Abstract
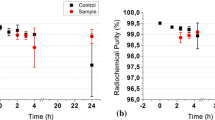
The aim of this work was to investigate the influence of pH, recovery volume, type of saline solution plastic or glass container, used to prepare the technetium-99m-d,l-hexamethylpropyleneamineoxime ([99mTc]Tc-d,l-HMPAO] radiopharmaceutical starting from generator produced sodium pertechnetate in nuclear medicine routine preparations. We observed that, neither the container type, nor the pH of 0.9% NaCl, used for diluting the [99mTc]pertechnetate, influenced [99mTc]Tc-d,l-HMPAO radiochemical purity (RCP) and stability, that decreased proportionally with the final volume of the preparation. In particular, the RCP of 1 mL kits preparations, at 30 min was found to be less than 80%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Technetium-99m (99mTc) (T 1/2 = 6.02 h; E γ = 140 keV) is a gamma-emitting medical radionuclide employed in the majority of diagnostic nuclear medicine procedures carried out every year in the world [1] and is also one of the most studied radionuclides in the research field, in order to obtain new radiopharmaceuticals that are increasingly compatible with the concept of personalized medicine [2, 3]. The technetium-99m-d,l-hexamethylpropyleneamineoxime ([99mTc]Tc-d,l-HMPAO) is routinely used for brain imaging investigations and for in vitro leukocytes labeling [4,5,6,7,8,9,10]. This compound is prepared from the commercial lyophilized kit, Ceretec™ that requires specific quality of the generator produced [99mTc]\({\text{TcO}}_{4}^{ - }\) eluate used for the reconstitution of the kit, that has to be no more than 2 h old and coming from a 99Mo/99mTc generator eluted no more than 24 h before.
The preparation of this radiopharmaceutical takes place instantly at room temperature and it must be injected within 30 min from the preparation. The particular caution that must be used in preparing this tracer results from the instability of the complex, which is the basis of the cerebral retention mechanism that distinguishes it. The neutral and lipophilic [99mTc]Tc-d,l-HMPAO complex rapidly diffuses across the blood–brain barrier (BBB) or the cell membrane and once deposited, the lipophilic specie is converted, in presence of glutathione [7], to hydrophilic products that is unable to re-cross the BBB [11, 12]. Unfortunately [99mTc]Tc-d,l-HMPAO reaches the instability quite quickly when stored at room temperature, and therefore its use is limited to a very short period of time after its preparation. For these reasons, the manufacturer requires that the reconstituted kit must be used within 30 min from the preparation and its radiochemical purity (RCP) must be not less than 80% [8]. Attempts to improve the use of labelled product have been reported, some of them in order to fractionate a reconstituted vial, others to overcome the restrictions on eluate [13], but most of them were focused to extend the shelf-life by the use of chelating, free radical scavengers or buffers [13,14,15,16,17,18,19]. Some years ago, a new commercial kit with shelf-life of 6 h containing cobalt chloride has been developed, but the new kit formulation results not appropriate for cell labeling [9, 20]. Moreover it has been reported that the presence of free radicals, caused by the γ decay of 99mTc, plays an important role in the [99mTc]Tc-d,l-HMPAO molecule stability that can be increased with minimal modification of manufacturer’s instructions and no chemicals addition to the kit commercial formulation [21]. Recently the effect on the [99mTc]Tc-d,l-HMPAO RCP, prepared using both exposed to light normal saline and stored in plastic ampoules (PAs), has been reported [22]. The study revealed that the exposition to light of normal saline contained in PA, causes a decrease in the [99mTc]Tc-d,l-HMPAO RCP, 30 min from Ceretec™ reconstitution. Other investigations were conducted in order to study in detail the effect of pH, buffer and ligand concentration on the stability of [99mTc]Tc-d,l-HMPAO [23], and it is a fact that the decomposition of the lipophilic compound are: its own instability, high radiation doses, Sn2+ ions excess and high pH (>9) [24]. During a preliminary study, not reported in this paper, we observed randomly that the RCP of [99mTc]Tc-d,l-HMPAO was <80% when normal saline coming from PA stored away from light was used for Ceretec™ kit reconstitution. On the contrary, this effect has not been observed when normal saline coming from glass ampoules (GAs) kept in the dark was used. We also noted that when the vial of HMPAO was reconstituted with 5 mL [99mTc]pertechnetate, previously diluted with PA normal saline, the final preparation pH was significantly lower (about pH 8) than the value indicated by the manufacturer (9.0–9.8). Starting from these observations, in this work we have reported the study of the RCP, stability and pH of [99mTc]Tc-d,l-HMPAO related to pH, type of container (PA or GA) and storage conditions of normal saline used for the Ceretec™ reconstitution.
