Abstract
A crown-ether based extraction chromatography resin, Eichrom Pb, has been characterized for separations of the flerovium (Fl) pseudo-homolog Hg. Previous results on the batch uptake of Pb(II) and Sn(IV) are compared with newly investigated Hg(II) in HCl matrices. It was determined that all three elements can be strongly retained by the resin at different HCl concentrations. To assess the feasibility for performing an experiment on the chemical properties of Fl using this resin the extraction kinetics were studied. A separation method for the isolation of the homologs Pb(II) and Sn(IV) and pseudo-homolog Hg(II) has been established using 2 mL pre-packed Pb resin columns under vacuum flow.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Work presented here is a continuation of the work in Ref. [1], “Characterization of the homologs of flerovium with crown ether based extraction chromatography resins: studies in hydrochloric acid.” As mentioned in [1] some predictions indicate that Fl, element 114, has a volatility and inertness greater than the other group 14 elements, which might lead to a potential chemical behavior more like Hg or a noble gas like behavior, which leads to Hg being a pseudo-homolog of Fl [2,3,4,5]. It was therefore necessary to see if the Eichrom Pb resin showed any affinity for Hg, following its strong affinity for Pb and Sn demonstrated in Ref. [1]. If it does, then it implies that Eichrom Pb resin could form the basis of a chemical extraction system for the isolation and study of Fl in aqueous solution.
While no reports exist that show the Eichrom Pb resin extractant (4′,4″(5″)-di-tert-butyldicyclohexano-18-crown-6 (DtBuC18C6)) having an affinity for Hg, there have been reports of similar 18-crown-6 extractants complexing with Hg [6]. Therefore, it was assumed that the Eichom Pb resin should be capable of extracting Hg, and potentially separating Pb, Hg, and Sn, the homologs and pseudo-homologs of Fl, using column chromatography methods.
As in [1], batch experiments were performed to determine the extraction behavior [7], and compare that to the extraction behavior previously reported [1] of Pb and Sn in their most stable oxidation states (+ 2 and + 4, respectively [8,9,10]) from an HCl matrix. The extraction kinetics for Hg(II), and a column separation of Pb(II), Hg(II), and Sn(IV), were established and the results are compared to those presented in [1]. The primary focus of this work was to determine if it was possible to use the Pb resin as a method for characterizing both the homologs and pseudo-homologs of Fl together from a simple HCl system. The results are used to comment on the potential design of a future Fl experiment.
Experimental
Reagents and materials
As with the previous publication [1], the Pb resin (50–100 µm, 40% w:w, Eichrom Industries, Inc.) was used for batch, kinetic, and column studies [11]. Acids were prepared from trace-metal grade acids and de-ionized water (18 MΩ cm). Tracer solutions of 212Pb, 113Sn and 197mHg were prepared with activity concentrations ranging from 2 to 10 cps per 20 µL. The 113Sn and 197mHg were produced and isolated carrier-free as described in Ref. [12]. The 232U (legacy material, Lawrence Livermore National Laboratory (LLNL)) decay chain was used to obtain 212Pb as a tracer for all studies by milking the 212Pb from a generator [13].
Activity measurements
Activity measurements were determined similarly to [1] using the same high purity germanium (HPGe) detector. Spectral lines with the highest relative yields were chosen for determining the activity of each radionuclide and analyzed with Maestro spectral hardware: 212Pb (t1/2 = 10.64 h), 286.6 keV (43.3%); 113Sn (t1/2 = 115.1 d), 391.6 keV (64.0%); and 197mHg (t1/2 = 23.8 h), 133.98 keV (33.5%) [14]. Since the activity of 113Sn is derived from its 113In daughter, post-extraction counting for Sn was performed 24 h after conclusion of the experiment to allow the In to reach secular equilibrium, as described in Ref. [1].
