Abstract
149Gd was produced from the 12C induced reaction on natural praseodymium target. No-carrier-added (nca) 149Gd was separated from the bulk target matrix by liquid–liquid extraction (LLX) using cation exchanger di-(2-ethylhexyl)phosphoric acid (HDEHP) dissolved in cyclohexane. High separation factor of 2,450 was achieved at the optimal experimental condition when 1% HDEHP and 0.1 M HCl were used as organic and aqueous phases respectively. The result was also compared with the previous reports.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The applications of lanthanide radionuclides are limited compared to that of other elements in the Periodic Table. One of the reasons of this fact may be due to the non-availability of suitable radionuclides of lanthanide elements in the no-carrier-added (nca) form. Neutron activation leads to radionuclides of low specific activity. Light ions, like α or p, activation on lanthanide elements leads to nca radionuclides but the separation becomes a colossal task due to the similar physicochemical properties of the adjacent lanthanides, especially when the nca radionuclide is in ultra-trace amount and bulk target is of milligram quantity. To get rid of this painstaking problem in separating nca radiolanthanides, for the first time, Lahiri et al. [1] proposed heavy ion activation, which essentially introduced large difference in atomic number between the product and the target elements, and in turn the separation of nca radiolanthanide from the bulk target became easier. Since then a large number of publications came out on the heavy ion assisted production of nca radionuclides including the radiolanthanides from the same group in a continuous endeavor.
153Gd radionuclide has found applications in the diagnostic nuclear medicine including scanning of lumbar vertebrae and femoral neck. The 153Gd–DTPA tracers act as potential MRI contrast agent. 153Gd is a candidate radionuclide for the detection of brain tumour and in scanning bone marrow without any toxicity. The uptake of Gd–DTPA in acute myocardial infarction may be a marker of acute myocardial necrosis [2, 3]. However, 153Gd has long half-life of 241.6 days, which is certainly a disadvantage for clinical applications. In this circumstance, comparatively short-lived 149Gd (9.4 days), having intense γ-rays, may act as a potential alternative of 153Gd. Production of nca 149Gd and its purification from the target matrix is therefore important for in vivo applications. The present report aims for the high purity nca 149Gd from natural praseodymium target by 12C activation.
The literature on the separation of Gd from other lanthanides, either as nca product or in trace scale is not too rich. There are very few reports on such separations [4]. Earlier the extraction and extraction chromatographic behaviors of 153Gd along with Sm, Eu and Tb in the di-(2-ethylhexyl)phosphoric acid (HDEHP)–decane–HNO3 system were studied by Melnik et al. [5]. They obtained purification factor for gadolinium of about 100 with respect to Sm and Tb impurities and about 2 with respect to Eu impurity. Nayak and Lahiri [6] studied liquid–liquid extraction (LLX) of trace level cerium and gadolinium (141Ce and 153Gd) from HCl and HNO3 media with liquid cation exchanger, HDEHP. A quantitative separation of the elements from an admixture of the two radioisotopes was achieved with 0.1% HDEHP and 0.01 M HCl solution. Later on authors separated nca gadolinium isotopes 147,149Gd from 80 MeV 12C activated natCeO2 target. They achieved separation of nca Gd (with 55% yield) by 1% HDEHP dissolved in cyclohexane from 10−4 M HCl solution [7]. Earlier, the same group had demonstrated the production of 147,149Gd and 147Eu in 70 MeV 11B irradiated praseodymium foil target [8]. The nca gadolinium and europium radionuclides were separated from bulk target by LLX using HDEHP. In this paper, we describe an alternative approach for the production of nca Gd by irradiating praseodymium foil with 12C beam and compared the results with our earlier reports.
Experimental
Production of nca Gd
A natural praseodymium (141Pr) foil of 99.9% purity, procured from Alfa Aesar, was used to prepare a self supporting metal target of 15 mg/cm2 thickness by proper rolling. The natPr target was irradiated by 71.5 MeV 12C6+ ions at the BARC-TIFR Pelletron facility, Mumbai, India, for 560 min. At the end of bombardment (EOB), a total charge of 1,428 μC was measured by an electron suppressed Faraday cup placed at the rear of the target. An aluminum catcher foil of 3 mg/cm2 was used to stop the recoiled evaporation residues, if any, in the beam direction.
In order to monitor the fate of the bulk praseodymium in the radiochemical separation, it is important to produce praseodymium radionuclides, which are hardly produced in the 12C + natPr reaction. Therefore, a separate natPr foil was irradiated in the neutron flux of 5.0 × 1012 n cm−2 s−1 for 4 h in the CIRUS reactor, BARC, Mumbai, India, to produce 142Pr (19.12 h).
At the EOB, target foils were assayed by off line γ-spectrometry using an n-type HPGe detector (CANBERRA) coupled with a PC based multi-channel analyzer, PCA2 (OXFORD), to obtain the activity of the product radionuclides. The detector had energy resolution of 2.13 keV at 1,332 keV. Efficiency calibration of the detector was performed using standard sources, 152Eu (13.506 years), 133Ba (10.54 years) and 137Cs (30.0 years) of known activity. Nuclear spectroscopic data of the radionuclides discussed in this report are tabulated in Table 1 [9].
