Abstract
Separation of no-carrier-added (NCA) 88Zr from natural yttrium target irradiated with proton beam has been studied using nature-resourced material, potato peel, which is a commonly available domestic waste. Here, an attempt has been undertaken to exploit natural resource as separating agent. Good separation of NCA 88Zr from bulk Yttrium target could be achieved at 0.01 M HCl concentration, when extraction was carried out using 20 mg potato peel charcoal—a natural bio-sorbent. 88Zr was extracted in the solid phase leaving behind bulk Y in the aqueous phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The no-carrier-added (NCA) radioisotopes are integral part of various applications in nuclear medicine since several decades. NCA radionuclides having high specific activities have applications both in therapeutic and diagnostic studies and also provide the necessary prerequisite for in vivo applications. For example, no-carrier-added 89Zr (T1/2 = 3.286 d) produced by the nuclear reactions, 89Y(p,n)89Zr and 89Y(d,2n)89Zr have been widely used for immuno-PET studies, labeling of monoclonal antibodies, proteins, etc. [1]. The relatively longer half-life of such radionuclides makes them suitable in localized radiation treatment of cancer and for long-term measurements in bio-distribution studies [2]. Also, 86Y (T1/2 = 14.7 h) − 90Y (T1/2 = 64 h) is utilized for theranostic purposes in combination, where 86Y is a positron emitter [1]. Table 1 lists some of the medically important Y and Zr radioisotopes. Apart from use in nuclear medicine, 88Y having T1/2 = 106.6 d, is also used as single radionuclide standard for calibration purposes [3].
Earlier, radiochemical separation of Y–Zr has been carried out using liquid liquid extraction (LLX) techniques like theonyl-trifluoro-acetone (TTA) in xylene; triphenyl phosphine oxide (TPPO) in chloroform, etc. [2]. Separation of NCA 89Zr was achieved using di-(2-ethylhexyl) phosphoric acid (HDEHP) in cyclohexane as liquid cation exchanger and also using high molecular weight amines like trioctyl amine (TOA) in cyclohexane, where Zr was extracted in the organic phase and Y remained in the aqueous phase [4, 5]. In case of solid liquid extraction (SLX) for separation of Zr from bulk Y, quite a few methods have been followed, like combination of Dowex 1-X8 and 4,4,4-trifluoro-1-(2-thionyl)-1,3-butanedione; Dowex 1-X8 and 12 M HCl as eluent; Dowex 50W-X8, Dowex 21 K resin, etc. [2, 6]. Though LLX can effectively separate NCA radioisotopes, but organic reagents used in LLX often are harmful to the environment.
Twelve principles including reduction of waste end-products, hazards, energy, environmental impact and production cost form the mandates of green chemistry. Although certain methods abide by the mandates of green chemistry, sometimes the production route of the chemicals used may not be environment-friendly. Therefore, for radiochemical separations, focus has gradually shifted towards nature-resourced-radiochemistry, which depends on the use of natural resources [7]. Nature resourced chemistry focuses on the utilization of the natural products and materials, which may also be effectively used for radiochemical separation purposes. In last few years, natural chemicals obtained from food products have been utilized in our laboratory effectively. For example, piperine, an alkaloid extracted from Piper nigrum (black pepper) has been studied for gold accumulation [8]; seed protein extracted from Erythrina variegate was found to bind effectively with Hg, Tl and Bi from multi-elemental matrix [9]; seed proteins of Mimusops elengi was studied for Au adsorption [10], etc. Recently, the efficacy of hesperidin, extracted from orange peel has been assessed towards its binding affinity for 89Zr, so as to design isotope-ligand complexation [11]. Further hesperidin was also studied for hesperidin-208Po association [12].
For long time, the use of natural bio-sorbents some of which are normally discarded as solid waste particularly the peels, husk or seeds of fruits and vegetables have been exploited explicitly for metal removal/adsorption activities [13, 14]. These sort of wastes generated can thus be effectively transformed into useful reagents having low operating cost, thus replacing the use of toxic chemicals. Potato peel is common domestic waste, also a waste product of agro-based industries and is easily available. It is reported that the presence of carboxylic (hemicelluloses, pectin, lignin), phenolic (lignin, lipids, waxes), hydroxyl (cellulose, lignin, pectin) groups on potato peel charcoal surface [15,16,17] often participates in metal extraction phenomena. Hence, many reported works in the past have utilized dried and grinded potato peel or its charcoal form for uptake of metal ions from complex multi-elemental milieu effectively [18,19,20,21] but never probed as an separating reagent for zirconium from bulk yttrium. Hence, the present study for the first time makes an attempt to valorize the efficacy of a household waste—potato peel for radiochemistry experiments.
