Abstract
Poly(glycolic acid) (PGA) is an essential biopolymer due to its thermal and mechanical properties and biodegradability which provide utility for medical applications and renewable industry. For biomedical applications, production of PGA with high molecular weight is an essential factor to possess adequate mechanical stability. Primary pathways for PGA synthesis are ring-opening polymerization of glycolide (ROP), direct polycondensation of glycolic acid, and solid-state polycondensation of halogen acetates. For PGA synthesis, different systems have been developed with using varying parameters including catalysis, initiators, solvents, and reaction temperature. This review summarizes the different synthesis pathways and physicochemical properties of PGA. Biomedical applications of PGA are also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biopolymers have been used in a wide range of applications such as surgical operations and regenerative medicine [1]. Synthetic biopolymers may have better mechanical properties and thermal stability compared to some natural polymers [2]. Poly(α-hydroxy acids) group of synthetic biopolymers has been studied extensively and the most widely used polymers in this category are poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lactic-co-glycolic acid) (PLGA) which are degradable under in vivo conditions and serve as proper matrix for regenerative medicine. Among them, PGA is precious biopolymer because of the its high cost regarding no technology found for cost-efficient high-scale production.
PGA is a highly crystalline polymer (45–55%) with a high melting point (220–225 °C) and a glass-transition temperature (Tg) about 35–40 °C [3, 4]. PGA has a faster degradation rate and higher mechanical properties compared to PLA and PLGA with different PGA/PLA ratio. PGA is insoluble in many solvents due to its high crystallinity but just soluble in highly fluorinated solvents such as hexafluoroisopropanol (HFIP) until a molar mass of 45,000 g/mol [5]. Synthesis of PGA and its industrial production is challenging especially in the case of obtaining high molecular weight due to unstable and easily degradable nature of PGA [6]. Glycolic acid and glycolide are used as monomers for PGA synthesis (Fig. 1). A method for synthesizing high-molecular-weight PGA via ring-opening polymerization of glycolide is valid however it allows small quantity of PGA at high cost [7]. Yamane and coworkers reported that PGA can be also produced via condensation of glycolic acid using a dehydrating reaction which is a comparatively cost-efficient method. However, obtaining of high-molecular weight PGA with this method has an obstacle due to the equilibrium between glycolide and chain extension for the hydroxyl termination of PGA [8]. Additionally, the ring-opening polymerization of glycolide was appeared for synthesizing high-molecular-weight PGA. However, there are still obstacles in the industrial scale of high-molecular-weight PGA such as deposition of glycolide on the inside wall of the distillation lines [8].
According to the reported market research by Global Market Insights Inc., the PGA is estimated to grow with a value of more than $6 billion to over $9 billion by 2024. The global PGA market is becoming increasingly important especially in the medical industry. The main applications of the PGA in the medical area are absorbable sutures and other advanced materials such as biodegradable bone grafts, dental materials, scaffolds in tissue engineering and drug delivery vehicles. It is estimated that PGA market will increase in near future with the advances of PGA in different applications such as packaging industry and shale gas extraction [9].
The first application of PGA as a biomaterial has been declared in 1954 [10], and synthetic and biodegradable sutures made of PGA called Dexon® was developed in 1962 by American Cyanamide company [11, 12]. Dexon® suture loses its mechanical stability in up to several weeks and quickly absorbed by body [13]. Dexon® as an biomedical application of PGA has been approved by the US Food and Drug Administration (FDA) in 1969 and interest in PGA has rapidly increased over time in terms of its use in other biomedical applications [14,15,16,17]. PGA and its copolymers have been used in medical fields such as in drug delivery systems, dental and orthopedic applications due to their good biocompatibility and biodegradability in addition to the sufficient mechanical characteristics [1]. PGA is used in the production of medical materials like screws, stents, and grafts for regenerative medicine applications. Higher molecular weight reduces the degradation rate of the biopolymer and this feature is crucial for tissues having longer regeneration period such as bone and cartilage [18]. In addition, the polymer gains mechanical stability with increase in molecular weight and crystallinity [19]. Applications of PGA was restricted to the medical field because of the high price of PGA. By decreasing the cost, new applications area can appear related to the certain characteristics of PGA including high gas-barrier properties and mechanical strength.
Achieving and producing high molecular weight PGA (Mn > 45,000 g/mol and Mw > 93,000 g/mol) is important for further processes [20]. In addition, sufficient mechanical stability of PGA is generally obtained at molecular weights >30,000 g/mol [20, 21]. It is very hard to achieve to the desired molecular weight with direct condensation method. For this purpose, the glycolide chain opening polymerization is used. However, there are some difficulties in this method such as necessity of high temperature and expensive raw material [22]. Additionally, catalysis used during ring-opening polymerization of glycolide could indicate toxic effect. For example, tin (II) octanoate/benzyl alcohol catalyst system give high molecular weight PGA but tin compound is known to be toxic and should rather be removed from the produced PGA [23].
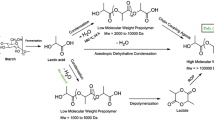
Despite its importance and use in clinical applications, when we look at the literature there are fewer reports on PGA because PGA and glycolic acid are more expensive that compared to lactic acid and PLA and PGA have highly crystalline properties that it is almost insoluble in widely used organic solvents such as tetrahydrofuran, chloroform, toluene, or dimethyl sulfoxide thus it is hard to characterize and process [22, 24]. However, PGA remains the most remarkable material among biopolymers due to its high mechanical strength and biocompatibility and obtaining high molecular weight has been a focus of researchers. Different approaches have been developed for PGA synthesis. Those synthesis approaches can be divided into three main parts: Ring-opening polymerization of glycolide, direct polycondensation of glycolic acid and solid-state polycondensation of halogenoacetates [7] (Fig. 2).
PGA with different physicochemical properties can be obtained according to the selected reagents, reaction initiators and synthesis procedures which affect the characteristics of the PGA including hydrophilicity, crystallinity, melting and glass transition temperatures, molecular weight, distribution of molecular weight, terminal end groups, and the residual monomers and additives [19]. Different approaches have been investigated for the production of high molecular weight PGA [7, 22, 23]. As mentioned above, high molecular weight PGA has unique properties, which opens the door to new applications of PGA. In this study, synthesis procedures and biomedical applications of PGA will be reviewed. The copolymers of PGA such as PLGA is not concern of this review.
Different pathways for the synthesis of PGA
There are various polymerization approaches in PGA synthesis depending on factors such as initiator catalysis, solvent, pressure, and temperature. Because the mechanical properties of PGA were directly related to the molecular weight, researchers have mainly focused on obtaining high molecular weight PGA. Synthesis approaches of PGA are shown in Fig. 3.
