Abstract
The extraction behavior of the Rf homologs, Zr and Hf, has been studied in HCl, HNO3, and H2SO4 media using TEVA® (a trioctyl and tridecyl methyl ammonium-based resin) and UTEVA® (a diamyl amylphosphonate-based resin). All six systems were considered for the future chemical characterization of Rf. Batch uptake studies were first performed to determine which systems could separate Zr and Hf and these results were used to determine what acid concentration range to focus on for the column studies. The batch uptake studies showed that UTEVA separates Zr and Hf in all media, while the intergroup separation was only observed in HCl media with TEVA. Both HCl systems showed viability for potential extraction chromatographic studies of Rf.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of the heaviest elements probes the upper limits of the periodic table, where relativistic effects may significantly alter the periodicity of the elements. These effects increase as Z 2 [1], so the transactinide elements (Z > 103) are expected to have the greatest effect. Chromatographic separations have frequently been used to investigate the strength of complex formation of transactinides in comparison to their homologs [2–5], since the extraction sequence correlates with the strength of complex formation. 261Rf is often used for these studies [2–8] because it has a relatively long half-life of 75 ± 7 s [9] and the 248Cm(18O, 5n)261Rf reaction has a relatively large cross section of 12 ± 3 nb [10]. In previous experiments, the automated ion-exchange separation apparatus coupled with the detection system for alpha spectroscopy (AIDA) was used to investigate the anion-exchange behavior of Rf in 4.0–11.5 M HCl and 8.0 M HNO3 using the anion-exchange resin MCI GEL CA08Y (also known as CA08Y) [3]. The online results suggested that the strength of chloride complex formation is Rf > Zr > Hf. In an 8.0 M HNO3 solution, Rf did not form anionic complexes, behaving similarly to Zr and Hf, and not like its pseudohomologs Pu(IV) and Th(IV). The neutral extractants tributylphosphate (TBP) [2, 4] and trioctylphosphine oxide (TOPO) [5] have also been used to study the complex formation strength of Rf. Günther et al. used a microcolumn containing TBP on an inert support, and in 8 M HCl the extraction sequence obtained was Zr > Rf > Hf [2]. Haba et al. then repeated the TBP chromatographic separation in 7.2–8.0 M HCl using AIDA and determined an extraction sequence of Zr > Hf ≈ Rf, while overall extraction increased with HCl concentration [4]. These authors interpreted this as evidence that the Zr and Hf tetrachlorides form more stable TBP complexes than Rf [4], under the assumption that the sequence of chloride complexation is Rf > Zr > Hf [3]. A chromatographic extraction using TOPO was also performed using AIDA and obtained an extraction sequence of Zr > Hf ≥ Rf [5]. TOPO was chosen because it was expected to form a stronger complex with group 4 elements than TBP, but this was not observed with Rf.
In the current work, the commercially available extraction chromatography resins TEVA® and UTEVA® from Eichrom Technologies (Lisle, Illinois, USA) were investigated in HCl, HNO3, and H2SO4 media (six systems) as possible candidates for studying the chemistry of Rf. The chloride systems could be used to possibly clarify the ambiguity in the previous results [2–8]; the other systems were studied because the chemical behavior of Rf in a pure nitric acid and sulfuric acid solution has been investigated only on a limited basis. The TEVA resin is an aliphatic quaternary amine physisorbed to an inert polymeric support. The extractant molecule is a mixture of trioctyl and tridecyl methyl ammonium chlorides (also known as Aliquat 336) [11]. TEVA behaves like an anionic-exchange resin; however, the functional groups are in the liquid form, so the extractant can more easily interact with anionic complexes [11]. Therefore, TEVA may have better separation capabilities than CA08Y. Hulet et al. studied Rf with Aliquat 336 supported on an inactive fluorocarbon powder in 12.0 M HCl [12]. It was discovered that Rf behaved like Hf and not like Cm or Fm; nevertheless, the experiment did not study Zr and intergroup trends could not be inferred. The UTEVA resin is a phosphonate compound physisorbed to an inert polymeric support. The extraction molecule is diamyl amylphosphonate, a neutral extractant [13]. In general, the extraction ability of organophosphorus compounds is correlated to the number of carbon-phosphorus bonds in the molecule: phosphate < phosphonate < phosphinate < phosphine oxide [14]. As a phosphonate, UTEVA should have an extraction strength in between TBP and TOPO.