Batch uptake experiments
Batch extraction experiments were used to determine uptake parameters for Hg(II) on Pb resin in HCl solutions. The experiments were performed identically to the batch uptake experiments described in Ref. [1]: to a 1.5 mL centrifuge tube, 10–20 mg Pb resin were added along with 1 mL of HCl ranging in concentration from 0.001 M to concentrated; preconditioning of the resin was achieved by placing the vials on a rotary mixer for 1 h. The only difference is that the 20 µL spike contained only 197mHg in 2.0 M HCl. Batch extraction experiments were performed in triplicate and reported errors are based on the standard deviation of the replicates. The Pb resin capacity factor (k′) was determined from the measured distribution ratios as described in references [11, 15].
Uptake kinetics
The uptake kinetics for Hg(II) were determined using the procedure from [1]. The HCl concentration at which strong uptake occurs in the batch experiment (0.4 M HCl for 197mHg(II)) was chosen as the concentration for kinetics studies. The concentration for strongest uptake was not chosen for Hg(II) because the extremely high k′ values of > 5000 would have yielded poor counting statistics in the aqueous phase. Standards were prepared by placing 1 mL of 0.4 M HCl in a 1.5 mL centrifuge tube and adding a 100 µL spike of a stock 197mHg solution (also in 0.4 M HCl). Standards were counted for 300 s with an HPGe detector, and made in triplicate. Samples were preconditioned by adding 1 mL of 0.4 M HCl to a 1.5 mL centrifuge tube along with 10–20 mg Pb resin and mixing for 1 h. A 100 µL spike of the stock 197mHg solution was added to the samples, and each sample was mixed for the desired time interval before filtering to remove the resin from the solution. To maintain the original counting geometry, a 700 µL spike of each filtered solution was added to 400 µL de-ionized water. The samples were counted with the same HPGe detector as the standards for 300 s–2 h (depending on activity). All samples were done in triplicate.
Column experiments
Column extraction of mixed 212Pb(II), 113Sn(IV) and 197mHg(II) was performed with pre-packed, 2 mL cartridges containing dry Pb resin in the same manner as described in [1]. Load solutions were prepared by taking aliquots of each tracer and evaporating them to dryness before reconstituting in 1 mL of the appropriate HCl solution. The initial sample activity was determined via HPGe counting. A vacuum box (Eichrom, Darien, IL, USA) was used for the extraction with a flow rate of 2 mL/min. The resin was conditioned prior to loading with 20 mL of the desired HCl of the same concentration as the load solution. Extractions were performed with HCl concentrations based on results from the batch experiments. Two column experiments were performed. For the initial experiment, the radionuclides were loaded on the column in 3 M HCl, where all three were expected to be retained, and 113Sn was first eluted with 0.4 M HCl, then 212Pb was eluted with 8 M HCl and finally 197mHg was eluted with concentrated HCl. Three rinse fractions at 3 M HCl were collected followed by 1 mL elution fractions (× 8 for 113Sn, × 9 for 212Pb, and × 16 for 197mHg) of the desired HCl concentration. Column flow was stopped just as liquid was about to reach the top-frit so the column never ran dry and each elution fraction was maintained at 1 mL. Fractions were counted by HPGe-spectrometry.
Due to extremely slow sorption and desorption kinetics for Hg(II), and the fact that Hg(II) was observed to bleed through the column and elute with poor peak resolution (discussed below), a second column experiment was performed. The load solution was changed to 0.4 M HCl (closer to peak extraction of Hg(II)) and Sn(IV) was allowed to pass through the column without interaction. The column was then capped for 1 h after the elution of Sn(IV) to allow Hg(II) to be fully retained. The column was capped again for 1 h after changing to concentrated HCl to assist with eluting Hg(II) in a tight elution peak. The remaining parameters for this column were kept the same as in the first experiment with 113Sn(IV) eluted with 0.4 M HCl (× 7 fractions), 212Pb(II) eluted with 8 M HCl (× 9 fractions) and 197mHg eluted with concentrated HCl (× 12 fractions).