Synthesis of PGA by direct polycondensation
This commonly used method for obtaining PGA is simple, but it is not an effective approach for high molecular weight PGA production. This method is based on the procedure of obtaining PGA by heating the glycolic acid to 175-200 °C under atmospheric pressure and then maintaining the pressure at 150 mm-Hg for up to 2 h. PGA obtained with this procedure has low molecular weight less than 10,000 g/mol due to the water appeared during the synthesis which is hard to remove from the polycondensation process [25, 26]. Zhaoyang and his colleagues have stated that the synthesis of low-molecular weight PGA by conducting the reaction at 165 °C and 70 Pa during 10 h in the presence of tin(II) chloride as catalyst [27]. The obtained PGA with low molecular weight could be suitable for use in drug delivery systems.
Synthesis of PGA by azeotropic polycondensation
High molecular weight-PGA (Mw > 930,000 g/mol) can be obtained by polycondensation in azeotropic solution called azeotropic condensation polymerization [22, 28,29,30]. In this method, the problem of removing the water formed during the reaction is overcome with the selected organic solvent. In addition, azeotropic distillation has some advantages for industrial production such as low cost and high efficiency [22, 31]. In the patent study US5444143A stated that, polyhydroxcarboxylic acid having a high molecular weight (Mw = 20,000–460,000 g/mol) can be obtained by dehydration condensation of hydroxycarboxylic acid with azeotropic distillation method [32]. In another patent study high molecular weight (Mw = 50,000–200,000 g/mol) polyhydroxy acids were also obtained by using dean stark apparatus via azeotropic distillation method [33]. However, glycolic acid and lactic acid blend was used as hydroxycarboxylic acid in these studies and therefore, these studies give us an idea for obtaining high molecular weight PGA. In a recent study, high molecular weight-PGA (Mn = 32,100 g/mol) with high crystallinity (77%) and high solubility (147.3 mg/mL) was obtained from glycolic acid by azeotropic distillation with Dean stark apparatus. In this study, solvent-catalyst binary interactions are found to be more effective than temperature of azeotropic distillation reaction [22].
Synthesis of PGA by acid and enzyme-catalyzed
Acid-catalyzed and enzyme-catalyzed reactions have been developed for the production of PGA. Masuda et al. conducted acid-catalyzed reaction with trioxane and paraformaldehyde used as reagent compounds and formaldehyde and carbon monoxide used as sources respectively, in the presence of chlorosulfonic acid as catalyst. The reaction is conducted at about 180 °C for 2 h which finally give a mixture of low and high molecular weight-PGA [34, 35]. Enzyme-catalyzed reactions are eco-friendly approach due to the non-synthetic pathway which carries out PGA synthesis via isolated enzymes. Kataoka et al. used ethylene glycol with Pichia naganishii which is a bacterium from isolated soil and obtained high molecular weight PGA [36].
Synthesis of PGA by oligomerization of glycolic acid and chain coupling reactions
A different approach to increase the molecular weight of PGA is oligomerization and chain coupling. In this method, a variety of chain linking and esterifying agents which react with hydroxyl or carboxyl functional groups on the molecular structure can be used [37]. However, the main drawback of this method is being a multi-step procedure including flammable solvents [38].
Synthesis of PGA by solid-state polycondensation of halogenoacetates
Another pathway for PGA synthesis is thermally induced solid-state polycondensation of halogenoacetates which is a solvent-free process. The homopolymerization of PGA is accompanied by the creation of sodium chloride. In this method, the obtained polymers usually have a lower degree of polymerization than from solution- or melt polymerization methods [39]. PGA can be obtained from 11 different halogenoacetate precursors with the general formula of MOOCCH2X which the composition of metal varies (M = Li, Na, K, Rb, Ag, Cs) and halogen (X = Cl, Br, I) [40]. In the study of Subramanyam, high molecular weight PGA (Mn = 100,000 g/mol) was obtained from halogenoacetates [41].
Synthesis of PGA by ring-opening polymerization
As to the ring-opening polymerization of glycolide method, which was first used by Carothers in 1932 to obtain poly(α-hydroxy acids), the product was low molecular weight PLA [42]. So far, different approaches have carried out on ring-opening polimerization for high molecular weight polymer synthesis and first attempt was high- molecular-weight-PLA obtained in 1950 using ring opening polymerization (ROP) technique with efficient monomer purification [31]. In a recent study, Schmidt and coworkers performed high-molecular-weight-PGA synthesis in supercritical carbon dioxide using ROP method. In this study, supercritical carbon dioxide (scCO2) was used as a reaction medium because of scCO2 allowed for a reduction in reaction temperature compared to conventional processes. Finally, they obtained PGA with number average molecular weight of 31,200 g/mol [43]. ROP process can be divided into four reaction subtypes according to the catalyst, initiator or reaction conditions and these are melt and/or bulk polymerization of glycolide, suspension or emulsion polymerization of glycolide, solution polymerization of glycolide, cationic and anionic polymerization.
Melt or bulk polymerization of glycolide
Regarding melt or bulk polymerization of glycolide reaction mechanism, in first step, the water is removed from polymer and oligomer is obtained low molecular weight polymer. Oligomer is heated under high vacuum and to prepare raw monomer is uses proper catalysts which known as chain coupling agents. Finally, high molecular weight polymer is obtained after several purification steps. The most important issue in this process that water must be removed which composed in the reaction process otherwise water molecules attack ester bond on the polymer, which occur decreases molecular weight of polymer. Takahashi and colleagues used the melt/solid state polycondensation method of glycolic acid to obtain high molecular weight PGA [20]. In this method, firstly, low molecular weight oligomer was obtained from glycolic acid at 190 °C and then suitable catalysts were selected and continued reaction at the same temperature finally obtained PGA which possess high molecular weight. After scanning catalysis, they found that zinc acetate dihydrate is the most suitable catalyst and as a result, they have been synthesized PGA with an average molecular weight of 91,000 g/mol. Shen et al. were achieved using two different catalysis, zinc acetate dihydrate and tin dichloride dehydrate, the PGA with a molecular weight of 45,000 g/mol in their work [44].
Solution polymerization
Although the melt/bulk polymerization method has a simple procedure and high molecular weight polymer is obtained according to the selected reaction conditions, the high temperature formed during synthesis cannot be removed due to poor heat conduction. In a study by Leenslag and Pennings, it is stated that the heat produced during the reaction reduces the molecular weight [45]. There are two types of polymerization methods where this problem is relatively uncommon. These are suspension and solution polymerization methods. In one study [46], solution and suspension polymerization methods of lactide and glycolide were studied, advantages or disadvantages of these studies were compared with melt and/or bulk polymerization methods.
