was eluted with 20 ml of 0.1 M HCl from the DGA resin.
The Cm solution also contained Am and lanthanide impurities, which were removed as described in previous work [16]. An aliquot of 2 ml for the sample solution was evaporated to dryness. The residue was re-dissolved with 5 ml of 4 M NH4SCN + 0.1 M formic acid solution. The solution was passed through a TEVA resin. The resin was rinsed with 10 ml of 4 M NH4SCN + 0.1 M formic acid solution. The actinides were eluted from the resin using 20 ml of 1 M HCl. The absence of radioactive lanthanides (mainly Eu isotopes) was verified by gamma spectrometry. The separation was repeated if needed.
Americium needed to be removed because its amount was too high for being introduced into the AMS. The separation of Am and Cm was done using a method previously developed [24]. The solution containing Am and Cm was evaporated to dryness and re-dissolved with 10 ml of 0.16 M Na2S2O8 + 0.005 M AgNO3 in 0.01 M HNO3. The solution was heated to 80 °C to oxidize Am(III) to Am(V). The solution was then passed through a DGA resin, which selectively retained Cm(III) from Am(V). Curium was eluted from the resin using 15 ml of 0.1 M HCl. A small aliquot of the solution was used to verify the absence of Am by alpha spectrometry (~ 0.05 ml). The alpha thin-layer source was prepared using a CeF3 micro-precipitation as described in reference [25]. The separation was repeated if needed until no Am was detected.
AMS target preparation
Solutions containing 0.1 mg of Fe(III) and 0.4 mg of Ti(IV) were added to 2 ml of the purified Cm solution. The pH was raised to pH 9 using ammonium hydroxide. The precipitate was centrifuged and the supernatant was discarded. The precipitate was transferred into a quartz crucible using methanol. The methanol was evaporated and the residue was heated to 600 °C for approximately 4 h and then cooled down. The sample was mixed with 5 mg of Nb powder directly in the quartz crucible using a disposable pestle. The mixed sample was pressed onto an aluminum ball in an AMS sample holder.
No recovery tracer was added to prevent contamination of the sample with 244Cm, 245Cm, and 246Cm. Therefore, the method chemical recovery was not determined. The chemical recovery was sufficiently high to be able to measure the Cm isotopes needed for this work with an acceptable precision.
Model age calculation
Calculations of the model age and related uncertainties have been done as described in previous work [16]. In short, net counting rates were calculated for each isotope by subtracting the blank counting rate from the measured counting rate. The net counting rates were corrected for radioactive decay using the known time elapsed since the end of irradiation of the fuel samples. Finally, the isotopic ratios of 244Cm/246Cm and 245Cm/246Cm at the end of irradiation were calculated from the decay corrected net counting rates. Gaussian error propagation was used to determine the uncertainties of the isotopic ratios, taking into account the uncertainties of both the measured counting rates and the average method blanks counting rate. The time elapsed since the end of irradiation including its uncertainty was calculated as described in our initial publication [16] using Eqs. (7) and (8).
Results and discussion
Cm ratios measured
The measured Cm count rates of the spent nuclear fuel samples presented in this study are shown in Table 2. These data were used to calculate the measured and decay-corrected at end of irradiation 244Cm/246Cm and 245Cm/246Cm ratios, which are presented in Table 3.
The 16 samples from the initial study [16] were re-analyzed together with two newly prepared AMS targets of sample E-4 of this study as external standard to obtain comparable results. In the re-analysis, the average measured and decay-corrected 244Cm/246Cm and 245Cm/246Cm molar ratios of sample E-4 were 890 ± 30 and 10.6 ± 0.4, respectively, which are 16% and 21% higher than the results for sample E-4 presented here. As a consequence, all 244Cm/246Cm and 245Cm/246Cm ratios of the initial study were divided by 1.16 and 1.21, respectively. The results of the normalized and decay-corrected isotopic ratios of the re-analysis study are listed in the Appendix (Tables 5 and 6).
Curium ratios relation
The 244Cm/246Cm ratios as a function of the 245Cm/246Cm ratios are shown in Fig. 1 for the re-analyzed initial study samples (normalized data) and the current study samples. The data from the initial study [16] are shown for comparison.