Results and discussion
Batch experiments
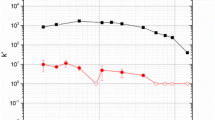
From previous results [1] the uptake of Sn(IV) and Pb(II) as a function of HCl concentration were measured for the Pb resin; Fig. 1 shows the effects of HCl concentration on the uptake of Hg(II) with the results for Sn(IV) and Pb(II) presented (dashed lines [1]).
The batch uptake (k′) of 197mHg(II) as a function of HCl media on Eichrom Pb resin (50–100 µm) with a 3 h equilibration time. Errors are from the standard deviation of replicates. Results are included for 212Pb(II) and 113Sn(IV) as previously described in [1]
Despite forming similar complexes in HCl as Pb(II), Hg(II) exhibits much stronger extraction over the same concentration ranges. Experiments reported in the literature conducted with the simpler dicyclohexano-18-crown-6 (DC18C6) as well as dibenzo-18-crown-6 (DB18C6) and the un-substituted 18-crown-6 (18C6) indicated that the following extraction mechanism is dominant for mercury [6]:
where CE = 18C6, DC18C6 or DB18C6. This indicates that the planar HgCl2 molecule is extracted between two crown ether molecules. While this previous study was conducted with Hg concentrations of 10−5 M instead of the carrier-free mercury used in this study, it does indicate a more complex extraction for Hg(II) than the traditional cavity based extraction exhibited by Pb(II). Decreasing extractability of these crowns, which was reported as 18C6 > DC18C6 > DB18C6, was attributed to the cavity sizes, which are 2.6 to 3.2 Å for 18C6 and DC18C6 [16] but increase to 4 Å for DB18C6 [17], and the decreasing basicity of the crowns over that same sequence. Mercury(II) has an ionic radius of 1.1 Å, which is much closer to the cavity diameter of 18C6 and DC18C6, and therefore receives more stabilization from those cavities than the larger DB18C6 cavity [6]. The observed slight decrease in extraction with DC18C6 compared to 18C6 was attributed to increased steric hindrance lessening the effect of cavity stabilization [6]. Therefore, Hg most likely extracts as HgCl2·2DtBuC18C6, and the decrease in mercury extraction at higher HCl concentrations may be attributed to the formation of ionic HgCl3− and HgCl42− [6].
Kinetics experiments
The data obtained from the batch studies indicate that Hg(II), Pb(II), and Sn(IV) can be separated from each other using a pure HCl matrix with the Pb resin at an equilibration time of 3 h. Due to the short-lived isotopes of Fl and the goal of an on-line chemical separation, flow rates of mL min−1 will be required. Therefore, the kinetics of extraction must be suitable for such flow rates. The kinetics of Pb(II) and Sn(IV) were previously explored (see Ref. [1]) and indicated suitable uptake for a second time scale separation. The kinetics of uptake for Hg(II) on the Pb resin at 0.4 M HCl (a point near the maximum extraction but not at maximum extraction), were investigated (Fig. 2). Sorption of Hg(II) was the slowest with full equilibrium not reached until greater than 8 h and very little sorption observed until at least 1 h had passed. Potential reasons for the slow equilibration of Hg(II) can be attributed to the extraction mechanism discussed above. Due to more complex multiple ligand extraction and the fact that the crown ether cavity is still believed to stabilize the extracted mercury species [6], the overall rate of this extraction is very slow on the resin based system. This is potentially made even slower due to the requirement of two crown ethers required to be in the correct orientation, which might be less favorable due to the rigid resin backbone.
Column experiments
Column experiments were used to determine if a stepwise extraction of Pb(II), Sn(IV) and Hg(II) could be achieved by varying only the HCl concentration. Based on the batch study results, the load solution for an initial column study of 3 M HCl was chosen due to the fact that Pb(II), Sn(IV) and Hg(II) should all be retained by the Pb resin at this concentration. The goal was to elute Sn(IV) with 0.4 M HCl, then Pb(II) with 8 M HCl and finally Hg(II) with concentrated HCl, Fig. 3.