This work was also carried out with the aim to support our upcoming studies about cyclotron produced 99mTc-pertechnetate radiopharmaceuticals [25,26,27]. In particular, for our forthcoming studies involving cyclotron-produced [99mTc]Tc-d,l-HMPAO, taking into account the sensibility of [99mTc]Tc-d,l-HMPAO to the environmental conditions, we consider important to exclude in advance variables, that do not belong to the pertechnetate production route, that might compromise the final RCP of the radiopharmaceutical.
Experimental
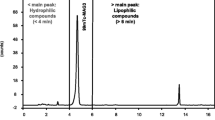
[99mTc]Tc-d,l-HMPAO was prepared by adding to the HMPAO kit (Ceretec™, GE Healthcare) 1, 2 or 5 mL of no more than 2 h old sodium [99mTc]pertechnetate (0.074–0.22 GBq/mL) eluted from a generator in turn eluted, with normal saline, no more than 24 h before. In the HMPAO kit, 0.5 mg of the tetradentate ligand d,l-4,8 diaza-3,6,6,9-tetramethylundecane-2,10-dione bisoxime (d,l-HMPAO), 7.6 µg of SnCl2·2H2O, and 4.5 mg of NaCl, freeze-dried and stoppered under nitrogen were present. 99Mo/99mTc generator was purchased from Drytec GE, Healthcare, UK. The radioactive concentration specified from the manufacturer was achieved by diluting generator eluted sodium [99mTc]pertechnetate with both normal saline contained in PAs (Fresenium Kabi AG, Germany) and normal saline contained in GAs (Fresenium Kabi AG, Germany). The labeling reaction is almost instantaneous and takes place in few seconds at room temperature. The [99mTc]Tc-d,l-HMPAO RCP and stability was measured at different time after reconstitution (2, 15 and 30 min) by the following chromatographic methods: (1) a thin-layer chromatography strip impregnated with silicic acid (TLC-SA, Agilent Technologies, USA) as stationary phase developed with methyl–ethyl-ketone (Sigma-Aldrich, Milan, Italy) as mobile phase and (2) TLC-SA developed with saline. TLC strips were analyzed, with a Cyclone Plus Storage Phosphor System instrument equipped with a phosphor imaging screen and an OptiQuant image analysis software (PerkinElmer, USA). The RCP and stability of [99mTc]Tc-d,l-HMPAO was calculated by subtracting the percentage activity measured at the solvent front with chromatographic system (1) (% [99mTc]pertechnetate + % [99mTc]Tc-d,l-HMPAO) from the percentage activity at the top of the TLC-SA strip determined with chromatographic system (2) (%[99mTc]pertechnetate). An electronic calibrated glass micro pH-meter (HI 122/Hanna Instrument, Cinisello Balsamo, Italy) was used for the pH determination of generator eluted [99mTc]pertechnetate, normal saline and for [99mTc]Tc-d,l-HMPAO.
Results and discussion
The radiopharmaceutical [99mTc]Tc-d,l-HMPAO was prepared introducing 1, 2, or 5 mL of generator eluted [99mTc]pertechnetate into Ceretec™ kit according to the procedure specified by the manufacturer. With the aim to obtain a radioactive concentration included in the range of 0.074–0.22 GBq/mL, pertechnetate was diluted before the reconstitution of the kit using normal saline contained in PAs or GAs, both kept away from sources of light. Moreover, for each volume of diluted [99mTc]pertechnetate three different radioactive concentration were used (0.074, 0.15 and 0.22 GBq/mL, as described in Tables 1, 2 and 3, respectively). For each type of ampoule (PA and GA), and radioactivity concentration, 15 kits were respectively reconstituted with 1, 2 or 5 mL of diluted sodium pertechnetate. The RCP of [99mTc]Tc-d,l-HMPAO, evaluated at different time after reconstitution, is reported in the last columns of Tables 1, 2 and 3. In the first column of each table the results have been divided into three main groups, based on the diluted [99mTc]pertechnetate volume used for the Ceretec™ reconstitution. For each diluted [99mTc]pertechnetate volume, the results, expressed as mean ± standard deviation, have been reported subdivided on the base of container type (PA or GA) of the normal saline used. We reported, for the sake of completeness, also pH and volume of normal saline used for diluting pertechnetate in the third and fourth column, respectively. Finally, in the fifth column the pH of the radiopharmaceutical preparation is reported.