Tracer-scale concentrations of the long-lived Rf homologs 88,89Zr and 175Hf have been used to mimic the “atom-at-a-time” nature of transactinide chemistry. Both resins’ extraction capabilities were quantified as resin capacity factor (the number of free column volumes to peak maximum), k′, for both Zr and Hf in various acid concentrations (1.0 M to concentrated). The column chromatographic separation of the two elements was measured with the goal of determining which of the six systems could preferentially extract one of the Rf homologs over the other, and in turn be used for future chemical characterization studies of Rf.
Experimental
Material
TEVA and UTEVA in the free resin form (50–150 μm particle size) were purchased from Eichrom Technologies. Prepackaged 2 ml dry pack cartridges of both TEVA and UTEVA resin (50–100 µm particle size) were also purchased from Eichrom Technologies. All standardized acids were made from reagent grade HCl, HNO3 and H2SO4 acids purchased from VWR International (used without further purification) and de-ionized water (18 MΩ cm).
Tracer solutions
Group 4 elements are known to predominantly form mononuclear species at metal ion concentrations <10−4 M [15], so carrier-free solutions of these radioisotopes had to be used to approximate the single-atom nature of Rf chemistry experiments. The radionuclides 88,89Zr and 175Hf were used as tracers and their decay properties are given in Table 1. Carrier-free 89Zr in a 1.0 M oxalic acid solution was purchased from PerkinElmer (Waltham, Massachusetts, USA). A small amount of 88Zr (<0.5 % of total activity) was present in the sample. The 88,89Zr oxalate was converted to the chloride form by adding 2 ml of La(NO3)3 carrier solution (10 mg ml−1) and 2 ml of 10 % NH4OH solution to the sample, causing the 88,89Zr to coprecipitate with La(OH)3. The solution was centrifuged and the supernatant was removed. The precipitate containing 88,89Zr was then dissolved in concentrated HCl, completing the chloride conversion. The 88,89Zr was isolated from the La carrier using a Dowex 1 × 8 anion-exchange column according to the procedure in [16]. The dissolved precipitate was loaded onto the column, concentrated HCl was passed through the column to remove impurities, and then the 88,89Zr was eluted using 2 M HCl. The 88,89Zr was stored in a 2 M HCl solution in a glass bottle.
175Hf was produced via the natLu(p, x)175Hf reaction [17] at the Center for Accelerator Mass Spectrometry at Lawrence Livermore National Laboratory (LLNL). The irradiated natLu target (99.9 %, <10 ppm natHf) was dissolved in concentrated HCl at 40–50 °C for approximately 30 min. The solution was allowed to cool, causing most of the natLu to precipitate out of solution. The sample was immediately centrifuged, and the supernatant containing the majority of the 175Hf was removed (and confirmed by gamma spectrometry). The 175Hf solution was then purified using the same method described above for 88,89Zr [16]. The 175Hf was also stored in a 2 M HCl solution in a glass bottle.