The initial column elution presented in Fig. 3 shows some bleeding of Hg(II) throughout the Sn(IV) and Pb(II) fractions before it is eluted in concentrated HCl, presumably due to the slow kinetics. The reason for the double elution peaks during the concentrated HCl fractions is due to capping the column for 1 h between each elution peak once it was realized that the slow desorption kinetics would make a quick elution of Hg(II) impossible. Results indicated that 3 M HCl was too high a concentration for a load solution due to the slow sorption kinetics of Hg(II); therefore, a second column experiment was designed with a load solution of 0.4 M HCl (thus having Sn(IV) pass through the column), which would be closer to the maximum extraction point for Hg(II). An additional change for this second column was to cap the column for 1 h after elution of Sn(IV) to ensure Hg(II) was retained and to cap the column after the first concentrated HCl fraction was added to aid in desorption of Hg(II) in a tight band, Fig. 4.
The change of load solution to 0.4 M HCl and the hour gaps between loading and eluting of Hg enabled a clean separation of Pb(II) from Sn(IV) and Hg(II). The Hg(II) elution peak was still slightly broad but did elute as expected. In both cases, Figs. 3 and 4, the Pb(II) and Sn(IV) behaved as they did in the initial Sn(IV) and Pb(II) separations from Ref. [1]. While it was observed that Pb(II) and Sn(IV) can be separated on the second time scale required for a Fl experiment, the addition of the pseudo-homolog Hg(II) made the separation impossible to perform on these short time scales. It would be possible to determine whether Fl was more chemically similar to Pb or Sn like (or neither); however, a definitive Hg-like conclusion as to its behavior would be nearly impossible with the current system due to the slow kinetics of Hg sorption and desorption.
Conclusions
The extraction behavior of Hg(II) from HCl media was studied using Eichrom Pb resin, which contains the 4′,4″(5″)-di-tert-butyldicyclohexano-18-crown-6 extractant, and was compared to that of previously reported data for Pb(II) and Sn(IV), Ref. [1]. The batch results show Hg(II) extracts at low HCl concentrations, from 0.04 to 2 M. Mercury is most likely extracted as HgCl2 between two crown cavities.
Results showed that the reaction kinetics for Hg(II) were much slower than that of Pb(II) and Sn(IV), with strong uptake not observed until at least 1 h had passed and full equilibrium occurring at greater than 8 h, compared with minutes to 1 h for Pb(II) and Sn(IV), respectively. As a result, the three elements could not be separated from one another on the second time scale using the system based on Eichrom Pb resin.
Column studies aimed at separating the homologs Pb(II) and Sn(IV) as well as the pseudo-homolog Hg(II) on the second time scale from a pure HCl matrix were also performed. Results indicated that with large equilibration times between column loading and elution of Hg(II), clean elution fractions could be obtained. However, when the column was run with ~ 2 mL/min flow rates, Hg(II) bled through the column when the flow was not halted to allow for it to fully adsorb and desorb. As a result, while it would be possible to discern if Fl was Pb or Sn like, the use of Eichrom’s Pb resin would yield inconclusive data as to whether Fl is Hg like.
It is possible with more work that at very low HCl concentrations Hg(II) may be retained by the column at large flow rates. In this case an experiment with a load solution of 0.001 M HCl would retain both Pb(II) and Hg(II) while Sn(IV) passes through. Under these conditions an observed Fl atom could be confirmed to be Sn(IV) like. Due to the extremely high Hg(II) extraction at this low of a concentration, changing to 8 M HCl to elute Pb(II), during which a Fl atom could be considered Pb(II) like, might be possible while retaining Hg(II) like atoms on the column. Experiments were performed loading the column at 0.4 M HCl and waiting 1 h before changing to 8 M HCl to elute Pb(II). Without this 1 h wait it was observed that approximately 20–30% of the Hg(II) bled off the column. While the extraction is stronger at 0.001 M as seen in Fig. 4, it would be expected that due to the slow kinetics at least some Hg(II) would bleed off the column in the Pb(II) fraction. Therefore, while these conditions would enable Sn(IV) like character to be elucidated from Pb(II) and Hg(II) it would still be difficult to discern if there is any true Hg(II) like character in Fl.
