Abstract
The aim of this study was to evaluate the effect of relative humidity on the stability of the 99mTc ethyl cysteinate dimer radiopharmaceutical. The radiochemical purity and pH of samples stored under controlled temperature and humidity conditions were assessed and compared to a negative control. 99mTc-Sestamibi samples were also analyzed under the same conditions for comparison purposes. The comparative analysis of results by Box and Whisker plots indicated the influence of high relative humidity (90 %) in the 99mTc-ECD stability. No significant changes were observed in the pH of the samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main reason for conducting stability studies of pharmaceuticals is the concern about the public health, since the degradation of a pharmaceutical can cause loss of therapeutic effect or increase toxicity. The widespread use of compounds labeled with technetium-99m (99mTc) in scintigraphy reveals a clear need of radiopharmaceuticals stability evaluation in various conditions, considering intrinsic and extrinsic factors that may affect the stability of these products [1].
According to the Brazilian Health Surveillance Agency (ANVISA) Resolution 01/2005, guide for conducting stability studies, the stability of pharmaceutical products can be compromised by environmental factors such as temperature, humidity and light, and intrinsic factors, such as physical and chemical properties of active substances and pharmaceutical excipients, pharmaceutical form and composition, manufacturing process, type and properties of packaging materials [1]. The stability lost is mainly characterized by the occurrence of chemical reactions that induce phenomenon such as hydrolysis and oxidation–reduction. Specifically in liquid pharmaceuticals, these reactions can also cause physical changes such as turbidity, clarity loss, viscosity, phase separation and discoloration [2]. Chemical reactions promoted by humidity specially affect drugs in solid and semi-solid forms as vitamin C [3] and may occur directly, when water acts as a reactant promoting the formation of free radicals, and indirectly, when water is adsorbed and dissolves the product surface, changing its physical state and reactivity [4]. In the case of radiopharmaceuticals, specifically, the radiolysis must be considered as a possible factor for molecule degradation. This phenomenon is characterized by the dissociation of water molecules in the presence of ionizing radiation, triggering the formation of free radicals [5].
Single-photon emission computed tomography (SPECT), along with the use of radiopharmaceuticals labeled with technetium-99m (99mTc), are important tools in the detection of a variety of diseases including disorders of the central nervous system. Neutral lipophilic radiotracers are used for cerebral perfusion examinations due to its ability to cross the blood brain barrier and be retained in the brain parenchyma. One of these compounds is the 99mTc-ethyl cysteinate dimer (99mTc-ECD) that has excellent retention and infusion characteristics [6]. Some factors may compromise the effectiveness of these drugs during the process of labeling, such as the quality of the generator eluate, lyophilized reagent components or procedures improperly performed, in disagreement with the manufacturer guidelines. These factors may cause the formation of impurities in which stands out the pertechnetate (99mTcO4 −), a consequence of non-reduction, and the reduced hydrolyzed technetium (99mTcO2), due to the reduction and non-metal complexation [7]. Some chromatographic techniques, such as thin layer chromatography (TLC), are used for quality control in order to identify and quantify those impurities [8].
According to the the Brazilian sanitary regulations, for registration purposes, all pharmaceuticals must take a long-term study of 12 months or an accelerated stability study of 6 months [1]. It is clear that this statement could not be applied to radiopharmaceuticals due their half-lives. To overcome this difficulty, the ANVISA Resolution 64/2009, which refers to the radiopharmaceuticals registration, requires at least five test points to be made, from time zero to the end of the experiment. The ANVISA Resolution considers humidity in stability studies of liquid pharmaceuticals, however, no scientific studies proving this influence were found. The aim of this study is to evaluate the effect of relative humidity (RH) on the radiolytic stability of the 99mTc-ECD radiopharmaceutical through periodic assessments of radiochemical purity and pH of samples stored under conditions of controlled temperature and humidity.
Experimental
Ethyl cysteinate dimer (ECD) samples [9], labeled with injectable solution of sodium pertechnetate (Na99mTcO4) were evaluated. The 99mTc-ECD was prepared according to the manufacturer’s instructions. An 1.0 ml saline solution (NaCl 0.9 % sterile) was added to the vial A and 1.0 ml of the solution to vial B. The contents were stirred to dissolve the product and then were added from 1.0 to 2.0 ml of solution of sodium pertechnetate (Na99mTcO4). To finish the reaction the solution was stirred again and left at room temperature for 30 min. The labeling of the 99mTc-Sestamibi was also made according to the pharmaceutical instructions. After adding from 3 to 6 ml of injectable solution of sodium pertechnetate, the volume of the vial was shaken to dissolve the product. Then the solution was heated on a water bath for 10 min and left at room temperature for more 10 min. Leftover radioactive eluate and radiopharmaceuticals were used after the withdrawals of doses for examinations in nuclear medicines services and for that reason it was not possible to standardize the radioactive concentrations utilized. Radioactive concentrations were used between 15.4 and 48 mCi and this factor was not taken into consideration in this study, since the recommendation of the manufacturers was followed in order to not use radioactive concentrations higher than 100 mCi (3700 MBq) for the labeling of the 99mTc-ECD and 400 mCi (14,800 MBq) for the 99mTc-Sestamibi.