In the solution polymerization process, the reaction is initiated with a relatively low concentration of monomer than compared melt/bulk polymerization and as the lower viscosity allows mixing during the reaction, heat transfer takes place and thus the heat generated in exothermic reactions can be avoided. However, this method has some disadvantages as in other polymerization methods. For example, the solvent used should be removed after the polymers has been synthesis. Another disadvantage is that racemization occurs during solution polymerization. In this study by Nieuwenhuis, they stated that obtained 90,100 g/mol molecular weight polymer by using solution polymerization method with a yield between 96 and 100% [46].
Suspension or emulsion polymerization
The basis of the suspension or emulsion polycondensation methods is the vigorous mixing of water-insoluble or slightly soluble monomers to prevent the particles from interacting with each other during the polymerization process. Larger particles (20 μm and above diameters) are obtained compared to emulsion polymerization, which can be easily isolated by filtration or sedimentation [47]. British patent GB825335A is describes a suspension polymerization method for the polymerization of lactide and glycolide. In this study, inert liquid hydrocarbons with boiling point between 80 °C and 250 °C were used. However, the explosive effects of these liquids make it difficult to study. Silicone-oil was selected for stabilizer and one, two, four, five, six and seventh groups of metal halides were used as catalyst and low molecular weight polymer was synthesized at the end of the reaction [48]. Nieuwenhuis carried out various suspension polymerization studies based on the system mentioned in the patent study. In one experiment, he used gasoil-L lactide stannous octoate system, maintained the reaction temperature between 80 and 160 °C and continued the reaction time which a period of 1–2 h to 20–30 h, after that was obtained polymer which have 175,000 g/mol molecular weight [46].
Cationic and anionic polymerization
In polymerization there is a direct link between the selected catalysis and the quality of the final product. Cationic and anionic compounds can be used in the polymerization of glycolic acid or glycolide as the monomer and are separated as cationic-anionic polymerization methods. Protonic acids such as sulfuric acid and phosphoric acid are used in cationic polymerization, also Lewis acids such as zinc chloride, ferric chloride, aluminum chloride, titanium tetrachloride, boron triflouride etherate, and antimony trifluoride can be used, which provide high mass polymers [49].
There are a few studies in the literature for the polymerization of glycolide with cationic and anionic compounds used as catalysts, but there are many methods with lactone. Studies with lactones may provide ideas for preparing PGA [50]. In another study carried out recently, high molecular weight PGA (Mn = 600,000 g/mol) was synthesized in two steps by carbon monoxide (CO) and formaldehyde cationic copolymerization method [7]. In this study, biomethanol or biogas was used as a sustainable raw material source for carbon monoxide (CO), while trioxane was used as a source of formaldehyde. In first step, oligomer (Mn ∼ 1800 g/mol) obtained from cationic polymerization esterification and then Zn(OAc)2·2H2O was used as catalysis in the second step of this study. Surprisingly, polymer having a molecular weight of approximately Mn ∼ 600,000 g/mol was synthesized with this method. However, they explained that this number must be juxtaposed with the solubility limits of PGA in HFIP [7]. In other study based on the previous study, under the same reaction conditions, additionally epoxides derived from long-chain fatty acids were used this study and approximately, obtained PGA with number average molecular weight 49,000–132,000 g/mol in this study and it has also been stated that massively advanced the solubility and reduced the melting temperature of the PGA [23]. Reyhanoglu and Gokturk, similar to their previous studies, they have been used polyethylene glycol (PEG) as an epoxy derivative in the system compared previous studies and finally, were produced up to weight average molecular weight 194,000 g/mol PGA [51].
As regards anionic polymerization, catalysts which are effective for lactones include alkali metals, alkali metal oxides, alkali metal naphthalene complexes and crown ethers can be given sample. The reaction is initiated by a nucleophilic attack of a negatively charged initiator to the carbon of the carbonyl group or alkyl-oxygen, resulting in linear polyester formation [52]. Anionic polymerization of lactic and glycolide have been less studied than the coordination-insertion approach. In a study conducted with alkali metal solutions, in polymerization of ß-lactone, it is stated that a polymer having with number average molecular weight 110,000 g/mol and with over 90% yield is obtained [53]. In addition to the abovementioned methods, PGA was synthesized by different polycondensation methods using clay, ionic liquids, diphenyl bismuth bromide as catalysis [6, 54, 55]. Different approaches have been developed for PGA synthesis and low or high molecular weight PGA synthesis has been made with these methods (Table 1).
Although the ring-opening polymerization method of glycolide has drawbacks such as expensive raw material and the toxic effects of the catalysis, this method has been used still industrially. However, the relatively expensive monomer limits production of PGA and synthesis methods of PGA which have low cost, high yield and high mass has always been of interest to researchers and leads to new alternative approaches. Azeotropic condensation polymerization method with Dean-Stark apparatus is remarkable and stands out as an alternative approach for industrial applications. This method can replace ring-opening polymerization method in the future.
Evaluation of physicochemical properties of PGA
PGA and its degradation products has been shown to be non-toxic and biocompatible. The importance of PGA comes from its physical and chemical properties provided that opportunity to use of this material in different regions where are from range pharmaceutical and biomedical applications to packaging targets [57]. The properties of PGA are associated with its high molecular weight, highly crystalline structure, melting temperature, glass-transition temperature, modulus, tensile strength, elongation-at-break, and actually it is the most hydrophilic molecule according to other polyesters (Table 2).
Effect of chemical structure on the degradation rate of PGA
The degradation rate of biopolymers depends on some significant factors such as the implanted area, molecular weight and distribution, mechanical properties, chemical structure, crystallinity, morphology, surface roughness, porosity, surface charge, surface free energy, pH, presence of additives etc. As such, biopolymers displays dissimilar degradation rates from each other, give rise to different degradation duration range from days to months [19]. The degradation characteristics of PGA depend on many factors such as accessibility to amorphous phase and degree of crystallinity. X-ray diffraction, Raman and Infrared spectroscopy can give us information about crystalline structure of PGA. Two glycolic units depends on each other through orthorhombic cell. Glycolic acid macromolecules form a planar zigzag conformation passing the orthorhombic unit cell with the dimensions of a = 5.22 Å, b = 6.19 Å, and c = 7.02 Å being the fiber axis. High density of PGA (1.69 g/cm3) demonstrate that it has crystallite structure which consist of the strict molecular packing and the close access of the ester groups can stabilize the crystal cage and lead to the high melting point of this polymer. Additionally, the configuration lead to the insolubility of PGA in common organic solvents [61, 62].