Experimental
Materials and method
Potato peels were randomly collected from local domestic market. Collected peels were first washed thoroughly under running tap water to get rid off unwanted dirt on the peel surfaces. The peels were then soaked in deionized water for 2 h followed by rinsing in 0.1 M HCl for making the surface of the adsorbent more protonated [22]. After this the peels were air-dried overnight and transformed to charcoal by slowly heating in hot plate. The charcoal thus obtained was grinded to fine-powder, sieved (mesh no. 100) and preserved in desiccator for future use.
Irradiation
Natural yttrium foil (99.9%) procured from Alfa Aesar and of thickness 37 mg cm−2 was irradiated with 18 MeV proton beam for 8.6 h with an average beam current of 1.2–1.5 μA (integrated charge = 27,000 μC) to produce 88Zr (T1/2 = 83.4 d) and 88Y (T1/2 = 106.6 d) by natY(p,2n) and natY(p,pn) reactions respectively. The irradiation was performed at the Variable Energy Cyclotron Centre, Kolkata, India. The total current has been measured by a current integrator via the electron suppressed Faraday cup present at the backend of target holder flange. The irradiated foil was kept aside for more than ~ 3 months to decay out all short-lived radionuclides. Afterwards the same source was used for couple of radiochemical experiment. In this case, we have used the source after a year of irradiation. The present separation study has been carried out by monitoring 88Zr and 88Y (Table 1).
Measurement
For the present experiment, Canberra p-type high purity germanium (HPGe) detector with 30% relative efficiency and 2.3 keV resolution at 1.33 MeV was used. Genie 2 k software was used for the spectral analysis. Energy and efficiency calibration was done using single standard sources of 152Eu (13.53 a), 133Ba (10.55 a), 137Cs (30.08 a) and 60Co (5.27 a). Prior to radiochemical analysis, the preserved yttrium target was dissolved in minimum volume of 1 M HCl. The mother stock was diluted properly to carry out different experimental variations. Since the proton irradiated Y target has been kept for several months, during the present experiment, the γ-spectrum of the target showed the presence of only 88Zr and 88Y (Fig. 1).
Solid–liquid extraction
The elemental uptake behavior of finely ground potato peel charcoal (PPC) was examined using batch mode. For extraction of NCA 88Zr from bulk Y target, the batch included 20 mg of potato peel powder exposed to 3.5 mL of HCl solutions of different strengths (3, 1, 10−1, 10−2, 10−3, 10−4 and 10−5 M) containing 0.5 mL of radioactive stock solution. This set was shaken for 30 min and centrifuged for 15 min at 5000 rpm. 1.5 mL supernatant was taken in eppendorf and assayed by gamma-spectrometry. 88Zr and 88Y were radiometrically identified from the respective γ-peaks at 392.8 and 898.6 keV. The extent of adsorption by PPC was calculated by comparing against reference solution. At the best pH obtained, weight of PPC was varied (10, 20, 40, 60, 80 and 100 mg), and extraction behavior was observed in each of the cases. Different shaking times of 0, 15, 30, 45, 60 min were considered to obtain the maximum separation condition.
Result and discussion
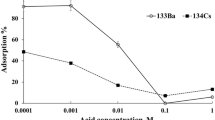
Extraction behavior of 88Zr and bulk Y with bio-sorbent PPC revealed that extraction of 88Zr increased with increase in acid concentration with maximum being extracted at 0.01 M HCl. Slight decrease in extraction was observed at higher range of acid concentration (Fig. 2). However, extraction of Y remained minimal over the wide range of acidity. Better separation of 88Zr could be observed at higher acidic condition of 1 M and 3 M, where the interference from bulk Y is minimum (< 5%). However, at higher acidic condition, often other simulations may not get effectively executed therefore we preferred the next best condition at 0.01 M, where NCA 88Zr has been completely extracted with ~ 9% contribution from 88Y. The separation factor achieved at 0.01 M HCl concentration is ~ 1.3 × 104.