After the removal of an aliquot for negative control, samples were stored in a climatic chamber (Ethik Technology, 420 CLDTS model) and submitted to a 40 °C temperature and a minimum relative humidity of 20 % and maximum of 90 %. Additionally, for comparative purposes, samples of 99mTc-Sestamibi radiopharmaceutical were stored under the same conditions mentioned above. The radiochemical purity of the samples was analyzed by TLC, using silica gel plates and Whatman paper as stationary phases. The mobile phases used were 20 % NaCl and acetone to 99mTc-ECD and methanol and 0.9 % NaCl to 99mTc-Sestamibi. After the chromatographic runs, the plates were read in radio chromatograph Raytest/Minigita with gamma detector Raytest/BGO-V, to identify and quantify possible impurities. The pH of the samples was also evaluated using pH paper indicator (2.0–9.0) with scale division of 0.5. Five test points were performed, the first one after the labeling procedure and the last one, 24 h after the tracing. In each analysis the sample results were compared to the negative control.
Results and discussion
Each result shown below corresponds to the arithmetic mean of measurements performed in triplicate. According to the data presented in Fig. 1a, b, samples of 99mTc-Sestamibi with initial radiochemical purity (RCP) above 99.2 %, remained stable for 24 h, presenting results higher than 97 % at the end of the experiment, for both humidity values tested. In Fig. 2a, it was compared results of 99mTc-Sestamibi control and samples submitted to 20 % RH and in Fig. 2b results of control and samples submitted to 90 % RH. The intersection between the data groups in the Box and Whisker plots indicated that there was no significant difference between control and samples for both relative humidity values tested.
Samples of 99mTc-ECD subjected to a RH of 20 % presented RCP of 97.6 % after 24 h and the percentage decreased to 88.7 % (Fig. 3a). When subjected to a RH of 90 %, the RCP of 99mTc-ECD samples initially decreased from 98.6 to 88.8 % at the end of the experiment (Fig. 3b). In the Fig. 4a, the comparison between results of control and samples submitted to 20 % RH showed similarity between the date groups. In the Fig. 4b, the date groups were located in different positions in the plot indicating variation between control and samples submitted to 90 % RH means. In Figs. 5 and 6 the results of RCP and identified impurities in the initial time and after 24 h of the experiment were showed.
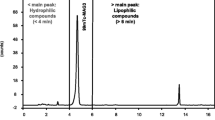
The chromatograms, in Fig. 7a, b, refer to the 99mTc-ECD control and samples analysis performed after 24 h. The two peaks observed in the chromatograms correspond to the percentage of 99mTcO2 deducted at the origin of the chromatographic plate (20 mm; Rf ~0.0) and in the front of plate the percentage of the compound 99mTc-ECD complexed along the possible presence of free 99mTcO4 − (60 mm; Rf ~1.0). The pH of the samples remained within the range specified by manufacturers throughout the experiment, between 5.0 and 6.0 for 99mTc-Sestamibi and 6.0–8.0 for 99mTc-ECD.
Sanchez et al. tested kits provided by three different manufacturers of 99mTc-Sestamibi. All samples were prepared in accordance with the manufacturer’s instructions and RCP was evaluated after 8 and 24 h. All samples showed a RCP higher than 90 % for activities in the range of 738–774 mCi. Norenberg et al. evaluated the influence of factors such as the amount of activity added to the reagent kit, the generator ingrowth time, the generator manufacturer, the age of the eluate, and the age of the formulated kit. The mean RCP remained greater than or equal to 90 % for 6 h at radioactivity levels ranging from 5.550 to 37.000 MBq [10, 11]. These findings are corroborate on the present work, where the RCP of 99mTc-Sestamibi samples were assessed up to 24 h in order to verify the influence of humidity. The results obtained showed no evident influence of this parameter on 99mTc-Sestamibi stability.