Glycolic acid which is degradation product of PGA is a natural metabolite. This feature, with PLA, give rise to make its attractiveness among biopolymers in medical applications (Fig. 4). PGA degrades in mainly two processes: Firstly, water penetrates in the amorphous regions of the PGA matrix and hydrolytic chain scission occurs in main chains. In the second stage, when the amorphous regions are corroded, its biodegradation process maintain with degradation of the crystalline regions. The first stage of biodegradation of Dexon® sutures continued along 21 days, while the second stage continued 28 days under in vitro conditions. Weight of polymer decreased almost 42% at the end of the biodegradation stage and PGA losses all of mechanical properties [63] due to the bulk degradation of PGA leading to rapidly reduce of mechanical properties. In addition, PGA can degrade enzymatically in the presence of enzymes which display esterase activity [64]. Degradation product (glycolic acid) is removed via urinary system from the body. Despite of natural characteristics of glycolic acid, it has an acidic nature and decrease pH of the area at which the degradation process occurs and may led to necrosis.
Thermal and mechanical properties of PGA
Different synthetic pathways and catalysts supply options to modify the molecular properties of PGA such as molecular weight, molar mass dispersion, end groups, form of GA units, and chain structure. Those factors decide substantially the physico-chemical features of PGA such as, elongation-at-break, tensile strength, modulus, thermal properties and density [65]. Thermal properties including melting, crystallization and glass transition temperature are essential for processing conditions of polymers, although they are not directly relevant to biocompatibility. Differential scanning calorimetry (DSC) is used to determine the thermal properties of polymers which are important for polymers applications and give information about mechanical behavior and degradability. Glass transition temperature (Tg) value is relevant to biodegradability of biopolymers. If the Tg of a biopolymer is close to the body temperature, biopolymers can be more elastic in implanted area [66]. Melting temperature (Tm) is also another significant parameter of thermal characteristics. Upon Tm, whole polymer chain mobility happens and mechanical features of a biopolymer are nearly decreased to zero [67]. With regard to crystallization temperature (Tc), the rate of crystallization a polymer is affected by Tc [68]. High crystallinity of polymer indicate that it has high Tc at the same time [58].
Mechanical properties (Young’s modulus, tensile strength, and elongation-at-break) are another important properties for biopolymers applications [69]. The mechanical properties and crystallization behavior of PGA are very dependent on the Mw and its molecular chain structure [21]. Characteristics of PGA including thermal, mechanical and degradation properties are given in Table 2. Tg of PGA is in the range of the body temperature (37 °C) which gives elasticity at the implanted area. Both Tm and Tc of PGA are high due to the its high crystallization structure.
Biomedical applications of PGA
The developments in regenerative medicine and drugs as well as requirements for biocompatible and safer materials have accelerated the tendency towards use of biodegradable materials for medical applications (Fig. 5). The general purposes of biodegradable materials use in medical applications include drug release (anti-infection, anti-tumor), operative assist (hemostasis, blood flow arrest), damage healing (organ regeneration, tissue growth), isolation (anti-adhesion), synthesis (suture, surgical bonding), reinforcement (regeneration of ligament), scaffold (cartilage, vessels), and incorporation (slow release) [70]. PGA is one of the typical biodegradable synthetic material used in medical applications through its biocompatible nature and toxicological safety due to being metabolic molecules found in humans [70, 71]. However, there are significant concerns about PGA due to its rapid degradation causing loss of mechanical strength [71]. Besides, high levels of glycolic acid leads to an inflammatory response after being resorbed by cells via the citric acid cycle [72]. PGA is also unsuitable for preventing intra-pericardial adhesions [73] and facilitating colonic anastomosis formation [74]. The studies related to the medical applications of PGA are explained in detail below.
Barrier membranes
Polymer barrier membranes are necessary for methods of guided tissue regeneration (GTR) and guided bone regeneration (GBR) [75]. A barrier membrane has important role which prevents epithelial or undesirable tissues migration in healing process of GTR and GBR applications [76]. Many barrier membranes have been improved, which are generally used in clinical applications [77]. A barrier membrane should possess specific properties such as biocompatibility, space-making, cell-occlusiveness, tissue integration and clinical manageability [78]. Also, the barrier membrane must match mechanical properties of the target tissue. Generally, these membranes are divided according to their degradation as resorbable and non-resorbable membranes [75, 77]. Non-resorbable membranes include expanded polytetrafluoroethylene material and titanium mesh which have restriction due to the requirement of a second surgical procedure to remove the biomaterial [79, 80]. Collagen and polyhydroxy acids such as polyglycolic acid (PGA) or polylactic acid (PLA) are the most used polymers in degradable membranes [81]. Their main advantage is non-requirement for a second surgical procedure to remove the biomaterial in addition to the elimination of potential effects of stress shielding of the regenerated tissue [82].
A barrier membrane such as artificial skin should maintain the humidity inside and should resistant to infection without inducing inflammation [70]. For this purpose, bio-based polymers like collagen and chitosan are preferred due to their high cell affinity and antimicrobial properties. However, biodegradable synthetic polymers like PGA is also used as artificial skin [83] successfully formed a joint like tissue including bone and cartilage by seeding chondrocytes and tenocytes into PGA scaffold. Besides, McVicar et al. proposed a self-reinforced PGA membrane for orbital floor repair [84]. The authors indicated that nobody faced clinical consequences because of hydrolysis of self-reinforced PGA membranes. In these applications, the tensile strength of self-reinforced PGA reduces within 4 weeks because the membrane is replaced by reorganizing tissue [85].
Drug delivery applications
A range of different materials have been employed for controlled release of drugs with a desirable physical property. The general requirement for a material to be used in drug delivery system includes being inert, free of impurities, having appropriate physical structure, and processability. In recent years, biodegradable polymers have been chosen as drug carriers for various formulations and medical use due to their deradable nature within the body [86]. These materials have been accepted due to the fact that the properties can be enhanced or changed by incorporating varius molecules such as ester, orthoester, anhydride, carbonate, amide, urea, and urethane in their basic structure [87]. Langer has studied various polymers for drug delivery and characterized the release of macromolecules from polymers [88]. Generally, PLA, PGA and their copolymers PLGA have been designed for drug delivery applications [89]. It is also possible to modify the mechanical, thermal, and biological properties of these polymers by altering its stereochemistry.
PGA has been widely used as absorbable sutures as a step for controlled drug delivery systems [90,91,92]. The advantage of PGA use as a drug delivery material is that PGA is broken down into metabolized molecules by body and removed from body through normal metabolic pathways. On the other hand, the insolubility in many common solvents, high melting point, cannot forming films/rods/capsules, unsuitable for solvent or melting based methods, and rapid degradation of PGA has resulted in limited studies on drug delivery systems [71, 93].