To explain the extraction behavior, probable distribution of different species of Zr and Y was plotted at various H+ concentrations with the help of the information obtained from CHEAQS software [23] (Fig. 3). At lower acid concentration, Zr mainly exists as neutral species of [Zr(OH)4]aq. The concentration of cationic Zr species (Zr(OH)3+, Zr(OH) 2+2 , Zr(OH) +3 ) on the other hand, increases with increasing H+ concentration. In extreme acidic aqueous solutions the existence of polymeric Zr species [Zr4(OH)8(H2O)16]8+ have been reported by Hagfeldt et al. [24]. However, Y distribution is totally different as compared to Zr. Yttrium mainly exists as free Y3+ throughout the acidic range (Fig. 3). The uptake of Zr by PPC can be explained by ion exchange mechanism. The highly protonated functional groups of PPC facilitate exchange of protons with cationic zirconium species. The bulkier cationic Zr species (Zr(OH)3+, Zr(OH) 2+2 , Zr(OH) +3 ) are selectively extracted by ion exchange mechanism over Y3+. However, possibility can also be the adduct formation between the functional groups with O− donor ligands (free and bound form of phenolics and acidic moieties that are present in PPC) and the cationic metal species [25].
At pH 2, weight variation was carried out. From Fig. 4 it might be observed that separation was maximum when 20 mg of PPC was taken. With further increase in PPC amount, it was observed that both 88Zr and bulk Y were extracted by the PPC. Studies were carried out for shaking time variation (5, 15, 30, 45, 60 min) with fixed amount of 20 mg PPC. Figure 5 shows the adsorption trend of 88Zr, and bulk Y with 20 mg PPC. It was observed from Fig. 5 that with increase in the shaking time, along with 88Zr, Y uptake also increased. Hence, the optimal condition for separation of NCA 88Zr from bulk Y was observed with 20 mg PPC at 30 min shaking time, where ~ 93% 88Zr was extracted with only negligible (3%) interference from bulk Y. The errors calculated in the experiments are < 1% and hence not visible in the presented graphs. Figure 6 shows the gamma-spectrum of the supernatant where the peaks of 88Y (898.2 and 1836 keV) are visible but 392.7 keV peak of 88Zr is absent, as it was extracted by PPC. Figure 7 provides the flowchart of the experimental procedure.
From the present experiment, it was observed that PPC extracts Zr effectively from the radioisotopic mixture of Y–Zr. At pH = 2, extraction of Zr was found to be maximum with 1.3 × 104 separation factor. The separation factor is quite comparable with other conventional techniques, for example, earlier, with LLX method (10% HDEHP/cyclohexane and 5 × 10−4 M H2SO4), a separation factor of 4.7 × 103, could be achieved. With SLX method (Dowex 50W-X8 H+ and 1.5 M H2SO4), separation factor of 1.1 × 107 was achieved [6]. Thus, it may be suggestive that the present nature-based resourced separation technique may be useful in separation of NCA Y from Zr target in future, where Zr would be extracted by the solid matrix leaving behind NCA Y in the supernatant. For example, NCA 86,90Y are produced from natZr target, via the nuclear routes of 90Zr(n,p) 90Y; 90Zr(γ,3n + p) 86m/gY; 92Zr(d,α4n) 86gY. In all these cases, PPC would be nice natural resource for obtaining NCA Y in the aqueous phase, free from bulk Zirconium.
Conclusion
In separation science, the gradual development of radiochemistry to radio-green chemistry is interesting, productive and sucessful. At present, the trend is proceeding towards utilisation of nature friendly techniques that may be exploited effectively. This study reports a humble attempt in utilising nature resourced material i.e. potato peel charcoal (PPC) in separation of 88Zr and bulk Y.