244Cm/246Cm as a function of 245/246Cm ratio for the new data (filled purple triangles), normalized initial data (filled black triangles), and the initial data (open squares) for comparison. The updated reference curve (straight line) considers only the new data and the normalized data from the initial study (filled purple and black triangles). The linear fit to the initial study data is shown for comparison (dashed line)
A refined reference curve for the Cm chronometry model was created by linearizing the data set and applying a linear fit that takes into account errors in the x and y directions [26]. The fit parameters of the underlying power law y = c xb were determined to be c = 48.63 ± 1.87 and b = 1.359 ± 0.024. A reduced Chi-squared value of 2.27 was calculated when comparing the measured data with the fit and taking into account the analytical uncertainties of the data points. This value was larger than the upper limit of 1.73 given by 19 degrees of freedom and 95% confidence limit, indicating that the scatter of the data was larger than the analytical uncertainties. By quadratically adding an external uncertainty to each data point the external scatter could be determined. By adding an external uncertainty of 2.6% to the data set, a reduced Chi-squared value of 1.73 was obtained. Consequently, for the calculation of the fit parameters and their uncertainties an external uncertainty of 2.6% was quadratically added to each data point.
Combining the current study data together with the normalized data from the initial study led to a more precise reference curve for the Cm chronometry model. The uncertainties of the fit parameters c and b were reduced from 6.3% and 3.2% (initial study) to 3.8% and 1.7% (this study), respectively, which should translate into more precise ages for unknown spent nuclear fuel samples. Indeed, when treating all samples as unknowns, the uncertainty of the calculated ages is reduced from 3.9 yr (initial study reference curve) to 2.7 yr with the new reference curve. In addition, the current data (E series) align relatively well with the initial data using completely different fuels, which supports the validity of the Cm age-dating radiochronometry model.
Spent nuclear fuel ages.
The calculated and actual spent nuclear fuel ages since last irradiation are shown in Table 4 and plotted in Fig. 2. The data from the initial study [16] are reported in Table 4 and Fig. 2 and decay-corrected for comparison. Also, the differences between the actual and calculated discharge ages from both studies are shown in Table 4. Note that the fuel discharge age uncertainty is sometimes significantly higher than the measurement uncertainty because the fuel age uncertainty depends on the accuracy of the reference curve.
The mean absolute differences between the calculated and actual discharge ages of the fuel for the samples from the initial study (fuels A to D) remained essentially the same (3.4 ± 2.2 yr and 3.3 ± 2.8 yr, respectively) (Table 4). Therefore, adding data for new fuels to the curve (E series) did not improve the accuracy of the initial study data (fuels A to D). When considering only the new fuels that had continuous irradiation (E series), the mean absolute difference with the expected ages of 1.2 ± 1.2 yr was significantly lower. The accuracy of the calculated ages was substantially improved for these data only. This could be because the fuel had continuous irradiation, but more data would be needed to confirm this hypothesis due to the limited amount of data available (n = 6). Finally, the mean absolute age difference of all the normalized data was 2.7 ± 2.6 yr, which is slightly better than the 3.4 ± 2.2 yr of the initial study. The overall mean relative average was lower solely because of the addition of the new fuel data (E series).
The reference curve cannot be prepared using only the current study data because the new data span over only a small range on the 245Cm/246Cm axis (Fig. 1). The extrapolation of the fit into regions far beyond this range would substantially increase the uncertainty of the calculated ages. Fuels with a wider range of burnups, i.e., lower and higher than the burnup values of the current study, would be needed to improve the reference curve.
Performance of the Cm radiochronometer
The 241Am/241Pu, 242Pu/240Pu vs 241Pu/239Pu and 90Sr/88Sr radiochronometers can predict the model discharge age of nuclear spent fuels with an accuracy of a few years (e.g. ± 1–6 yr) [12,13,14]. This is comparable with the Cm radiochronometry age model developed. Radiochronometers developed to determine model ages of purified U and Pu materials have a much higher accuracy of up to 1 month [7], mainly because the parent radionuclide and its progeny have been separated at t = 0, which is not the case with spent nuclear fuel. If the Cm radiochronometry method developed is applied to reprocessed fuels, consideration needs to be made of the possible contribution of Cm isotopes from the first irradiation, and thus the model discharge date would be a composite of the two irradiations.
A potential solution to increase the accuracy of the Cm radiochronometer could be to have correction factors derived from other isotopes to account for the discontinuous irradiation in the reactor. It has been suggested [27] that 147Sm could be used to estimate how long the reactor was shut down, but this has never been experimentally demonstrated. Note that 147Sm has not been measured for this project and cannot be verified with the current data.