The release behavior of PGA, and thus degradation, depends on its’ initial molecular weight, crystallinity, and porosity [94]. The drug delivery rates of PGA can be altered by controlling the molecular weight distribution via changes in the routes of PGA synthesis or via addition of crystals to change particle size distribution [40]. Moll and Ries explained that the release from a low molecular weight structure occurred over within a few hours rather than days, suggesting that the polymer was solubilized immediately [95]. Hurrel and Cameron showed that the release of a model drug, theophylline, from polyglycolide also depended on buffer concentration, pH and particular buffer ions on the hydrolysis reaction [96]. PGA has a great potential on and widely use as wound closure materials, surgical sutures, and tissue engineering scaffolds. However, its limited potential on drug release systems was also studied by researchers by using different parameters [97,98,99].
Dental applications
Biodegredable polymers are generally used for two different dental applications: as a void filler and as a guided tissue regeneration membrane. Tooth extraction is followed by packing into the cavity as a healing agent with the help of porous polymer particles [100]. On the other hand, biodegredable polymers are applied as in the film form for GTR membrane applications. The polymer film is positioned by periodontal surgery to exclude epithelial migration with the aim of supporting, slower growing tissue (also connective and ligament cells) to proliferate [101]. The crystalline structure and high molecular weight of PGA limits the clinical use of PGA for some dental applications such as osteosynthesis due to being susceptible to degradation [102, 103]. A typical complete bone healing requires more than 7 weeks, however within 4–7 weeks PGA generally loses its mechanical strength [104, 105]. On the other hand, it has been reported some negative effects arising from the difficulties in clearing the accumulated acid-degradation products [102, 105]. Okuyama and colleagues studied PGA sheets using fibrin glue to cover open wounds after resection of oral mucosal lesions of selected patients between 2010 and 2016 [106]. The authors observed a significant risk for granuloma like neoplasm (GLN) development when PGA was used on the raw surface of the tongue while no immediate abnormal postoperative bleeding (APB) was observed. It was stated that among these minor complications, GLN did not involve the recurrence of a tumor. However, APB occurred due to PGA sheets with fluctuating adhesive forces and fibrin glue might sometimes induce life threatening situations.
The physico-chemical properties of PGA made it suitable for sustaining drug release under in vivo conditions. However, its faster degradation rates and solubility problem have limited the use of PGA-based drug delivery systems [107]. During the degradation of PGA, glycolic acid released to medium and inflammatory response might occur. Thus, medical structures including anti-inflammatory drugs, surface modifiers, or agents for enhancement of periodontal regeneration would be beneficial [108]. The mechanical integrity of PGA can be lost easily due to the rapid degradation, allowing to the release of glycolic acid, which might cause inflammatory response [107]. PGA in different forms such as fiber meshes has been accepted as attractive candidate to transplant cells however the resistance of PGA to compressional forces have been found as low [109]. Thus, PGA has been stabilized by different techniques such as copolymerization. The tensile strength and modulus of PGA are around 89 MPa and 7 GPa, respectively [110] and shows strong and flexible structure with a potential use in periodontal and oral mucosa grafts [111]. For instance, Mizutani et al. studied the suitability of polymers such as PGA as screw post materials in primary teeth and reported that PGA had appropriate strength and hydrolysis ability [112].
Orthopedic applications
Human bone exposes to different mechanical forces such as daily activities, exercises, weight bearing in a dynamic environment [113]. Bone tissue engineering and orthopedics have some successful application in addressing issues associated with three-dimensional scaffold materials and architecture [114]. PGA and their copolymers have been widely used for orthopedic applications such as bone, tendon, ligament, articular-cartilage scaffolds [113]. PGA degrades by simple hydrolysis or non-specific enzymes and produced some molecules, which can be excreted in urine or enter the tricarboxylic acid cycle [115].
A tissue-engineering alternative to cancellous bone have been developed towards mimicking structure and properties by some researchers with bioresorbable polymers such as polymer microspheres [116, 117] and porous polymer scaffolds [118, 119]. The biodegradable polymers used for tendons and ligaments replacements should maintain their tensile strength for at least 12 months for fibrous tissue to regenerate [113]. Cooper and Lu reported PGA implant showed the highest time zero mechanical strength but showed the lowest cell attachment in tendon and ligament applications [120, 121]. PGA was evaluated for tendon and ligament repair due to the limit of strength of widely used collagen [113]. On the other hand, nonwoven structures such as PGA has been also studied for cartilage repair [122]. Mikos et al. have studied a coated PGA nonwoven mesh to overcome the loss of mechanical strength arising from proliferation and reported greater mechanical properties [123]. Besides, Moran et al. showed the effect of coating on strength and degradation and reported a less chondrocyte attachment on coated scaffold compared with PGA controls [124].
PGA is one of the suitable material for bone internal fixation devices however the hydrolytic degradation of PGA leads to the loss of mass within 12 months and loss of strength within 2 months [16]. Some researchers reported a lack of sufficient mechanical integrity in vivo with significant decrease in performance within 4 weeks when a more soluble form of PGA was used [125, 126]. On the other hand, Liu et al. indicated that PGA produced granulomatous inflammation after brain resection [127]. In the study of Hosseini et al., PGA microfibrillar scaffold was coated with poly(4-hydroxybutirate) (PHB) acid and then aligned before heating above the glass transition temperature [128]. The heat application is followed by stretching the coated scaffold to obtain an aligned and three dimensional (3D) porous fibrillar scaffolds. The authors aimed to obtain a partial welding of randomly oriented microfibers at their intersection points by coating polymer to a non-woven mesh (Fig. 6). The results showed that fibroblasts cell alignment along the direction of the PGA fibers was increased under in vitro conditions.
F-actin (magenta) and cell nuclei (cyan) alignment analysis of cells on pre-stretched PGA scaffolds after 1 week in cell culture: a Maximum z-projection intensity of multi-plane confocal images of cells, b Respective actin fiber alignment quantifications, c %actin fibers and d) %cell nuclei within ±10° of the strain axis [128]. PGA fiber autofluorescence are indicated as cyan. Copyright 2017. Reproduced with permission from Wiley Online Library
Kodama et al. studied islets tissue engineering from cultured cells for pancreatic islet transplantation for treatment of type 1 diabetes [129]. For this purpose, the authors enzymatically dissociated rat pancreatic islets into a single-cell suspension followed by seeding onto a PGA scaffold. To collect the tissue engineered islets, PGA and cells were isolated from medium including epidermal growth factor, nerve growth factor, and insulin-like growth factor to suspend in a thermos-reversible gelatin polymer (TGP) with insulin, transferring and selenous acid. Collected cells were transplanted beneath the kidney capsule of Streptozotocin-induced diabetic nude mice. Authors concluded that the use of PGA and TGP has high potential implications for the treatment type 1 diabetes.
