Abstract
In this study, trace amounts of thorium ions were extracted using a highly sensitive, selective, rapid and simple micro-funnel magnetic stirring-assisted liquid–liquid microextraction method, and determined by UV–Vis spectrophotometry. Tri-n-octylphosphine oxide was used as the complexing agent during the microextraction process, and Arsenazo III was applied as the chromogenic reagent through spectrophotometric determination. The effective parameters on extraction efficiency were optimized by experimental design methods. Under the optimum conditions, detection limit, relative standard deviation and the enrichment factor were 0.98 μg L−1, 0.69% and 152, respectively. The calibration graph was linear over the range of 5–250 μg L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thorium is one of the significantly radioactive elements that still occur naturally at low concentrations (10 μg L−1) in the earth’s crust. The main sources of thorium in nature are soil, rocks, plants, sand and water. This element is used in various products and processes, including the production of ceramics, carbon arc lamps, as an alloying material and as the light source in gas mantles [1, 2]. The toxicity of this element and its compounds is very high which causes irreversible consequences in the human body. The continuous exposure to thorium may increase the chance of developing types of cancer [3]. Therefore, developing a simple and sensitive method is very necessary and important for the separation/preconcentration and determination of thorium at trace amounts in environmental samples. Various analytical techniques such as inductively coupled plasma [4,5,6], optical sensor [7, 8], electrochemistry [9, 10], chromatography [11, 12] and spectrophotometry [12,13,14,15] have been developed for determination of Th(IV). Spectrophotometry is very simple, rapid and cost-effective for determination of elements in a variety of samples in comparison with the other techniques. However it is either not selective or it has a poor selectivity. In most of these techniques the direct measurement of trace amounts of elements is difficult hence separation and preconcentration steps are required. Several extraction techniques were applied for this purpose, such as liquid–liquid extraction (LLE) [16], solid-phase extraction (SPE) [17] and cloud point extraction (CPE) [18]. However, these methods are relatively expensive, time-consuming and in some cases require a large amount of hazardous organic solvent. In 2006, Rezaee et al. introduced dispersive liquid–liquid microextraction (DLLME) [19] which has many advantages such as high enrichment factor, simplicity, rapidity, easy to operate, low sample volume, low cost, and low consumption of organic solvents. In this method the extracting solvents are limited only to chlorinated solvents which have higher density than water.
Recently, great efforts were made to use low-density solvents in the microextraction methods [20,21,22,23,24]. However a common issue with these solvents is the difficulty of withdrawing very little amounts of it from the aqueous solvent surface. Therefore bell-shaped extraction device assisted liquid–liquid microextraction (BSED–LLME), was introduced by Radomir Cabala et al. [25]. In this technique, the extraction of organic compounds from mineral and drinking water samples was done.
In our previous study [26], we reported micro-funnel magnetic stirring-assisted dispersive liquid–liquid microextraction technique for determination of 3-chloro-4-(dichloromethyl)-5-hydroxy-2 (5H)-furanone in aqueous samples by GC-ECD.
The aim of this paper is extraction of trace amounts of thorium using a modified micro-funnel device coupled with UV–Vis spectrophotometry. A homemade micro-funnel vessel is used for the microextraction procedure by collecting the organic solvent in narrow neck glass tube. In this study Tri-n-octylphosphine oxide (TOPO) was used as the complexing agent during the microextraction process, and Arsenazo III was applied as the chromogenic reagent through spectrophotometric determination. Experimental parameters which may affect the extraction efficiency were identified by Plackett–Burman Design (PBD) and optimized by the Box–Behnken Design (BBD).
Experimental
Instrumentation
A Hewlett–Packard 8453 diode array spectrometer controlled by a Hewlett–Packard computer was used for spectrophotometric measurements. A vortex mixer (IKA Vortex Genius 3, Germany) was used for completely mixing the sample solution. Stirring the sample solution was carried out by Magnetic stirrer (IKA RH basic 2, Germany). A homemade microfunnel vessel was used for extraction process.