Batch study procedure
Before the experiments, Zr and Hf samples were separately prepared by transferring 1.0 ml of the stock solution into a 12 × 55 mm polypropylene (PP) vial. The samples were evaporated to dryness in a water bath under a stream of compressed air and then reconstituted with 1.0 ml of the acid concentration of interest. Standardized acids were prepared of HCl, HNO3 and H2SO4 that spanned a wide concentration range (1.0 M to concentrated). The extraction of Zr and Hf was not studied in acid concentrations <1.0 M due to the tendency of group 4 elements to undergo extensive hydrolysis in this concentration range [15]. It was discovered that a variable amount of the activity was still sorbed to the PP vial after the sample was reconstituted, so the samples were transferred to a fresh PP vial before the initial activity was measured. 10–20 mg of the resin of interest was then weighted into each sample vial, and the samples were mixed by a shaker for 1 h at room temperature. The slurry was filtered using a 0.45 µm (pore size) polytetrafluoroethylene 25 mm diameter syringe tip filter. The filter paper and the resin absorbed a variable amount of the solution depending on the acid concentration of the sample. To correct for this effect, a measured aliquot, typically 50–65 % of the original sample, was collected and transferred to a fresh PP vial. Water was added to maintain the same 1.0 ml counting geometry as the initial count. The sample was counted a second time to determine the amount of Zr or Hf that had been extracted by the resin. The extraction capabilities of TEVA and UTEVA resin were studied in all 3 acid media (HCl, HNO3 and H2SO4) using the method described above. Each acid concentration was studied in triplicate.
Column study procedure
Extraction chromatography was performed using prepackaged 2 ml dry pack cartridges of the TEVA and UTEVA resins (50–100 µm particle size). These cartridges have a free column volume equal to 65–68 % (1.3–1.4 ml) of the bed volume [11, 13]. The load samples were prepared and measured for initial activity using the same method described for batch studies. A vacuum box purchased from Eichrom Technologies was used to control the eluent flow rate to 1.0 ml min−1 per recommendation of Eichrom. All column studies were performed at room temperature. The column was conditioned by running 10.0 ml of the same acid concentration as the load solution through the column, and a 1.0 ml load sample was loaded onto the column. Due to the free column volume size, the elution profile was determined using 1.0 ml fractions, the first of which was the load sample. The acid concentration of the load fraction and the next five 1.0 ml fractions was chosen based on the batch study results such that the separation factor was very high, while one of the radioisotopes’ k′ was small (<5). This maximizes the probability that exactly one of Zr or Hf will elute completely within the first six fractions. Six additional 1.0 ml fractions of an acid concentration where the resin did not retain either Zr or Hf were collected in order to elute any remaining radionuclides. The 12 individual fractions were collected in PP vials and counted to determine the elution profile. All column studies were repeated in triplicate.
Activity measurements
A PerkinElmer Wizard2 2480 automatic well-type NaI gamma counter with a 1000-sample capacity was used to assay the majority of the samples due to its high throughput and detection efficiency. The individual radionuclides had to be studied separately due to the large specific activity differences between 89Zr and 175Hf (see Table 1). Mixed radionuclides column studies using 88Zr and 175Hf together were preformed to confirm promising results obtained when studying the radioisotopes separately. 88Zr was used instead of 89Zr due to its lower specific activity (see Table 1). A high-purity Ge (HPGe) detector was used to assay the mixed samples. A detector with greater resolution was needed because of spectral interference between the prominent gamma lines of 88Zr and 175Hf (see Table 1).
Data analysis
The uptake was quantified as k′ for each batch study and calculated by first determining the weighted distribution coefficient, D w, in ml g−1 according to Eq. (1) [13, 18]:
where A o is the initial activity of the sample and A s is the final activity of the sample after the sample has interacted with the resin; the difference is the amount of activity the resin retained. W is the mass of the resin in grams and V is the volume of the sample in milliliters. D w can be converted to k′ by dividing by the “resin factor” for the individual resins [13, 18]. The TEVA and UTEVA resin factors are 1.9 and 1.7, respectively [11, 13]. The batch study k′ results discussed below are weighted averages of the replicates.
The column study data analysis to determine the elution profile was considerably simpler. For each of the 12 individual fractions, the fraction of activity eluted was determined as the ratio between activity measured in the collected fraction and the activity measured in the load sample. The elution profiles shown in this work are an average of the column study replicates. The minimum fraction of activity eluted that could accurately be measured was determined using the “minimum detectable activity,” Eq. (6) in [19].