All measurements have been normalized using sample E-4, which makes it more challenging for interlaboratory comparison. The development of a reference material would enable other laboratories to make comparable measurements and provide a way to correct for laboratory, process, and instrument specific bias between laboratories. The Cm isotopic composition of the reference material could be established using mass spectrometric methods such as inductively coupled mass spectrometry (ICP-MS), thermal ionization mass spectrometry (TIMS), and AMS.
Conclusions
A three Cm isotopes system was refined to determine the model discharge ages of spent nuclear fuels. This radiochronometer is applicable for spent fuel not purified at time of discharge. A discontinuous irradiation of the nuclear fuel was presumed to be a limitation of the Cm chronometry reference curve due to the significant decay of 244Cm when the irradiation is stopped. Six fuel samples that were continuously irradiated were used to verify this assumption. Adding these new data improved the precision of the 244Cm/246Cm as a function of 245Cm/246Cm fit curve. It also slightly improved the accuracy of the fuel age determination from 3.3 ± 2.8 to 2.7 ± 2.6 yr, respectively, on average. The model ages determined with the added fuel samples from the current study, 1.2 ± 1.2 yr, were on average more accurate than the initial study. The results of this study suggest that a discontinuous irradiation of the fuel adds to the uncertainty of the determined age. Further improvements of the reference curve would require a much larger number of data. Comparison of this radiochronometer to others would be interesting, including for the case of reprocessed fuel.
References
Keegan E, Kristo MJ, Toole K, Kips R, Young E (2016) Nuclear forensics: scientific analysis supporting law enforcement. Anal Chem 88:1496–1505
Mayer K, Wallenius M, Ray I (2015) Nuclear forensics—a methodology providing clues on the origin of illicitly trafficked nuclear materials. Analyst 130:433–441
Moody KJ, Grant PM, Hutcheon ID (2015) Nuclear forensics analysis, 2nd edn. CRC Press, Boca Raton
Straub MD, Arnold J, Fessenden J, Kiplinger JL (2021) Recent advances in nuclear forensics chemistry. Anal Chem 93:3–22
Charbonneau L, Benoit JM, Jovanovic S, St-Amant N, Kiser S, Cooke MW, Mercier JF, Nielsen K, Kelly D, Samuleev P, Galea R, Moor K, Saull PRB, Chamberlain DB, Steeb JL, Graczyk DG, Tsai Y, Sullivan VS, Dimayuga IC, Shi Y, Rao R, Lariviere D (2014) A nuclear forensic method for determine the age of radioactive cobalt sources. Anal Methods 6:983–992
Steeb JL, Graczyk DG, Tsai Y, Mertz CJ, Kimberlin A, Chamberlain DB (2016) Age-dating methodology for 137Cs ceramic sources. J Radioanal Nucl Chem 309:999–1019
Sturm M, Richter S, Aregbe Y, Wellum R, Mialle S, Mayer K, Prohaska T (2014) Evaluation of chronometers in plutonium age determination for nuclear forensics: what if the ‘Pu/U clocks’ do not match? J Radioanal Nucl Chem 302:399–411
Kayzar-Boggs TM, Luitjohan KE, Imhoff SD, Edwards MA, Krajewski KJ, Denton JS, Engel JR, Hudston LA, LaMont SP, Sanborn ME, Wende AM (2023) Results from a controlled depleted uranium metal casting experiment designed to investigate nuclear Forensic Radiochronometry Signatures. J Radioanal Nucl Chem 332:1695–1706
Varga Z, Mayer K, Bonamici CE, Hubert A, Hutcheon I, Kinman W, Kristo M, Pointurier F, Spencer K, Stanley F, Steiner R, Tandon L, Williams R (2015) Validation of reference materials for uranium radiochronometry in the frame of nuclear forensic investigations. Appl Radiat Isot 102:81–86
Wallenius M, Lutzenkirchen K, Mayer K, Ray I, Aldave de las Heras L, Betti M, Cromboom O, Hild M, Lynch B, Nicholl A, Ottmar H, Rasmussen G, Schubert A, Tamborini G, Thiele H, Wagner W, Walker C, Zuleger E (2007) Nuclear forensic investigations with a focus on plutonium. J Alloys Compd 444–445:57–62
Nicolaou G, Biegalski SR (2018) Discrimination of plutonium from thermal reactors in the frame of nuclear forensics. J Radioanal Nucl Chem 317:559–564