Reagent and chemicals
1-undecanol, 1-dodecanol, 1-decanol, n-octanol and toluene were purchased from Merck (Darmstadt, Germany). Tri-n-octylphosphine oxide (TOPO) and disodium salt of Arsenazo III (1, 8-dihydroxy-2, 7-bis (2 arsonophenylazo) naphthalene-3, 6-disulphonic acid) was purchased from Merck (Darmstadt, Germany). A 0.05% (w/v) stock solution of Arsenazo III (AIII) was prepared by dissolving 0.05 g of its disodium salt in 100 mL mixture of methanol and 3 mol L−1 HCl solutions. Analytical grade of thorium nitrate and nitrate salts of other cations (all from Merck, Darmstadt, Germany) were available in the highest purity. Methanol, ethanol, sodium chloride, sodium nitrate, nitric acid (HNO3) and hydrochloric acid (HCl) and perchloric acid (HClO4) were purchased from Merck (Darmstadt, Germany). The glassware was kept in a 5% (v/v) nitric acid solution overnight and thereafter washed with deionized water.
General procedure
Schematic diagram of the developed MF-MSA-LLME method steps is presented in Fig. 1. 50 mL of the sample solution containing 100 μg L−1 of thorium ion, NaNO3 (0.5% w/v) and HNO3 (3% w/v) were poured into cylindrical glass vial (13 cm height and 28 mm i.d.). 200 μL of TOPO solution (3.5% w/v in toluene) was injected on the sample solution surface and the micro funnel cap was placed on the cylindrical vial (Fig. 1a). Then the mixture was vigorously stirred at 1200 rpm for 5 min (Fig. 1b). This process greatly increased the contact area between the organic phase and aqueous phase, therefore the species was extracted into the fine droplets. Then the magnetic stirrer was stopped (Fig. 1c) and for separation of the two phases, the solution was kept immobilized for 1 min. Accordingly, the emulsified fine droplets of the extraction phase were collected on the top of the solution (Fig. 1d). Next, a few milliliters of doubly distilled water were injected into the vial through the rubber cap, which holds the micro-funnel to the glass tube. The floated organic solvent was moved into the capillary tube of micro-funnel (Fig. 1e). After that the organic solvent was collected using a 250 μL Hamilton syringe, transferred into the eppendorf tube and mixed with 10 μL of 0.04% w/v Arsenazo III solution as the chromogenic reagent. After vortexing for 3 min as rest time for the color to stabilize, Th4+–AIII complex was formed and was sedimented at the bottom of eppendorf tube. Afterward, toluene was carefully removed and sedimented phase was diluted to 50 μL by methanol. Finally the absorbance of the solution was recorded at λmax = 660 nm against a reagent blank (Fig. 1f).
Sample preparation
The water samples were collected from different part in Arak, Iran. The water samples (tap, river, well and mineral waters) were filtered using 0.45 μm micropore membranes, and stored in glass containers at 4 °C.
Optimization strategy
Different factors such as type and volume of extraction solvent, type and concentration of acid in sample solution, concentration of AIII, concentration of TOPO, extraction and vortex time and ionic strength can affect the extraction recovery in the MF-MSA-LLME procedure. So, an experimental design strategy is recommended for their optimization. Experimental design strategy was applied in two stages: (i) screening the significant factors affecting the extraction recovery using Plackett–Burman design and (ii) optimizing the significant factors using a Box-Behnken design. The results were analyzed using MINITAB Version 17 software.
Results and discussion
Selection type and volume of extraction solvent
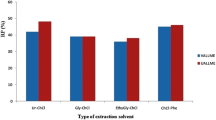
The extraction solvent should be immiscible with aqueous phase, have lower density than water and extraction capability of the target analytes. For this purpose, 1-octanol, 1-decanol, toluene, 1-undecanol and 1-dodecanol were examined. The obtained result showed that toluene has the highest extraction efficiency for the extraction of thorium (Fig. 2).