The γ-spectrometric analysis of irradiated target assured the production of nca 149Tb and 149Gd radionuclides in the target matrix. 149Gd was produced via 141Pr(12C,p3n)149Gd and 141Pr(12C,4n)149Tb(EC)149Gd reactions. After the complete decay of short-lived 149Tb (4.118 h), nca 149Gd was separated from the bulk praseodymium target.
Radiochemical separation
The 12C irradiated natPr foil and neutron irradiated natPr foil, were separately dissolved in the minimum volume of 0.1 M HCl. The 12C irradiated target solution was spiked with 142Pr tracer solution and evaporated to dryness in order to attain the same chemical form between radioactive 142Pr tracer and bulk Pr. The residue was then re-dissolved into 0.01 M HCl to prepare active uniform mixture of nca product and target matrix.
Separation of nca Gd from bulk praseodymium was approached by LLX. Series of LLX experiments were carried out using the cation extracting agent HDEHP (1% v/v) dissolved in cyclohexane as organic phase and dilute HCl as aqueous phase. In each extraction, 3 mL of organic solution was shaken for 10 min in a mechanical shaker with equal volume of aqueous HCl and 100 μL of activity. Then liquid mixture was left for the complete phase separation. In order to determine the distribution of activities, 2 mL of aliquot was collected from each phase for γ-spectrometric study. In an extraction series, concentration of HCl was varied from 10−5 to 1 M keeping the HDEHP concentration fixed to 1% (v/v) and in the next series, the concentration of HDEHP was varied with respect to fixed 0.1 M HCl. In the third set of extraction, concentration of HDEHP was varied at a fixed 0.1 M HCl in presence of H2O2. Nca Gd radionuclides were back extracted to the aqueous phase from HDEHP using both 6 M HCl and 0.1 M DTPA in 1 M NaOH separately. All the chemicals used for the chemical separation were of analytical grade.
Results and discussion
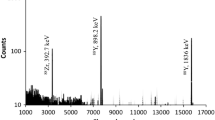
The 12C activated natural praseodymium target was assayed at different time intervals by γ-spectrometry. Analysis of the γ-spectra confirms the production of 149Tb, 150Tb, 151Tb and 149Gd in the Pr target matrix. The batch yield for 149Tb, 150Tb, 151Tb and 149Gd were found to be 86, 37, 7 and 70 kBq/μA h at EOB, respectively. The amount of nca 149Gd produced in the target matrix is comparable to that of nca 149Tb at EOB. Probably, the production route of nca Gd not only includes it’s decay through 141Pr(12C,4n)149Tb(EC/β+)149Gd but also includes the possibility of direct production through 141Pr(12C,p3n)149Gd reaction. However, theoretical calculation using Monte Carlo statistical simulation code, PACE II [10] shows very low cross section for the direct production of 149Gd (Fig. 1). 149Tb has two decay modes, EC (83.3%) and α (17.7%). The irradiation time in the present experiment was long (9.3 h), which is more than two half-lives of 149Tb (4.118 h), and consequently increased the amount of nca 149Gd (9.28 days) at EOB via electron capture. Another important observation is that 149Tb is the low spin (1/2+) state, and the high initial angular momentum involved in the reaction of 71.5 MeV 12C + 141Pr will result in preferential population of the high spin (11/2−) isomer (149mTb). The EC decay of this isomer to 149Gd is responsible for the high yield of the latter as observed in the present experiment. The γ-spectrum of the 12C activated natural praseodymium foil collected after 1.2 h of EOB is presented in Fig. 2. It is interesting to note that contrary to the PACE-II prediction, no evidence of the production of 145,146Eu was observed in the present work.