References
Qaim SM (2017) Nuclear data for production and medical application of radionuclides: present status and future needs. Nucl Med Biol 44:31–49
Kandil SA, Scholten B, Saleh ZA, Youssef AM, Qaim SM, Coenen HH (2007) A comparative study on the separation of radiozirconium via ion-exchange and solvent extraction techniques, with particular reference to the production of 88Zr and 89Zr in proton induced reactions on yttrium. J Radioanal Nucl Chem 274(1):45–52
Mann WB, Rytz A, Spernol A (eds) (2012) Radioactivity measurements: principles and practice. Elsevier, Oxford
Lahiri S, Mukhopadhyay B, Das NR (1997) Simultaneous production of 89Zr and 90,91m,92mNb in a-particle activated yttrium and their subsequent separation by HDEHP. Appl Radiat Isot 48:883–886
Lahiri S, Mukhopadhyay B, Das NR (1997) Simultaneous production of 89Zr and 90,91m,92mNb in a-particle activated yttrium and their subsequent separation by TOA. J Radioanal Nucl Chem 218:229–231
Dutta B, Maiti M, Lahiri S (2009) Production of 88,89Zr by proton induced activation of natY and separation by SLX and LLX. J Radioanal Nucl Chem 281:663–667
Lahiri S, Choudhury D, Sen K (2018) Radio-green chemistry and nature resourced radiochemistry. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-018-6240-3
Ghosh K, Lahiri S (2007) Radiometric study on bioaccumulation of gold by an alkaloid extracted from fruits of Piper nigrum. J Radioanal Nucl Chem 274:233–236
Datta Samanta T, Laskar S, Nayak D, Lahiri S (2007) Studies on metal–protein interactions: inter-comparison among various approaches. J Radioanal Nucl Chem 273:323–325
Nayak D, Hazra KM, Laskar S, Lahiri S (2008) Preconcentration of gold by Mimusops elengi seed proteins. J Radioanal Nucl Chem 275:423–426
Ghosh K, Naskar N, Choudhury D, Lahiri S (2017) Separation of no-carrier-added 88Zr from proton induced bulk yttrium target by naturally synthesized hesperidin. In: Proceedings of 13th DAE-BRNS biennial symposium on nuclear and radiochemistry-NUCAR 2017 KIIT Bhubaneswar
Lahiri S, Choudhury D, Naskar N, Ghosh K (2018) Studies on 208Po-Hesperidin association. In: 4th international conference on application of radiotracers and energetic beams in sciences-ARCEBS 2018 Ffort Raichak, Kolkata
Jain N (2015) Removal Of Heavy Metal By using different fruit peels, vegetable peels and organic waste: a review. Int J Adv Res 11(3):916–920
Fu F, Qi Wang (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418
Öktem YA, Soylu SGP, Aytan N (2012) The adsorption of methylene blue from aqueous solution by using waste potato peels; equilibrium and kinetic studies. J Sci Ind Res 71:817–821
Ezekiel R, Singh N, Sharma S, Kaur A (2013) Beneficial phytochemicals in potato—a review. Food Res Int 50:487–496
Jeddou KB, Chaari F, Maktouf S, Nouri-Ellouz O, Helbert CB, Ghorbel RE (2016) Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem 205:97–105
Chavan MA, Mane S (2013) Removal of copper and zinc from aqueous solutions by using low cost adsorbents. Int J Sci Res 4(7):3078–3080
Ahirwar B, Sharma AK, Sharma S (2017) Removal of copper from aqueous solution using low cost biosorbent (Potato Peel). Int J Appl Res 3(9):459–462
Massimi L, Giuliano A, Astolfi ML, Congedo R, Masotti A, Canepari S (2018) Efficiency evaluation of food waste materials for the removal of metals and metalloids from complex multi-element solutions. Materials 11:334
Sher Mohammed NM, Mohammed Salim HA (2017) Adsorption of Cr(VI) ion from aqueous solutions by solid waste of potato peels. Sc J Univ Zakho 5(3):254–258
Mutongo F, Kuipa O, Kuipa PK (2014) Removal of Cr(VI) from aqueous solutions using powder of potato peelings as a low cost sorbent. Bioinorg Chem Appl. https://doi.org/10.1155/2014/973153
Verweij, W.: Cheaqs Pro: a program for calculating chemical equilibria in aquatic systems. http://home.tiscali.nl/cheaqs/ (2005)
Hagfeldt C, Kessler V, Persson I (2004) Structure of the hydrated, hydrolysed and solvated zirconium(IV) and hafnium(IV) ions in water and aprotic oxygen donor solvents. A crystallographic, EXAFS spectroscopic and large angle X-ray scattering study. Dalton Trans 14:2142–2151
Greenwood NN, Earnshaw A (1997) Chemistry of the elements, 2nd edn. Butterworth-Heinemann, Oxford
Acknowledgement
Authors are grateful to Professor Susanta Lahiri for his guidance. SB and NN are thankful to Dr. Punarbasu Chaudhuri, Department of Environmental Sciences, University of Calcutta. NN is also grateful to UGC for providing her necessary fellowship. Authors are thankful to Variable Energy Cyclotron Centre (VECC) for the beam-time. DAE-SINP 12 5 year plan project Trace, ULtratrace analysis and Isotope Production (TULIP) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naskar, N., Choudhury, D., Basu, S. et al. Separation of NCA 88Zr from proton irradiated natY target: a novel approach using low cost bio-sorbent potato peel charcoal. J Radioanal Nucl Chem 322, 231–235 (2019). https://doi.org/10.1007/s10967-019-06637-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06637-z