The effect of toluene volume was also investigated in the range of 50–500 µL and the results are given in Fig. 3. The results showed that maximum extraction efficiency was in 200 µL. Therefore, 200 µL of toluene was selected as extraction solvent for further experiment.
Selection of type of acid in sample solution
The effect of the presence of different inorganic acids such as HNO3, HCl and HClO4 on the extraction of thorium ion using TOPO by the proposed method was investigated and results are shown in Fig. 4. Based on the results, HNO3 showed the highest efficiency in the MF-MSA-LLME of thorium ions using TOPO.
Effect of salt
The addition of salt to the aqueous sample may affect the extraction recovery due to salting-out. In order to evaluate influence of the ionic strength on the extraction efficiency, different amounts of NaNO3 (0–5% w/v) were investigated. The results indicated that the extraction efficiency was increased by increasing NaNO3 concentration up to 0.5% (salting-out effect) and then decreased (Fig. 5). Therefore, 0.5% of NaNO3 was selected for subsequent experiment.
Effect of HCl concentration
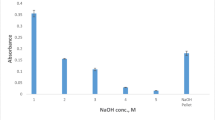
AIII has different active sites and it reacts with thorium in acidic medium. AIII solution in methanol creates a reddish purple color (λmax = 515 nm), while the Th4+–AIII complex (in acidic medium) is blue (λmax = 660 nm). So, effect of acidic medium on complexation of AIII with Th4+ was evaluated in the range of 0.5–5.0 mol L−1. The results showed that maximum absorbance of Th4+–AIII complex was achieved in 3.0 mol L−1 of HCl and then remained more or less constant. Therefore, 3.0 mol L−1 of HCl was chosen as the optimum value for next experiment.
Screening of significant factors using Plackett– Burman design
In this study, the Placket–Burman design (PBD) [27] was applied as a screening approach to recognize significant factors in extraction of thorium in aqueous samples. According to preliminary experiments, a PBD design was applied for 20 runs to estimate the significance of five factors [HNO3 concentration (X1, %v/v), concentration of TOPO (X2, %w/v), extraction time (X3, min), concentration of AIII (X4, %w/v) and vortex time (X5, min)], with two replicate at center point. Each factor was tested at two levels. The factors, their levels and the runs of PBD are shown in Table 1.
The standardized effects of factors in the screening experiments in the form of a Pareto chart in 95% confidence level are shown in Fig. 6. The Pareto charts of the standardized effects were drawn based on the results of analysis of variance (ANOVA) tests. According to results (Fig. 6), concentration of AIII, extraction time and concentration of TOPO had the most significant variables on the extraction efficiency. While, HNO3 concentration and vortex time, had no significant effect on the response and therefore were eliminated in BBD study and fixed at center point value.
Box–Behnken design
In the next step, a BBD was performed in order to determine the optimum conditions for the three screened significant factors (concentration of AIII, extraction time and concentration of TOPO). In this design the interaction effects, main effects, and quadratic effects were investigated and optimized. To perform BBD, the number of experiments (N) is obtained by the following equation:
where k is the number of factors and Co is the number of center points [28]. Thus, in the present study, 15 experiments were required including three center points, and they were performed randomly. The selected levels of factors and the design matrix with the responses are given in Table 2.
ANOVA was applied in order to evaluate the method and the results are shown in Table 3. P value and lack of fit (LOF) are important parameters in ANOVA table. The factors with P value less than 0.05 have more significant effect on the extraction process by MF-MSA-LLME method. According to the obtained results, P-value of lack-of-fit (0.575) was not significant (P > 0.05) which confirms a good fitting of model to responses. Also the coefficients of R2 (99.82%) and adjusted R2 (99.49%) indicate a good relationship between responses and the fitted model.