Results and discussion
TEVA experiments
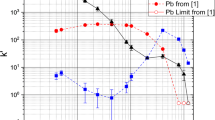
The batch study results for TEVA extraction of 89Zr and 175Hf from an HCl medium are shown in Fig. 1. In the range of approximately 2.0–6.0 M HCl there is little retention of both Zr and Hf, which is expected since both Zr and Hf are expected to form cationic and neutral species in this HCl concentration range [20]. Between 6.2 and 7.8 M HCl, Zr begins to have an affinity for TEVA while Hf stays completely in solution. This is evidence that Zr forms anionic species in a lower HCl concentration than Hf, in agreement with previous work [3]. Uptake of both Zr and Hf increases with HCl concentration above 7.8 M, while Zr continues to be more retained by TEVA. The highest separation factor, 18 ± 8, was measured in 8.4 M HCl; this acid concentration range was studied for viability in column separations (see the “Results and discussion” below).
The extraction of 89Zr and 175Hf from HNO3 and H2SO4 media using TEVA did not show promise for the chemical study of Rf. In both cases, the resin did not have any affinity (k′ < 1) for Zr or Hf in any of the concentrations studied (1.0–15.2 M HNO3 and 1.9–17.7 M H2SO4). The results in the HNO3 medium agree with previous work done with the anion exchange resin, CA08Y [3]. Work by Caletka et al. showed the anion exchange resin, Dowex 1 not retaining group 4 elements between 1.0 and 6.0 M H2SO4 [21], so it is not surprising that TEVA would not extract Zr or Hf in any of the concentrations considered. However, the formation of anionic species in this acid concentration range cannot be excluded due to the possibility of competing sorption of sulfate ions [22]. Based on these batch study results, neither of these systems were considered for column studies.
Based on the batch study results of TEVA in an HCl medium, three different column study conditions were tested. The acid concentration of the load solution and subsequent five fractions were 7.8, 8.1, and 8.4 M HCl; it was expected at these acid concentrations Hf would elute from the column while Zr would be retained. All column study conditions then used six fractions of 4.1 M HCl to remove any remaining radionuclides; at this acid concentration k′ of <0.9 and 1.6 ± 0.9 were measured for Zr and Hf, respectively. The most promising results were obtained using 8.1 M HCl to separate Hf from Zr and are shown in Fig. 2. Under these conditions, the majority of the Hf eluted in the first six fractions (90 ± 1 %) while almost none of the Zr was detected (<5 %). As expected, Zr was eluted from the column using 4.1 M HCl. A mixed radionuclides column study using 88Zr and 175Hf confirmed these results (also shown in Fig. 2). This system shows promise for a future extraction chromatographic study of Rf.
HCl elution profile for Zr and Hf using a 2 ml TEVA resin cartridge with a flow rate of ~1 ml min−1. The first fraction is the load fraction. The solid-lined results are averages from the triplicate column studies performed with 89Zr and 175Hf, separately. The dotted-lined results are from the mixed radionuclides, 88Zr and 175Hf, column study. Arrows indicate upper limits
UTEVA experiments
The batch study results for UTEVA extraction of 89Zr and 175Hf from all media studied are shown in Fig. 3. The HCl system results (Fig. 3a) are in agreement with previous work using neutral extractants [2, 4, 5]. Below ~4 M HCl, Zr and Hf did not have any affinity for UTEVA, and this is most likely due to the ions forming cationic species in this acid concentration range [20]. In ~5 M HCl, Zr begins to form neutral species and is extracted by the resin, while Hf does not form neutral species until ~6 M HCl. Zr and Hf affinity for UTEVA continues to increase with HCl concentration and reaches 100 % uptake by the resin in ~8 M HCl. From ~8 M to concentrated HCl, the retention of Zr and Hf by UTEVA is consistently 100 %, which was surprising since TEVA also had high retention in this acid concentration range (see Fig. 1). However, this can be explained by the equilibrium between neutral and anionic species being shifted whenever an extractant molecule retains a neutral Zr or Hf complex. When the neutral species is extracted by UTEVA, the equilibrium shifts to favor neutral complexes so an anionic complex is converted; this process continues until all of the Zr and Hf complexes are retained by the resin. A similar phenomenon has been seen with cationic and anionic exchange resins in low H2SO4 concentrations [22]. The highest separation factor, >9.4, was measured in 5.6 M HCl. This acid concentration range, 4.5–6.0 M, was studied for viability in column separations (see the “Results and discussion” below).