Hanson SK, Pollington AD (2021) Measuring signatures of fuel irradiation in large particle samples. J Anal At Spectrom 36:1018–1027
Savina MR, Isselhardt BH, Shulaker DZ, Robel M, Conant AJ, Ade BJ (2023) Simultaneous isotopic analysis of fission product Sr, Mo, and Ru in spent nuclear fuel particles by resonance ionization mass spectrometry. Sci Rep 13:5193
Steier P, Hrnecek E, Priller A, Quinto F, Srncik M, Wallner A, Wallner G, Winkler S (2013) AMS of the minor plutonium isotopes. Nucl Instrum Methods Phys Res Sect B 294:160–164
Tandon L, Kuhn K, Martinez P, Banar J, Walker L, Hahn T, Beddingfield D, Porterfield D, Myers S, LaMont S, Schwartz D (2009) Establishing reactor operations from uranium targets used for the production of plutonium. J Radioanal Nucl Chem 282(2):573–579
Christl M, Guérin N, Totland M, Gagné A, Kazi Z, Burrell S, Synal HA (2019) A novel chronometry technique for dating irradiated uranium fuels using Cm isotopic ratios. J Radioanal Nucl Chem 322(2):1611–1620
Osaka M, Koyama SI, Mitsugashira T (2012) Analysis of curium in mixed oxide fuel irradiated in the experimental fast reactor JOYO for the evaluation of its transmutation behavior. J Nucl Sci Technol 41(9):907–914
Gourgiotis A, Isnard H, Aubert M, Dupont E, AlMahamid E, Tiang G, Rao L, Lukens W, Cassette P, Panebianco S, Letourneau A, Chartier F (2010) Accurate determination of Curium and Californium isotopic ratios by inductively coupled plasma quadrupole mass spectrometry (ICP-QMS) in 248Cm samples for transmutation studies. Int J Mass Spectrom 291(3):101–107
Gourgiotis A, Isnard H, Nonell A, Aubert M, Stadelmann G, Dupont E, AlMahamid I, Tiang G, Rao L, Lukens W, Cassette P, Panebianco S, Letourneau A, Chartier F (2013) Bk and Cf chromatographic separation and 249Bk/248Cm and 249Cf/248Cm elemental ratios determination by inductively coupled plasma quadrupole mass spectrometry. Talanta 106(15):39–44
Radev R, McLean T (2014) Neutron sources for standard-based testing. Lawrence Livermore National Laboratory, Livermore
Table of nuclear data, Laboratoire national Henri-Becquerel. http://www.lnhb.fr/nuclear-data/nuclear-data-table/. Accessed 27 June 2023
Christl M, Gautschi P, Maxeiner S, Müller AM, Vockenhuber C, Synal HA (2023) 236U analyses with the ETH Zurich MILEA prototype system. Nucl Instrum Methods Phys Res B Beam Interact Mater At 534:61–71
Leppänen J, Pusa M, Viitanen T, Valtavirta V, Kaltiaisenaho T (2015) The serpent Monte Carlo code: status, development and applications in 2013. Ann Nucl Energy 82:142–150
Kazi Z, Guérin N, Christl M, Totland M, Gagné A, Burrell S (2019) Effective separation of Am(III) and Cm(III) using a DGA resin via the selective oxidation of Am(III) to Am(V). J Radioanal Nucl Chem 321:227–233
Dai X (2011) Isotopic uranium analysis in urine samples by alpha spectrometry. J Radioanal Nucl Chem 289(2):595–600
Bevington PR, Robinson DK, Robinson B (2002) Data reduction and error analysis for the physical sciences, 3rd edn. McGraw-Hill, Boston
Scott RS (2005) Nuclear forensics: attributing the source of spent fuel used in an RDD event. Thesis, Texas A&M University
Funding
This work was supported by Atomic Energy of Canada Limited under the Canadian Federal Science and Technology Work Plan (Project No. 51200.50.20.04.07). The ETH Zurich Laboratory of Ion Beam Physics is partially funded by its consortium partners EAWAG, EMPA, PSI, and WSL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial nor non-financial interests to disclose. The authors have no conflict of interest to disclose. The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Guérin, N., Christl, M., Gagné, A. et al. Validation and improvement of curium radiochronometry to determine the model discharge date of spent nuclear fuels. J Radioanal Nucl Chem 333, 2661–2669 (2024). https://doi.org/10.1007/s10967-024-09495-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09495-6