The enhancement and changes in molecular weight and crystallinity degree of PGA cause differences in mechanical strength. Generally, it was reported that PGA lost its mechanical strength within 6 weeks and resorbed in a few months depending on the molecular weight, purity, crystallinity and the size and shape of the implant [130]. In clinical applications, PGA structures should retain adequate tensile strength over the critical period requiring suitable conditions. Thus, it is necessary to determine the mechanical properties of PGA structures such as tensile strength, stiffness, toughness, etc., before, after or during implantation. For instance, the increase in crystallinity of PGA will increase the brittleness of implant, thus altering the resistance during healing [131]. In orthopedics applications, PGA has been also used to form a controlled release medium for drugs and other bioactive agents. Controlled release of active agents is achieved during the degradation of PGA over time. In scaffold applications, a porous structure releasing growth factor for repair could be obtained with a simultaneous loss in mechanical properties [132]. Braunecker et al. studied PGA scaffolds with drug release ability and reported that decreasing the molecular weight in addition to the increasing the pore size and volume led to accelerated drug release [94].
Stents
Stents made of biodegradable polymers have been used as a support of the arterial wall only during vessel healing. These materials gradually transfer the mechanical load to the tissue while the stent mass and strength decrease over time without a necessity for a second surgery to remove the device. They also provide longer-term delivery of drug and/or gene therapy to the vessel wall from an internal reservoir [133]. PGA is one of the most frequently used materials for biodegradable stents [134, 135]. PGA has less strength, but faster degradation rates among the other biodegradable polymers. PGA has been successfully combined with other materials as copolymers/blends to improve its flexibility [136, 137]. These materials can degrade by simple hydrolysis of the ester bond in the polymer backbone [133]. In typical formulations, PGA degrades over a period of 6–12 months. Van der Giessen et al. have studied the effects of five different biodegradable polymers, including PGA and reported extensive inflammatory responses within the coronary arterial wall, which might be due to the parent polymer compound, additives of polymer, biodegradation products, the implant geometry or bacterial or nonbacterial contamination [137].
Sutures
The first absorbable polymeric surgical sutures were made of PGA and have been introduced in 1970’s. All polyesters are degradable however, aliphatic polyesters such as PGA, can degrade over time required for suture materials [16]. PGA has been widely used as suture materials due to its low melting temperature (>200 °C) and impressive tensile strength (≈12.5 GPa) [138, 139]. However, the degradation can occur faster than expected, as in the case of infected urogenital tract. The reason for this behavior was explained as that the bacterial enzymes digests urea and decreases the pH, leading an acceleration on the degradation rate of PGA suture [140].
In pulmonary surgery, non-woven fabric made of PGA is preferred instead of non-degradable materials since foreign body granuloma often occurs due to non-degradable polymers. To overcome these problems, PGA has been developed as a reinforcing agent for suturing native tissues [141]. However, Munteanu et al. diagnosed foreign body granuloma at PGA suture site after 10 months from resection of a cerebral glioblastoma [142]. Chu studied the effect of buffer on the degradation of PGA in terms of mechanical properties [63]. The results of this study indicated that the tensile strength of buffered PGA reduced, probably due to Na2HPO4, which increases hydrolysis and loss of strength. Besides, the buffers with lower pH presented higher degradation rates, as in the case of tied-chain segments of macromolecules theory. Chu and Campell analyzed the morphological changes of PGA sutures when exposed to different dosages of γ irradiation (0, 2.5, 5, 10, 20, and 40 Mrad) at different immersion days (0, 7, 14, 28, 48, 60, and 90 days) by using scanning electron microscopy (Fig. 7) [143]. The authors reported that the increase in γ irradiation caused an increase in surface cracks on the filaments with the suture hydrolytic degradation whereas there were no cracks on the irradiated suture surface when any hydrolytic degradation was not applied. Additionally, Chu studied the effect of γ irradiation (0–20 mrad) on the enzymatic (esterase, α-chymotrypsin, and trypsin) and in vivo degradation of PGA sutures [144]. These sutures were and implanted in inbred black-and-white hooded hister rats (Liverpool strain). Different from in vitro study results, implanted PGA sutures maintained slightly higher tensile strength after in vivo degradation.
SEM images of Dexon sutures subjected to 90, 14 and 40 days-degradation under in vitro conditions (PBS at pH = 7.4, 37 °C), respectively: a unirradiated 2–0 Dexon sutures, b 10 Mrad irradiated 2–0 Dexon sutures, c 20 Mrad irradiated 2–0 Dexon sutures. Adapted from [143]
Jang et al. prepared PGA mesh to prevent T postoperative pancreatic fistula (POPF) after distal pancreatectomy and tested its effect on 97 patients (aged 20–85 years) with curable benign, premalignant, or malignant disease of the pancreatic body or tail between, on November 2011 and April 2014 [145]. The authors applied PGA with fibrin glue followed by wrapping the PGA mesh around the remnant pancreatic stump. It was reported that the rate of clinically relevant POPF was significantly lower in the PGA group than in the control group with the help of wrapping of the cut surface of the pancreas with PGA mesh. It was also stated that there was no significant difference between the PGA and control groups by means of male to female ratio, malignancy, pancreatic duct diameter, soft pancreatic texture, and thickness of the transection margin parameters.
Tissue engineering
In recent years, PGA has been utilized as fillers combined with other degradable polymers and has been used in short-term tissue engineering scaffolds such as scaffold for bone [146,147,148,149], tendon [150, 151], tooth [152], cartilage [153, 154], vaginal [155], intestinal [74], lymphatic [156], and spinal regeneration [157]. The archetypal tissue engineering technology using a biodegradable synthetic polymer scaffold involves seeding cultured cells onto a preformed porous scaffold, which is chemically designed to degrade over time in the physiological environment [158]. In vascular applications, PGA is one of the most commonly used degradable polymer scaffolds. PGA–polyglactin copolymers seeded in stages with autologous fibroblasts, smooth muscle cells and endothelial cells have been the first attempt in an ovine model [159]. In this study, the scaffold had been completely degraded in 11 weeks under in vivo conditions, but the resulting tissues were not deemed suitable for implantation in the higher pressure systemic circulation [159]. In another study, PGA has been used with polyhydroxyalkanoate to augment the longer term mechanical properties of the construct [160]. On the other hand, Niklason et al. showed that bovine and porcine cell seeded PGA scaffolds implanted into a porcine model had very promising results. In this study has used a bioreactor system, which showed that these engineered vessels could remain patent for up to 4 weeks, and that application of pulsatile strain in vitro improved patency rates as well as graft morphology and function [161]. Other studies include the modification of PGA scaffold to guide cell function and better control the biological response [162,163,164]. On the other hand, the degradation products of these systems may have some negative effects such as changing the environment by decreasing the pH leading damage to nearby cells [165] or activating the inflammatory and immune responses [165]. Wang et al. reported that PGA degraded in vitro and changed the tissue reconstruction inducing fibrosis [166]. Aghdam et al. improved a polymer composite made of poly(ɛ-caprolactone) (PCL) and PGA for soft tissue engineering applications [167]. The authors found that PGA inclusion into PCL caused an increase in the average diameter of the nanofibers (Fig. 8). Thus, PGA increased the hydrophilicity and water uptake of the nanofibrous scaffolds while improving the mechanical properties of prepared scaffold with a potential for soft-tissue engineering applications.