The relationship between the response value and the three variables were expressed with the following equation:
Figure 7a–c, show the three-dimensional response surface plots using Eq. 2, when one of the variables is fixed at the center point and two factors are allowed to vary. Figure 7a depicted the combined effect of concentration of AIII and concentration of TOPO on the response. Figure 7b showed the combined effect of extraction time and concentration of AIII at the fixed value of concentration of TOPO. Figure 7c described the combined effect of concentration of TOPO and extraction time at the fixed value of concentration of AIII. Based on the surface plots in Fig. 7a–c, extraction efficiency of thorium by MF-MSA-LLME method was increased with increasing concentration of TOPO up to 3.5% and then decreased. The results show that the maximum analytical signal was obtained in 0.04% of concentration of AIII. Also as can be seen, with increasing of extraction time up to almost 5 min, analytical signals increased and then decreased. Based on the BBD results the optimum conditions were: AIII concentration 0.04%w/v, concentration of TOPO 3.5 %w/v and extraction time 5 min.
Interference effect of foreign ions
To evaluate the selectivity of the proposed method for the extraction and determination of thorium ions in water samples containing different ions, the influence of several ions were studied. Table 4 represents the tolerance limits of the examined metal ions. According to the obtained results, most of the cations and anions did not interfere with the extraction and determination of thorium. However, Zr4+ has serious interfere in extraction and determination of thorium ions.
Analytical figures of merit
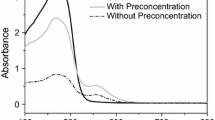
The analytical characteristics of the developed method, including linear dynamic range (LDR), limit of detection (LOD), repeatability and enrichment factor were obtained under optimum experimental conditions. The calibration curve for thorium was linear from 5 to 250 μg L−1, with correlation coefficient of 0.9981. LOD of the method for the determination of Th(IV) was defined as 3Sb/m where “Sb” is standard deviation of the blank signals for seven replicates and “m” is calibration curve’s slope of the MF-MSA-LLME method was 0.98 μg L−1. The precision of the method was evaluated by the relative standard deviation (RSD) (based on analysis of seven replicates containing 100 μg L−1 of Th(VI)) was found to be 0.69%. Enrichment factor which was calculated from the slope ratio of calibration curve with (5.328) and without (0.035) preconcentration by the MF-MSA-LLME procedure, was about 152.
Analysis of real samples
To evaluate the accuracy and applicability of the proposed method, it was applied to determine thorium ions from several water samples (tap, river, well and mineral waters were collected from Arak city, Iran). Recovery was tested by spiking known amount of thorium ions in the water samples and the results are given in Table 5. The results showed that the recoveries for the addition of different concentrations of thorium ions were in the range 95.08–100.32%.
Comparison of MF-MSA-LLME with some other method
Comparison of this method with those of some of the best previously preconcentration methods for determination of thorium is summarized in Table 6. As can be seen, the proposed method has good enrichment factors, wide linear dynamic range, and relative standard deviation and low LOD comparable with the most of reported methods. The results clearly indicate that MF-MSA-LLME is a reproducible, rapid, and simple technique that can be used for preconcentration and determination of trace amounts of thorium ions in water samples.
Conclusion
In this study, a novel, effective and sensitive MF-MSA-LLME method by the application of home-designed extraction vessel combined with UV–Vis spectrophotometry for the analysis of trace amount of thorium ions was developed. The proposed method was successfully applied without using disperser solvent and centrifugation step. Moreover, for optimization of the affective parameters in extraction efficiency, the PBD and BBD were successfully applied. This method is simple, rapid, inexpensive and low toxicity and has good enrichment factor, low LOD and high sensitivity and selectivity. The proposed method was successfully used to determine trace amounts of thorium ions in the real water samples with satisfactory results.