UTEVA extraction of 89Zr and 175Hf from an HNO3 medium showed significant separation of Zr and Hf (Fig. 3b). Both Zr and Hf do not have very high affinity for UTEVA in HNO3 concentrations <2 M; TEVA also did not show any uptake in this acid concentration range, so it can be inferred that both Zr and Hf are present in solution as cationic species. In the range 2.0–8.0 M HNO3, both Zr and Hf show increased affinity for UTEVA with increasing acid concentration. As seen in the HCl systems, Zr is extracted at a lower concentration than Hf, evidence that Hf forms cationic complexes in higher HNO3 concentrations than Zr. Above 8.0 M HNO3, UTEVA has complete retention of both Zr and Hf. These results agree with work by Omtvedt et al. on the online liquid–liquid extraction of Zr, Hf, and Rf with dibutyl-phosphoric acid into toluene from 6.0 M HNO3 [23]. The highest separation factor, 10 ± 1, was measured in 4.9 M HNO3. This acid concentration range was not considered for column separation because the measured k′ for Hf (20 ± 2) was too large (see “Experimental” in the section entitled “Column Study Procedure”). The second highest separation factor, 9 ± 4, was measured in 2.6 M HNO3; this acid concentration range was considered for column separation since Hf’s measured k′ was 2.4 ± 0.9 (see the “Results and discussion” below).
The results from the UTEVA in H2SO4 batch study were notable because it was the only system where Hf was shown to have a higher affinity for the resin than Zr (Fig. 3c). The speciation of group 4 elements in H2SO4 is more complicated because the successive formation reaction constants for sulfate complexes do not vary greatly [24]. Consequently, the cationic, neutral, and anionic species of Zr and Hf more readily coexist in this particular medium [22, 24]. In the range of approximately 2.0–6.0 M H2SO4, no uptake is observed with UTEVA for both Zr and Hf, evidence that Zr and Hf are not present in solution as neutral species. Between 6.0 and 8.0 M H2SO4, UTEVA begins to uptake Zr and Hf, evidence that neutral species are being formed. The highest k′ measured is 115 ± 10 for Hf in 12.1 M H2SO4; this corresponds to an uptake of only ~75 %. This could be confirmation that multiple species coexist in high H2SO4 concentrations. Above 15.0 M H2SO4, the results from the batch study showed UTEVA having no affinity for Zr and Hf; this could be an indication that Zr and Hf are not present in solution as neutral species. However, in the presence of a strong acid, the stationary phase (i.e., extractant) could separate from the inert support and then pass through the filter during the batch study [25]. Also, the extraction molecule, diamyl amylphosphonate, could degrade under these conditions. A color change from colorless to yellow was observed with the samples in this concentration range. Nevertheless, the highest separation factor, 2.0 ± 0.2, was measured in 10.0 and 12.1 M H2SO4, with Hf being preferentially extracted over Zr. These acid concentrations were not considered for column separations because Zr’s measured k′ (21 ± 2 and 57 ± 4, respectively) were too high.
The highest separation factor in an HCl medium, >9.4, was measured in 5.6 M HCl for the UTEVA resin. Various acid concentrations (4.6, 5.1, and 5.6 M HCl) were studied for column separation of Zr and Hf; at these acid concentrations it was expected (based on batch study results) that Zr would be retained by the column while Hf would elute. A k′ for both Zr and Hf of ≤1 was measured in 2.0 M HCl; this was the eluent concentration used to remove any remaining radionuclides from the column. The column study conditions with the best separation between Zr and Hf are shown in Fig. 4. The load and the subsequent six fractions were 5.6 M HCl; in these fractions 93 ± 2 % of the Hf eluted from the column while only ~8 % of the Zr was detected. As expected, Zr was eluted from the column using 2.0 M HCl. A mixed radionuclides column study using 88Zr and 175Hf confirmed these results (also shown in Fig. 4). These results show that group 4 elements can be separated using a UTEVA column in an HCl medium and might be applied to study Rf chemistry.