Generally we have found that, for the same kit reconstitution volume, the RCP of [99mTc]Tc-d,l-HMPAO is not affected by the type of ampoule containing the normal saline used for the dilution of [99mTc]pertechnetate. Moreover, RCP values proportionally decrease with reconstitution volume and, in particular, for 1 mL preparations, the RCP at 30 min after preparation, was found to be not consistent with manufacturer requirement. This is in agreement with what was reported by Hung et al. [23] and Jinmng et al. [28], who showed that an excess of stannous ions concentration in solution, as in the 1 mL preparation, if compared to 5 mL one, accelerate the decomposition of the lipophilic complex, decreasing stability of the [99mTc]Tc-d,l-HMPAO.
Conclusions
In conclusion, we found that container type and pH of normal saline used for [99mTc]pertechnetate dilution do not influence the RCP and stability of [99mTc]Tc-d,l-HMPAO at 30 min after its preparation. However, we observed a more rapid decomposition of the neutral lipophilic compound in kits reconstituted with [99mTc]pertechnetate volume smaller than 2 mL, typical for routine in vitro leukocyte labeling. In particular, we found that the kit reconstituted with a volume of 1 mL should not be used after 15 min post preparation. This suggests, in particular taking into account that no correlation among the [99mTc]pertechnetate radioactive concentration (allowed by the manufacturer) and RCP of the product seems to exist, that any protocols involving volume smaller than 2 mL of generator eluted [99mTc]pertechnetate for the kit reconstitution should be carefully evaluated in terms of stability of the product. In our investigation we also noted that the average pH is always lower than the one indicated by the manufacturer (9.0–9.8). This does not seem to compromise the stability of the final [99mTc]Tc-d,l-HMPAO complex.
References
Selivanova SV, Lavalleè E, Senta H et al (2017) Clinical trial using sodium pertechnetate 99mTc produced with medium-energy cyclotron: biodistribution and safety assessment in patients with abnormal thyroid function. J Nucl Med 58:791–798
Boschi A, Cazzola E, Uccelli L et al (2012) Rhenium(V) and technetium(V) nitrido complexes with mixed tridentate π-donor and monodentate π-acceptor ligands. Inorg Chem 51:3130–3137
Boschi A, Uccelli L, Pasquali M et al (2013) Mixed tridentate π-donor and monodentate π-acceptor ligands as chelating systems for rhenium-188 and technetium-99m nitride radiopharmaceuticals. Curr Radiopharm 6:137–145
Chaplin SB, Oberle PA, McKenzie EH et al (1986) Regional cerebral uptake and retention of 99mTc-tetramethyl and pentamethyl-propylene amine-oxime chelates. Nucl Med Biol 13:261–267
Peters AM, Danpure HJ, Osman S et al (1986) Clinical experience with 99mTc-hexamethyl-propyleneamineoxime for labeling leukocytes imaging inflammation. Lancet 2:946–949
Neirinckx RD, Canning LR, Piper IM et al (1987) Technetium-99m d,l-HM-PAO: a new radiopharmaceutical for SPECT imaging of regional cerebral blood perfusion. J Nucl Med 28:191–202
Neirinckx RD, Harrison RC, Forster AM et al (1987) A model for the in vivo behavior of Tc-99m d,l-HMPAO in man. J Nucl Med 28:559–563
GE Healthcare (2016) Ceretec technical leaflet. GE Healthcare, UK
De Vries EF, Roca M, Jamar F, Israel O, Signore A (2010) Guidelines for the labelling of leucocytes with 99mTc-HMPAO. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging 37:842–848
Boschi A, Paquali M, Uccelli L, Duatti A (2007) Novel Tc-99m radiotracers for brain imaging. Braz Arch Biol Technol 50:37–44
Ballinger J (1990) Preparation of Tc-99m HMPAO [comment on]. J Nucl Med 31:1892–1895