References
Yaftian MR, Zamani AA, Rostamnia S (2006) Thorium (IV) ion selective transport through a bulk liquid membrane containing 2-thenoyltrifluoroacetone as extractant-carrier. Sep Purif Technol 49(1):71–75
Aydin FA, Soylak M (2007) Solid phase extraction and preconcentration of uranium (VI) and thorium (IV) on Duolite XAD761 prior to their inductively coupled plasma mass spectrometric determination. Talanta 72(1):187–192
Suleymanov B, Ahmedov M, Safarova K, Steinnes E (2010) Metals in main rivers of azerbaijan: influence of transboundary pollution. Water Air Soil Pollut 213(1–4):301–310
Laferriere BD, Maiti TC, Arnquist IJ, Hoppe EW (2015) A novel assay method for the trace determination of Th and U in copper and lead using inductively coupled plasma mass spectrometry. Nucl Instrum Methods Phys Res 775:93–98
Deb SB, Saxena MK, Nagar BK, Ramakumar KL (2008) Determination of trace amounts of thorium in a uranium matrix by inductively coupled plasma mass spectrometry and validation of the separation procedure by standard addition and tracer techniques. At Spectrosc 29:39–44
Shrivastav P, Menon SK, Agrawal YK (2001) Selective extraction and inductively coupled plasma atomic emission spectrophotometric determination of thorium using chromogenic crown ether. J Radioanal Nucl Chem 250(3):459–464
Rastegarzadeh S, Pourreza N, Saeedi I (2010) An optical chemical sensor for thorium (IV) determination based on thorin. J Hazard Mater 173(1–3):110–114
Safavi A, Sadeghi M (2006) Design and evaluation of a thorium (IV) selective optode. Anal Chim Acta 567(2):184–188
Ganjali MR, Norouzi P, Faridbod F, Riahi S, Yaftian MR, Zamani A et al (2007) Highly selective and sensitive Th4+-PVC-based membrane sensor based on 2(diphenylphosphorothioyl)-N0, N0-diphenylacetamide. J Appl Electrochem 37(7):827–833
Arida HA, Ahmed MA, El-Saied AM (2003) A novel coated graphite rod Th(IV) ion selective electrode based on thorium oxinate complex and its application. Sensors 3(10):424–437
Kadam RB, Mali GG, Mohite BS (2013) Analytical application of poly [dibenzo-18-crown 6] for chromatographic separation of thorium (IV) from uranium (VI) and other elements in glycine medium. J Radioanal Nucl Chem 295(1):501–511
Rozmarić M, Ivsić AG, Grahek Z (2009) Determination of uranium and thorium in complex samples using chromatographic separation. ICP-MS and spectrophotometric detection. Talanta 80(1):352–362
Khan MH, Hafeez M, Bukhari SMH, Ali A (2014) Spectrophotometric determination of microamounts of thorium with thorin in the presence of cetylpyridinium chloride as surfactant in perchloric acid. J Radioanal Nucl Chem 301(3):703–709
Al-Kady AS (2012) Optimized and validated spectrophotometric methods for the determination of trace amounts of uranium and thorium using 4-chloro-N-(2, 6 dimethylphenyl)-2-hydroxy-5- sulfamoylbenzamide. Sens Actuators B 166–167:485–491
Niazi A (2006) Spectrophotometric simultaneous determination of uranium and thorium using partial least squares regression and orthogonal signal correction. J Braz Chem Soc 17:1020–1026
Zhijun G, Wangsuo W, Dadong S, Minyu T (2003) Liquid–liquid extraction of uranium (VI) and thorium (IV) by two open-chain crown ethers with terminal quinolyl groups in chloroform. J Radioanal Nucl Chem 258(1):199–203
Yousefi SR, Zolfonoun E (2016) On-line solid phase extraction using ion-pair microparticles combined with ICP-OES for the simultaneous preconcentration and determination of uranium and thorium. Radiochim Acta 104(11):801–807
Saha A, Deb SB, Sarkar A, Saxena MK, Tomar BS (2016) Simultaneous preconcentration of uranium and thorium in aqueous samples using cloud point extraction. RSC Adv 6:20109–20119
Rezaei M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116(1–2):1–9