HCl elution profile for Zr and Hf using a 2 ml UTEVA resin cartridge with a flow rate of ~1 ml min−1. The first fraction is the load fraction. The solid-lined results are averages from the triplicate column studies performed with 89Zr and 175Hf, separately. The dotted-lined results are from the mixed radionuclides column study. Arrows indicate upper limits
The best separation factor using UTEVA in an HNO3 medium was measured in 2.6 M HNO3. The concentrations studied for UTEVA column separation of Zr and Hf were 1.0, 1.5, 2.0, and 2.6 M HNO3. 1.0 M HNO3 was used to elute any remaining radionuclides from the column; for both Zr and Hf the k′ measured was <0.8 under these conditions. All column study experiments using ≤2.0 M HNO3 as the eluent for the first six fractions had a majority of both Zr and Hf completely eluted within the first three fractions. There was no separation of Zr and Hf, and these systems are not suitable to study the chemistry of Rf. When 2.6 M HNO3 was used to separate Zr and Hf, again a majority of the Hf eluted within the first three fractions. However, only 11 ± 1 % of the Zr was detected in these fractions. The partial elution of Zr with 2.6 M HNO3 could be due to multiple species, cationic and neutral, coexisting in solution. In the subsequent three fractions of 2.6 M HNO3, Zr was not detected. Six fractions of 1.0 M HNO3 were used to elute any remaining radionuclides from the column; surprisingly, Zr was also not detected in any of these fractions. This could be evidence that the kinetics of going from a neutral to a cationic species in 1.0 M HNO3 do not occur on a timescale adequate for columns with a flow rate of 1 ml min−1. Also, it is possible that under these conditions Zr is hydrolyzing on the column. Consequently, column separations using UTEVA in a pure HNO3 medium do not show promise for studying Rf chemistry. Samples containing Rf cannot be counted before the chemical study, so the Rf must elute from the column in at least one of the two acid concentrations.
Conclusions
The separation of Rf homologs using TEVA and UTEVA resins in HCl, HNO3 and H2SO4 media has been investigated. Batch study results showed that TEVA did not have any affinity for Zr or Hf in HNO3 and H2SO4 media, and these systems are not suitable for studying intergroup trends of group 4 elements. However, intergroup separation was observed in an HCl medium using TEVA. The highest separation factor, 18 ± 8, was measured in 8.4 M HCl. The best TEVA column separation of Hf from Zr was observed using 7.8 M HCl as the eluent. Within the first six fractions, 90 ± 1 % of the Hf eluted while a majority of the Zr stayed on the column (>95 %). The tracer-level separation of Zr and Hf using a TEVA column in an HCl medium is possible, and with further development this system could be used for chemical characterization of Rf.
UTEVA in an HCl medium also showed promise for a future extraction chromatographic study of Rf. The highest separation factor, >9.4, was measured in 5.6 M HCl for the UTEVA resin. The best UTEVA column separation of Hf from Zr was observed using 5.6 M HCl as the eluent. Within the first six fractions, 93 ± 2 % of the Hf eluted while a majority of the Zr stayed on the column (~92 %). The UTEVA batch study in an HNO3 medium showed evidence of good intergroup separation. Separation factors of 9 ± 4 and 10 ± 1 were measured in 2.6 and 4.9 M HNO3, respectively. However, the UTEVA column study in HNO3 medium did not show promise for studying Rf chemistry, because Zr retained by the column could not be recovered. Lastly, UTEVA in an H2SO4 medium was the only system where Hf was shown to have a higher affinity for the resin than Zr. In 10.0 and 12.1 M H2SO4 a separation factor of 2.0 ± 0.2 was measured. This system did not show promise for extraction chromatographic studies of Rf. However, a batch uptake study of Rf should still be considered, because almost all of the systems that have been applied to Rf have had a preference for Zr. A system that shows preference to Hf, UTEVA in H2SO4, could bring new insight to the chemistry of Rf.