Koslowsky et al. evaluated the stability of 99mTc-ECD and 99mTc-HMPAO stored in syringes for 8 h. The RCP of the 99mTc-ECD samples remained above 94 % at all times of the experiment, regardless the samples were stored in syringes or in the vial itself. The 99mTc-HMPAO samples showed higher instability in relation to 99mTc-ECD, the RCP samples decreased to 81.4 % at the end of the experiment, stored in vial for sample and decreased further for samples stored in syringes, resulting in an average of 74.0 % at the end of the experiment [12]. The samples were not submitted to any stress. Analysis of the results of 99mTc-ECD samples evaluated in this study suggests a profile of accelerated radiolytic decomposition compared to the negative control. The chromatograms obtained after analysis of the 24 h 99mTc-ECD indicated the presence of reduced hydrolyzed technetium (99mTcO2) as the main impurity responsible for the loss of RCP of the radiopharmaceutical. The 99mTc-ECD radiopharmaceutical remained approved for use for approximately 5 h in both tested humidity conditions. This period of stability corresponds approximately to the period of validity of the compound, which is 4 h. After this period, the radiochemical purity of the sample loss becomes more pronounced.
Conclusions
From the results obtained it can be concluded that 99mTc-ECD seems to be more sensitive to environmental factors than 99mTc-Sestamibi since the pronounced loss of radiochemical purity of the samples submitted to 90 % RH compared to the control shows the influence of high relative humidity in the compound stability. For a better understanding of the influence of relative humidity on the stability of radiopharmaceuticals, the following studies are suggested:
-
Carry out tests with other radiopharmaceuticals used in clinical routine.
-
Test different manufacturers.
-
Assess the possible influence of relative humidity in the successive withdrawals of radiopharmaceutical of multi-dose vials, simulating the conditions of nuclear medicine services routine.
References
Ministério da Saúde, Agência Nacional de Vigilância Sanitária—ANVISA, Diário Oficial da União, Brasília, DF, 01 de agosto de 2005
Brossard D (2013) Methodological guidelines for stability studies of hospital pharmaceutical preparations. Part 1: liquid preparations, Societé Française de Pharmacie Clinique, Groupe d’Evaluation et de Recherche sur la Protection en Atmosphère Contrôlée
Hiatt AN, Taylor LS, Mauer LJ (2010) Influence of simultaneous variations in temperature and relative humidity on chemical stability of two vitamin C forms and implications for shelf life models. J Agric Food Chem 58:3532–3540. doi:10.1021/jf9003342f
Yoshioka S, Stella VJ (2000) Stability of drugs and dosage forms. Kluwer Academic Publishers, New York
Turner JE (2007) Atoms, radiation and radiation protection. Weinheim, Wiley-VCH
Vallabhajosula S, Zimmerman RE, Picard M, Stritzke P, Mena I, Hellman RS, Tikofski RS, Stabin MG, Morgan RA, Goldsmith SJ (1989) Technetium-99m ECD: a new brain imaging agent: in vivo kinetics and biodistribution studies in normal human subjects. J Nucl Med 30:599–604. http://jnm.snmjournals.org/content/30/5/599. Acessed 20 March 2014
Marques FLN, Okamoto MRY, Buchpiguel CA (2001) Alguns aspectos sobre geradores e radiofármacos de tecnécio-99m e seus controles de qualidade. Radiol Bras 34(4):233–239
Almeida EV, Monteiro EG, Alves EV, Silva NG, Fukumori NTO, Barboza MF, Mengatti J, Matsuda MMN, Vasconcellos MBA (2010) Controle de qualidade de EC-99mTc: determinação de pureza radioquímica e investigação da influência de impurezas na biodistribuição. Rev Bras de Fís Méd 4(1):71–74
Zolle I (2007) Preparation and quality control in nuclear medicine. Springer, New York
Norenberg JP, Vaidya MP, Hladik WB III, Pathak DR, Born JL, Anderson TL, Born JL, Anderson TL, Carroll TR (2005) The effect of selected preparation variables on the radiochemical purity of 99mTc-Sestamibi. J Nucl Med Technol 33:34–41
Sanchez C, Zimmer M, Cutrera P, McDonald N, Spies S (2009) Radiochemical purity and stability of generic Tc-99m Sestamibi Kits. J Nucl Med 50:2235
Koslowsky IL, Brake SE, Bitner SJ (2014) Evaluation of the stability of 99mTc-ECD and stabilized 99mTc-HMPAO stored in syringes. J Nucl Med Technol 29:197–200. http://tech.snmjournals.org/content/29/4/197.full.pdf+html. Accessed 23 March 2014
Acknowledgments
The authors would like to thank the Brazilian Nuclear Energy Commission (CNEN); National Council for Scientific and Technological Development (CNPq); Nuclear and Energy Research Institute (IPEN) and Cardiac Emergency of Pernambuco (PROCAPE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
dos Santos, E.V., de Oliveira, M.L. & do Nascimento, J.E. Influence of humidity on radiochemical purity of 99mTc-ECD and 99mTc-Sestamibi. J Radioanal Nucl Chem 306, 751–755 (2015). https://doi.org/10.1007/s10967-015-4424-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4424-7