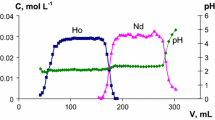
The radiochemical separations were started 2 days after the EOB ensuring complete decay of 149Tb. Figure 3 represents the extraction behavior of nca Gd and bulk Pr as a function of concentration of HCl at a fixed 1% (v/v) HDEHP dissolved in cyclohexane. At the low concentration of HCl, from 10−5 to 10−2 M region, nca Gd was extracted to the HDEHP phase along with bulk Pr. The high extractability of lanthanides may be attributed to the formation of cationic aquo-complex like, [Ln(H2O) x ]3+ [11] at the lower acidity and extracted to the liquid cation exchanger HDEHP. A sharp change in the extraction behavior was observed at 0.1 M HCl, where nca Gd was extracted ~79% to the organic phase leaving the bulk praseodymium in the aqueous phase. After 0.1 M HCl concentration, the extractions of both bulk Pr and nca Gd sharply decreased. At 0.1 M HCl and 1% HDEHP concentration, about 4% bulk Pr was extracted to the organic phase with the nca products. In order to improve the separation efficiency, a second series of extraction was carried out by varying the concentration of HDEHP with fixed 0.1 M HCl. The highest separation factor was observed as before, i.e. with 1% HDEHP and 0.1 M HCl (Fig. 4). The extent of separation was further dramatically improved with addition of H2O2 to the aqueous phase (Fig. 5). The maximum separation was achieved at 1% HDEHP and 0.1 M HCl in presence of H2O2, where about 80% nca 149Gd was extracted to the organic phase leaving the bulk praseodymium quantitatively in the aqueous phase. A high separation factor (D Gd/Pr) of 2.45 × 103 was achieved in this optimum condition. The addition of H2O2 increased the separation factor probably due to the fact that H2O2 helped Pr to obtain Pr4+, which eventually increased the difference of ionic radii between Pr4+ and Gd3+ and as a consequence the difference between the extraction behaviors of these two elements increased. Nca 149Gd was back extracted from the organic phase using 6 M HCl and 0.1 M DTPA in 1 M NaOH. The distribution coefficients and separation factors at the optimal conditions have been tabulated in Table 2. A schematic of the above separation is presented in Fig. 6. The extraction patterns of nca Gd and bulk matrix are quite confirmative with our earlier publications [7, 8]. It is noteworthy to mention that addition of H2O2 improved D Gd values and separation factor between nca Gd and bulk Pr compared to earlier works. A comparison of D Gd and separation factor values with earlier experiments have been tabulated in Table 3. The contamination from 150Gd (1.8 Myears) will be infinitesimally small due to very long half-life. In principle, presence of 151Gd (120 days) is expected from the decay product of its precursor 151Tb. However, no signature of 151Gd was observed during radiochemical separation due to the low yield of 151Tb and comparatively longer half-life of 151Gd. A finer adjustment in the target thickness may also reduce the production of impurity during irradiation.
Conclusion
Application of short-lived radionuclides requires reliable and faster method for the separation of desired lanthanide with high purity from the corresponding target matrix. The reported separation technique, which describes the separation of nca Gd from the bulk praseodymium target matrix by LLX using HDEHP requires about 80 min time. The indirect production of nca 149Gd is advantageous compared to earlier methods because of the following reasons (i) there are no co-produced nca radionuclides of other elements, therefore, high radiochemical purity for nca 149Gd is achievable (e.g. reference [8], where 147Eu was co-produced with nca Gd radionuclides) (ii) the present method also yields high radionuclidic purity for 149Gd (e.g. reference [8], where both 147Gd and 149Gd were produced) (iii) due to the absence of any other co-produced radiolanthanide, the separation scheme became easier. The reported method is simple, fast and reliable.
References
Lahiri S, Nayak D, Das SK, Ramaswami A, Manohor SB, Das NR (1999) Separation of carrier free dysprosium and terbium isotopes from 12C6+ irradiated Nd2O3. Appl Radiat Isot 51:27–32
Eichstaedt HW, Felix R, Dougherty FC, Langer M, Rutsch W, Schmutzler H (1986) Magnetic-resonance-imaging (MRI) in different stages of myocardial-infarction using the contrast agent gadolinium-DTPA. Clin Cardiol 9:527–535
Nayak D, Lahiri S (1999) Application of radioisotopes in the field of nuclear medicine part I: lanthanide series elements. J Radioanal Nucl Chem 242:423–432
Nayak D, Lahiri S (1999) Separation of the carrier free radioisotopes of lanthanide series elements. Solvent Extr Ion Exch 17:1133–1154
Melnik MI, Karelin EA, Filmonov VT (1995) Production of high purity 153Gd. 2. Removal of samarium, terbium and microamounts of europium from gadolinium by extraction chromatography. Radiochemistry 37:156–158
Nayak D, Lahiri S (1999) Extraction and separation of 141Ce and 153Gd with HDEHP. J Radioanal Nucl Chem 240:75–77
Nayak D, Lahiri S, Das SK, Ramaswami A, Manohor SB, Das NR (1999) Separation of carrier-free gadolinium produced in an 80 MeV 12C6+ irradiated CeO2 target. Appl Radiat Isot 51:1–7
Nayak D, Lahiri S, Ramaswami A, Manohor SB (1999) Separation of no-carrier-added 147,149Gd and 147Eu produced in 70 MeV 11B irradiated praseodymium foil target. Radiochim Acta 87:93–96
Firestone RB, Shirley VS (1996) Table of isotopes, 8th edn. Wiley, New York
Gavron A (1980) Statistical model calculations in heavy ion reactions. Phys Rev C 21:230
Greenwood NN, Earnshaw A (1989) Chemistry of the elements. Pergamon Press, Oxford
Acknowledgment
This work has been carried out as part of the Saha Institute of Nuclear Physics—Department of Atomic Energy, XI five year plan project “Trace Analysis: Detection, Dynamics and Speciation (TADDS)”. We sincerely thank the staff of BARC-TIFR pelletron facility, Mumbai, for their cooperation and help during the experiment. M. Maiti is thankful to the Council of Scientific and Industrial Research (CSIR) for providing necessary grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maiti, M., Lahiri, S. & Tomar, B.S. Separation of no-carrier-added 149Gd from 12C activated natural praseodymium matrix. J Radioanal Nucl Chem 291, 427–432 (2012). https://doi.org/10.1007/s10967-011-1195-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1195-7