SEM images of electrospun: a PCL, b PCL/PGA (80/20), c PCL/PGA (65/35), d PCL/PGA (50/50), and e PGA nanofibers [167]. Copyright 2012. Reproduced with permission from Wiley Online Library
Bailey et al. studied about a treatment for the temporomandibular joint (TMJ) disorders [168]. The authors used a spinner flask to seed PGA scaffolds with either TMJ condylar chondrocytes or mesenchymal-like stem cells derived from human umbilical cord matrix (HUCM). It was reported that HUCM constructs revealed higher amounts of collagen I and little amount of collagen II while TMJ constructs revealed little collagen I and no collagen II. This means that HUCM stem cells may therefore be an attractive alternative to condylar cartilage cells for TMJ tissue engineering applications. Weiser et al. utilized a model system based on 3 T3-L1 cells and tested this model for long term in vivo development of cellular constructs with varying stages of adipogenic development [169]. The authors used blank PGA fiber meshes, scaffolds seeded with uninduced 3 T3-L1 preadipocytes, and cell–polymer constructs precultivated under adipogenic conditions to implant subcutaneously into nude mice. It was reported that no fat formation occurred in constructs without adipogenic precultivation and implantation of mature fat pads resulted in adiponecrosis within the constructs. Besides, these engineered adipose tissues showed long-term survival over the period of 24 weeks.
Conclusions and future perspective
PGA is one of the most important biopolymer which has biocompatible, mechanically strong and biodegradable characteristics. The demand to PGA has an increasing trend with encouragement of environment-friendly polymer for packaging, increasing use of medical devices and extraction of shale gas. On the other hand, still, there is a few industrial producer of PGA due to extinction of cost-effective industrial scale production method. Thus, to make intense research activities, the main challenge is cost-effective production of high molecular weight PGA. Future studies will obviously focus on obtaining high molecular weight (Mn ≥ 45,000 g/mol) PGA with cost-efficient approaches. Different application areas and novel composites or processes will also be focus of the research in PGA in future.
References
Sharma A, Sharma G (2018) Biomaterials and their applications. AIP Conf Proc 1953:080041
Sionkowska A (2011) Progress in polymer science current research on the blends of natural and synthetic polymers as new biomaterials. Prog Polym Sci 36:1254–1276
Soni S, Gupta H, Kumar N, Nishad D, Mittal G, Bhatnagar A (2010) Biodegradable biomaterials. Recent Pat Biomed Eng 3:30–40
Nakafuku C, Yoshimura H (2004) Melting parameters of poly(glycolic acid). Polymer (Guildf) 45:3583–3585
Middleton JC, Tipton AJ (2000) Synthetic biodegradable polymers as orthopedic devices. Biomaterials 21(23):2335–2346
Lu Y, Schmidt C, Beuermann S (2015) Fast synthesis of high-molecular-weight Polyglycolide using Diphenyl bismuth bromide as catalyst. Macromol Chem Phys 216:395–399
Göktürk E, Pemba AG, Miller SA (2015) Polyglycolic acid from the direct polymerization of renewable C1 feedstocks. Polym Chem 6(21):3918–3925
Yamane K, Sato H, Ichikawa Y, Sunagawa K, Shigaki Y (2014) Development of an industrial production technology for high-molecular-weight polyglycolic acid. Polym J 46:769–775
(2019) Polyglycolic Acid (PGA) Market Research Report - Global Forecast till 2030. https://www.marketresearchfuture.com/reports/polyglycolic-acid-market-5749 (Accessed: August 2019)
Higgins NA (1954) Condensation polymers of hydroxyacetic acid. U.S. patent 2676945
Schmitt EE, Albert R, Chester P (1967) Surgical sutures. U.S. patent 3297033
May P, Polistina RA (1969) Process for polymerizing a Glycolide. U.S. patent 3442871
Reed AM, Road A (1980) Biodegradable polymers for use in surgery - poly(glycolic)/poly(lactic acid) homo and copolymers: 2. In vitro degradation Polymer (Guildf) 22:494–498
Ashammakhi N, Rokkanen P (1997) Absorbable polyglycolide devices in trauma and bone surgery. Biomaterials 18:3–9
Kehoe S, Zhang XF, Boyd D (2012) FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 43:553–572
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32:762–798
Suggs LJ, Moore SA, Mikos AG (2007) Synthetic biodegradable polymers for medical applications. In: Mark JE (ed) Physical properties of polymers handbook. Springer, New York, NY, pp 939–950
Jahno VD (2005) Síntese e caracterização do Poli (L-Ácido Láctico) para uso como biomaterial
Benatti ACB, Fla A, Xavier MV, et al (2019) Bioreabsorbable polymers for tissue engineering: PLA, PGA, and their copolymers. In: Holban AB, Grumezescu AM (eds) Materials for Biomedical Engineering. Elsevier, pp 83–116
Takahashi K, Taniguchi I, Miyamoto M, Kimura Y (2000) Melt/solid polycondensation of glycolic acid to obtain high-molecular-weight poly(glycolic acid). Polymer (Guildf) 41:8725–8728
Agrawal CM, Niederauer GG, Athanasiou KA (1995) Fabrication and characterization of PLA-PGA orthopedic implants. Tissue Eng 1:241–252
Sanko V, Sahin I, Aydemir Sezer U, Sezer S (2019) A versatile method for the synthesis of poly(glycolic acid): high solubility and tunable molecular weights. Polym J 51(7):637–647
Reyhanoglu Y, Sahmetlioglu E, Gokturk E (2019) Alternative approach for synthesizing Polyglycolic acid copolymers from C1 Feedstocks and fatty Ester epoxides. ACS Sustain Chem Eng 7:5103–5110
You Y, Youk JH, Lee SW, Min BM, Lee SJ, Park WH (2006) Preparation of porous ultrafine PGA fibers via selective dissolution of electrospun PGA/PLA blend fibers. Mater Lett 60:757–760