Zhang Y, Lee HK (2013) Low-density solvent-based vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction combined with gas chromatography–mass spectrometry for the fast determination of phthalate esters in bottled water. J Chromatogr A 1274:28–35
Farajzadeh MA, Djozan D, Khorram P (2011) Development of a new microextraction method based on a dynamic single drop in a narrow-bore tube: application in extraction and preconcentration of some organic pollutants in well water and grape juice samples. Talanta 85(2):1135–1142
Ezoddin M, Abdi K, Esmaeili N (2016) Ultrasound enhanced air-assisted surfactant liquid–liquid microextraction based on the solidification of an organic droplet for the determination of chromium in water, air and biological samples. Microchem J 129:200–204
Hosseini M, Heydari R, Alimoradi M (2014) Vortex and air assisted liquid–liquid microextraction as a sample preparation method for high-performed liquid chromatography determinations. Talanta 130:171–176
Heydari F, Ramezani M (2017) Application of response surface methodology for optimization and determination of palladium by in-tube ultrasonic and air-assisted liquid–liquid microextraction coupled with flame atomic absorption spectrometry. Sep Sci Technol 52(10):1643–1651
Cabala R, Bursova M (2012) Bell-shaped extraction device assisted liquid–liquid microextraction technique and its optimization using response-surface methodology. J Chromatogr A 1230:24–29
Farahani A, Ramezani M, Hassan J (2017) Microfunnel magnetic stirring-assisted dispersive liquid–liquid microextraction derivatization technique for the determination of 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (Mutagen X) in aqueous samples by GC-ECD. Sep Sci Technol 52(9):1505–1511
Asfaram A, Ghaedi M, Goudarzi A (2016) Optimization of ultrasound-assisted dispersive solid-phase microextraction based on nanoparticles followed by spectrophotometry for the simultaneous determination of dyes using experimental design. Ultrason Sonochem 32:407–417
Zhou Y, Song JZ, Choi FFK, Wu HF, Qiao CF, Ding LS et al (2009) An experimental design approach using response surface techniques to obtain optimal liquid chromatography and mass spectrometry conditions to determine the alkaloids in Meconopsi species. J Chromatogr A 1216(42):7013–7023
Ghasemi JB, Zolfonoun E (2010) Simultaneous spectrophotometric determination of trace amounts of uranium, thorium, and zirconium using the partial least squares method after their preconcentration by α-benzoin oxime modified Amberlite XAD-2000 resin. Talanta 80(3):1191–1197
Jain VK, Pandya RA, Pillai SG, Shrivastav PS (2006) Simultaneous preconcentration of uranium (VI) and thorium (IV) from aqueous solutions using a chelating calix[4] arene anchored chloromethylated polystyrene solid phase. Talanta 70(2):257–266
Veyseh S, Niazi A (2017) Flotation-assisted homogeneous liquid–liquid microextraction for determination of thorium in water samples by inductively coupled plasma–mass spectrometry and Box-Behnken design. Sep Sci Technol 52(7):1289–1297
Zolfonoun E, Salahinejad M (2013) Preconcentration procedure using vortex-assisted liquid–liquid microextraction for the fast determination of trace levels of thorium in water samples. J Radioanal Nucl Chem 298(3):1801–1807
Rezaee M, Khalilian F (2016) A novel method for the determination of trace thorium by dispersive liquid–liquid microextraction based on solidification of flotation organic drop. Quim Nova 39(2):167–171
Acknowledgement
Financial support from the Azad University of Arak is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heydari, F., Ramezani, M. Micro-funnel magnetic stirring-assisted liquid–liquid microextraction technique combined with UV–Vis spectrophotometry for determination of thorium in aqueous samples with the aid of experimental design. J Radioanal Nucl Chem 320, 27–36 (2019). https://doi.org/10.1007/s10967-019-06452-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06452-6