References
Despotopulos JD, Kmak KN, Gharibyan N, Henderson RA, Moody KJ, Shaughnessy DA, Sudowe R (2016) Characterization of the homologs of flerovium with crown ether based extraction chromatography resins: studies in hydrochloric acid. J Radioanal Nucl Chem 310:1201–1207
Pitzer KS (1975) Are elements 112, 114, and 118 relatively inert gases? J Chem Phys 63:1032–1033
Nash CS (2005) Atomic and molecular properties of elements 112, 114 and 118. J Phys Chem A 109:3493–3500
Liu W, van Wüllen C, Han YK, Choi YJ, Lee YS (2001) Spectroscopic constants of Pb and Eka-lead compounds: comparison of different approaches. Adv Quant Chem 39:325–355
Hoffman DC, Lee DM, Pershina V (2006) In: Vertes A, Klencsar Z (eds) The chemistry of the actinide and transactinide elements. Springer, Dordrecht, pp 1652–1752
Francis T (2002) Liquid-liquid extraction and separation of mercury from industrial wastes. (Doctoral dissertation). Regional Research Laboratory (CSIR), India
Roesmer J, Kruger P (1960) The radiochemistry of mercury. National Academy of Sciences-National Research Council, Washington D.C
Seth M, Faegri K, Schwerdtfeger P (1998) The stability of the oxidation state +4 in group 14 compounds from carbon to element 114. Angew Chem Int Ed Engl 37:2493–2496
Nervik WE (1960) The radiochemistry of tin. Subcommittee on radiochemistry. National Acadamy of Sciences-National Research Council, Washington D.C
Gibson WM (1961) The radiochemistry of lead. National Academy of Sciences-National Research Council, Washington D.C
Horwitz EP, Dietz ML, Rhoads S, Felinto C, Gale NH, Houghton J (1994) A lead-selective extraction chromatographic resin and its application to the isolation of lead from geological samples. Anal Chim Acta 292:263–273
Despotopulos JD, Kmak KN, Gharibyan N, Brown TA, Grant PM, Henderson RA, Moody KJ, Tumey SJ, Shaughnessy DA, Sudowe R (2015) Production and isolation of homologs of flerovium and element 115 at the Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry. J Radioanal Nucl Chem 308:567–572
Despotopulos JD (2015) Studies of flerovium and element 115 homologs with macrocyclic extractants (Doctoral dissertation). ProQuest Ann Arbor 3715057:224
“National Nuclear Data Center “NNDC” (2013) Brookhaven National Laboratory, [Online]. http://www.nndc.bnl.gov. Accessed 16 Oct 2016
Horwitz EP, Bloomquist CA (1972) Preparation, performance, and factors affecting band spreading of high-efficiency extraction chromatographic columns for actinide separations. J Inorg Nucl Chem 34:3851–3871
Pedersen CJ (1970) Crystalline salt complexes of macrocyclic polyethers. J Am Chem Soc 92:386–391
Pedersen CJ (1967) Cyclic polyethers and their complexes with metal salts. J Am Chem Soc 89:7017–7036
Acknowledgements
The authors would like to thank CAMS facility staff at LLNL, specifically Scott Tumey, Thomas Brown and Graham Bench for providing beam time and expertise to the production of radionuclides used in this study. This study was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. This work was funded by the Laboratory Directed Research and Development Program at LLNL under project tracking code 11-ERD-011 and 17-LW-035, as well as by the LLNL Livermore Graduate Scholar Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Despotopulos, J.D., Kmak, K.N., Gharibyan, N. et al. Studies of the homologs and pseudo-homologs of flerovium with crown ether based extraction chromatography resins. J Radioanal Nucl Chem 318, 1821–1826 (2018). https://doi.org/10.1007/s10967-018-6207-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6207-4