Seifert S, Muth O, Jantsch K, Johannsen B (1995) Radiochemical purity of Tc-99m HMPAO: critical parameters during kit preparation. Nucl Med Biol 22:1063–1069
Weisner PS, Bower GR, Dollimore LA, Forster AM, Higley B, Storey AE (1993) A method for stabilising technetium-99m exametazime prepared from a commercial kit. Eur J Nucl Med 20:661–664
Chattopadhyay S, Das MK, Vanaja R, Ramamoorthy N (2001) Purification and stabilization of 99mTc-d,l-HMPAO: role of organic extractants. Nucl Med Biol 28:741–745
Solanki C, Wraight EP, Barber RW, Sampson SB (1998) Seven-hour stabilization of 99mTc-exametazime (HMPAO) for cerebral perfusion. Nucl Med Commun 19:567–570
Chuang MH, Tsao WL, Wang YF (2003) Comparison of the stabilization of Tc-99m HMPAO in different salicylate preparations. Tzu Chi Med J 15:363–366
Pastor F, Leiva L, Martinez MT, Fuente T (2006) Modificación del procedimiento de marcaje de Tc-99m exametazima y su influencia sobre la estabilidad de la preparación. Rev Esp Med Nucl 25:29–32
García Seguí V, Roca Engroñat M, Armero Muñoz M et al (1998) Stabilization of 99mTc-HMPAO with methylene blue: its use in leukocyte labelling). Rev Esp Med Nucl 17:261–265
Billinghurst MW, Abrams DN, Lawson MS (1991) Stabilization of [99mTc]HMPAO-ethanolic preparation. Int J Radiat Appl Instrum 42:607–611
Grüning T, Franke WG (2001) Are leukocytes labelled with stabilized Tc-99m-HMPAO becoming activated during labelling? J Nucl Med 42:685–689
Martinez T, Miñana E, Martín-Falquina TC, Fuente T (2014) Influence of radiolytic-produced hydrogen peroxide on the stability of commercial kit 99mtechnetium-exametazime preparation. J Label Compd Radiopharm 57:49–52
Craig F, Beatti LA, O’Brein LM, Millar AM (2009) Preparation of 99mTc radiopharmaceuticals: the effect on radiochemical purity of using sodium chloride injection from plastic ampoules that have been exposed to light. Nucl Med Commun 30:868–871
Hung CY, Corlija M, Volkert A, Holmes RA (1988) Kinetic analysis of Tc-99m-d,l-HM-PAO decomposition in aqueous media. J Nucl Med 29:1568–1576
Ramamoorthy N, Pillai MRA, Troutner DE (1993) Studies on some aspects of the instability of 99mTc-hexamethylpropylene amine oxime (99mTc-HMPAO). Nucl Med Biol 20:307–310
Esposito J, Vecchi G, Pupillo G, Taibi A, Uccelli L, Boschi A, Gambaccini M (2013) Evaluation of 99Mo and 99mTc productions based on a high-performance cyclotron. Sci Technol Nucl Install. Article ID 972381
Martini P, Boschi A, Cicoria G, Uccelli L, Pasquali M, Duatti A, Pupillo G, Marengo M, Loriggiola M, Esposito J (2016) A solvent-extraction module for cyclotron production of high-purity technetium-99m. Appl Radiat Isot 118:302–307
Uccelli L, Boschi A, Pasquali M et al (2013) Influence of the generator in-growth time on the final radiochemical purity and stability of radiopharmaceuticals. Sci Technol Nucl Install. Article ID 379283. ISSN 1687-6083
Jinming Z, Jiahe T, Qiongfang L et al (1996) Kinetic analysis of 99mTc-d,l-HMPAO decomposition and the method of stabilisation. J Radioanal Nucl Chem 206:85–89
Acknowledgements
Products are furnished by Nuclear Medicine Unit of University Hospital of Ferrara.
Disclosures
We declare that we have not received funding, contracts or other forms of personal or institutional funding, with companies whose products are mentioned in the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uccelli, L., Martini, P., Pasquali, M. et al. Radiochemical purity and stability of 99mTc-HMPAO in routine preparations. J Radioanal Nucl Chem 314, 1177–1181 (2017). https://doi.org/10.1007/s10967-017-5437-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5437-1