References
Pyykkö P, Desclaux JP (1979) Relativity and the periodic system of elements. Acc Chem Res 12:276–281
Günther R, Paulus W, Kratz JV, Seibert A, Thörle P, Zauner S, Brüchle W, Jäger E, Pershina V, Schädel M, Schausten B, Schumann D, Eichler Β, Gäggeler HW, Jost DT, Türler Α (1998) Chromatographic study of rutherfordium (element 104) in the system HCl/tributylphosphate (TBP). Radiochim Acta 80:121–128
Haba H, Tsukada K, Asai M, Goto S, Toyoshima A, Nishinaka I, Akiyam K, Hirata M, Ichikawa S, Nagame Y, Shoji Y, Shigekawa M, Koike T, Iwasaki M, Shinohara A, Kaneko T, Maruyama T, Ono S, Kudo H, Oura Y, Sueki K, Nakahara H, Sakama M, Yokoyama A, Kratz JV, Schädel M, Brüchle W (2002) Anion-exchange behavior of Rf in HCl and HNO3 solutions. J Nucl Radiochem Sci 3:143–146
Haba H, Tsukada K, Asai M, Toyoshima A, Ishii Y, Toume H, Sato T, Nishinaka I, Ichikawa T, Ichikawa S, Nagame Y, Sato W, Matsuo K, Kitamoto Y, Tashiro Y, Shinohara A, Saito J, Ito M, Ikezawa T, Sakamaki M, Goto S, Kudo H, Kikunaga H, Arai M, Kamataki S, Yokoyama A, Akiyama K, Sueki K, Oura Y, Schädel M, Brüchle W, Kratz JV (2007) Extraction behavior of rutherfordium into tributylphosphate from hydrochloric acid. Radiochim Acta 95:1–6
Toyoshima A, Kasamatsu Y, Tsukada K, Asai M, Ishii Y, Toume H, Nishinaka I, Sato TK, Nagame Y, Schädel M, Haba H, Goto S, Kudo H, Akiyama K, Oura Y, Ooe K, Shinohara A, Sueki K, Kratz JV (2010) Extraction chromatographic behavior of Rf, Zr, and Hf in HCl solution with styrene-divinylbenzene copolymer resin modified by TOPO (trioctylphosphine oxide). J Nucl Radiochem Sci 11:7–11
Kratz JV (2011) Aqueous-phase chemistry of the transactinides. Radiochim Acta 99:447–502
Czerwinski ΚR, Gregorich ΚΕ, Hannink ΝJ, Kacher CD, Kadkhodayan BA, Kreek SA, Lee DM, Nurmia MJ, Türler A, Seaborg GT, Hoffman DC (1994) Solution chemistry of element 104: part I. Liquid–liquid extractions with triisooctylamine. Radiochim Acta 64:23–28
Czerwinski ΚR, Kacher CD, Gregorich ΚE, Hamilton TM, Hannink ΝJ, Kadkhodayan ΒA, Kreek SA, Lee DM, Nurmia MJ, Türler A, Seaborg GT, Hoffman DC (1994) Solution chemistry of element 104: part II. Liquid–liquid extractions with tributylphosphate. Radiochim Acta 64:29–35
Sylwester ER, Gregorich KE, Lee DM, Kadkhodayan B, Türler A, Adams JL, Kacher CD, Lane MR, Laue C, McGrath CA, Shaughnessy DA, Strellis DA, Wilk PA, Hoffman DC (2000) On-line gas chromatographic studies of Rf, Zr, and Hf bromides. Radiochim Acta 88:837–843
Murakami M, Goto S, Murayama H, Kojima T, Kudo H, Kaji D, Morimoto K, Haba H, Kudou Y, Sumita T, Sakai R, Yoneda A, Morita K, Kasamatsu Y, Kikunaga H, Sato TK (2013) Excitation functions for production of Rf isotopes in the 248Cm + 18O reaction. Phys Rev C 88(024618):1–8
Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Maxwell SL, Nelson MR (1995) Separation and preconcentration of actinides by extraction chromatography using a supported liquid anion exchanger: application to the characterization of high-level nuclear waste solutions. Anal Chim Acta 310:63–78
Hulet EK, Lougheed RW, Wild JF, Landrum JH, Nitschke JM, Ghiorso A (1980) Chloride complexation of element 104. J Inorg Nucl Chem 42:79–82
Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Essling AM, Graczyk D (1992) Separation and preconcentration of uranium from acidic media by extraction chromatography. Anal Chim Acta 266:25–37
Manchanda VK, Pathak PN, Mohapatra PK (2010). In: Moyer BA (ed) Ion exchange and solvent extraction: a series of advances vol 19, p 73
Pershina V, Trubert D, Naour CL, Kratz JV (2002) Theoretical predictions of hydrolysis and complex formation of group-4 elements Zr, Hf and Rf in HF and HCl solutions. Radiochim Acta 90:869–877
Kraus KA, Nelson F (1956) Anion-exchange studies of the fission products. In: Proceedings of the international conference on the peaceful uses of atomic energy, 1955. United Nations Publishers, New York, pp 113–125
Bennett ME, Mayorov DA, Chapkin KD, Alfonso MC, Werke TA, Folden CM III (2012) Measurement of the natLu(p, x)175Hf excitation function. Nucl Instrum Methods Phys Res B 276:62–65
Horwitz EP, Chiarizia R, Dietz ML (1992) A novel strontium-selective extraction chromatographic resin. Solvent Extr Ion Exch 10:313–336
Storm DJ, Stansbury PS (1992) Minimum detectable activity when background is counted longer than the sample. J Health Phys 63:360–361
Kim JI, Lagally H, Born HJ (1973) Ion exchange in aqueous and in aqueous-organic solvents: part I. Anion-exchange behaviour of Zr, Nb, Ta and Pa in Aqueous HCl-HF and in HCl-HF-organic solvent. Anal Chim Acta 64:29–43
Caletka R, Hausbeck R, Krivan V (1990) Anion-exchange behavior of 12 elements in H2SO4 and HF-H2SO4 medium. J Radioanal Nucl Chem 142:383–391
Guseva LI, Tikhomirova GS (2002) Ion-exchange procedure developed for isolation of element 106 (seaborgium) and study of its chemistry in H2SO4 and H2SO4/HF solutions, with W as imitator. Radiochemistry 44:337–341
Omtvedt JP, Alstad J, Breivik H, Dyve JE, Eberhardt K, Folden CM III, Ginter T, Gregorich KE, Hult EA, Johansson M, Kirbach UW, Lee DM, Mendel M, Nähler A, Ninov V, Omtvedt LA, Patin JB, Skarnemark G, Stavsetra L, Sudowe R, Wiehl N, Wierczinski B, Wilk AP, Zielinski PM, Kratz JV, Trautmann N, Nitsche H, Hoffman DC (2002) SISAK liquid-liquid extraction experiments with preseparated 257Rf. J Nucl Radiochem Sci 3:121–124
Connick RE, McVey WH (1949) The aqueous chemistry of zirconium. J Am Chem Soc 71:3182–3191
Siekierski S (1975) In: Braun T, Ghersini G (eds) Extraction chromatography. Elsevier, New York, pp 77–79
Acknowledgments
The authors would like to thank J. D. Despotopulos, K. J. Moody and E. E. Tereshatov for their informative discussions on this work. The authors would also like to thank the heavy element group at LLNL for providing the 175Hf. This work was supported by the Robert A. Welch Foundation under grant number A-1710.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alfonso, M.C., Bennett, M.E. & Folden, C.M. Extraction chromatography of the Rf homologs, Zr and Hf, using TEVA and UTEVA resins in HCl, HNO3, and H2SO4 media. J Radioanal Nucl Chem 307, 1529–1536 (2016). https://doi.org/10.1007/s10967-015-4256-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4256-5