Kariduraganavar MY, Kittur AA, Kamble RR (2014) Polymer synthesis and processing. In: Laurencin CT, Deng M (eds) Sangamesh GK. Elsevier, Natural and Synthetic Biomedical Polymers, pp 1–31
Lowe CE (1954) Preparation of high molecular weight polyhydroxyacetic ester. U.S. patent 2668162
Zhaoyang W, Yaoming Z, Yurong Y et al (2004) Hecheng Xianwei Gongye. Hecheng Shuzhi Ji Suliao/China Synth Resin Plast 27:1–20
Fumiaki I, Mineo K, Masahiro O, et al (1994) Process for preparing polyhydroxycarboxylic acid. U.S. patent 5440008
Kameoka R, Higuchi C, Ajioka M, et al (1995) Aliphatic polyester and preparation process thereof. U.S. patent 5428126
Yoshida Y, Miyamoto M, Obuchi S, et al (1998) Preparation process of polyhydroxycarboxylic acid. U.S. patent 5770683
Singh V, Tiwari M (2010) Structure-processing-property relationship of poly (glycolic acid) for drug delivery systems 1: synthesis and catalysis. Int J Polym Sci 2010:1–23
Obuchi S, Elias HG (1995) Preparation process of polyhydroxycarboxylic acid. U.S. patent 5444143
Enomoto K, Ajioka M, Yamaguchi A (1994) Preparation process of polyhydroxycarboxylic acid thereof. U.S. patent 5310865
Masuda T, Kagami K, Murata K et al (1982) Copolymerizatıon of carbon-monoxide with formaldehyde using Trioxane or paraformaldehyde as a formaldehyde source in the presence of the Chlorosulfuric acid catalyst. Nippon Kagaku Kaishi 2:257–262
Ishihara K, Ohara S, Yamamoto H (2000) Direct Polycondensation of carboxylic acids and amines catalyzed by 3,4,5-Trifluorophenylboronic acid. Macromolecules 33:3511–3513
Kataoka M, Sasaki M, Hidalgo AGD, Nakano M (2001). Glycolic Acid Production Using Ethylene Glycol- Oxidizing Microorganisms 65(10):2265–2270
Buchholz B (1994) Process for preparing polyesters based on hydroxycarboxylic acids. U.S. patent 5302694
Bonsignore PV (1995) Production of high molecular weight polylactic acid. U.S. patent 5470944
Herzberg O, Epple M (2001) Formation of polyesters by thermally induced polymerization reactions of molecular solids some background information on polyesters. Eur J Inorg Chem 2001(6):1395–1406
Epple M, Herzberg O (1997) Polyglycolide with controlled porosity : an improved biomaterial. J Mater Chem 7:1037–1042
Pinkus A, Subramanyam R, Pinkus AG, Subramanyam R (1984) New high-yield, one-step synthesis of polyglycolide from haloacetic acids. J Polym Sci Polym Chem Ed 22:1131–1140
Xiguang D, Li C, Chen Q, Xuesi C (2003) Dongbei Shida Xuebao. Dongbei Shida Xuebao / Ziran Kexue Ban 35:119
Schmidt C, Behl M, Beuermann S (2014) RSC advances synthesis of high molecular weight polyglycolide in supercritical carbon dioxide. Macromol Chem Phys 4:35099–35105
Shen K, Yang S (2013) Preparation of high-molecular-weight poly (glycolic acid) by direct melt polycondensation from glycolic acid. Adv Mater Res 821:1023–1026
Leenslag JW, Pennings AJ (1987) Synthesis of high-molecular-weight poly( clactide) initiated with tin 2-ethylhexanoate. Die Makromol Chemie Macromol Chem Phys 188:1809–1814
Nieuwenhuis J (1992) Synthesis of polylactides, polyglycolides and their copolymers. Clin Mater 10(1):59–67
Dawkins J V (1989) Aqueous suspension polymerization in comprehensive polymer science. In: Pergamon press, Oxford. Pp 231
Boehringer A, Liebrecht I, Liebrecht J LW (1957) Improvements in or relating to the polymerization of cyclic esters. U.S. patent 825335
Chujo K, Kobayashi H, Suzuki J, Tokuhara S, Tanabe M (1967) Ring-opening polymerization of glycolide. Die Makromol Chemie Macromol Chem Phys 100:262–266
Bourissou D, Martin-Vaca B, Dumitrescu A, Graullier M, Lacombe F (2005) Controlled cationic polymerization of lactide. Macromolecules 38:9993–9998
Reyhanoglu Y, Gokturk E (2019) Polyglycolic acid copolymers from one-step cationic polymerization of formaldehyde, carbon monoxide, and epoxides derived from PEG. Polym Adv Technol 30:1789–1795
Albertsson AC, Varma IK (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4:1466–1486
Jedlinski Z, Kurcok P, Kowalczuk M (1985) Polymerization of. Beta.-lactones initiated by potassium solutions. Macromolecules 18:2679–2683
Murugan KD, Radhika S, Baskaran I, Anbarasan R (2008) Clay catalyzed synthesis of bio-degradable poly (glycolic acid). Chinese J Polym Sci 26:393–398
Gharbi REL, Fradet A, Dali S (2006) Synthesis of poly (glycolic acid) in ionic liquids. J Polym Sci Part A Polym Chem 44:3025–3035
Epple M, Kirschnick H (1996) The thermally induced solid-state polymerization reaction in Halogenoacetates. Chem Ber 129:1123–1129
Amass W, Amass A, Tighe B (1998) A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym Int 47:89–144
Yu C, Bao J, Xie Q, Shan G, Bao Y, Pan P (2016) Crystallization behavior and crystalline structural changes of poly(glycolic acid) investigated: via temperature-variable WAXD and FTIR analysis. Cryst Eng Comm 18:7894–7902
Agrawal A, Saran AD, Rath SS, Khanna A (2004) Constrained nonlinear optimization for solubility parameters of poly (lactic acid) and poly (glycolic acid)—validation and comparison. Polymer (Guildf) 45:8603–8612
Van de Velde K, Kiekens P (2002) Biopolymers: overview of several properties and consequences on their applications. Polym Test 21:433–442
Kister G, Cassanas G, Vert M (1997) Morphology of poly(glycolic acid) by IR and Raman spectroscopies. Spectrochim Acta - Part A Mol Biomol Spectrosc 53:1399–1403
Chatani Y, Suehiro K, Ôkita Y, Tadokoro H, Chujo K (1968) Structural studies of polyesters. I. Crystal structure of polyglycolide. Die Makromol Chemie Macromol Chem Phys 113:215–229
Chu CC (1981) The in-vitro degradation of poly (glycolic acid) sutures—effect of pH. J Biomed Mater Res 15:795–804
Williams DF, Mort E (1977) Enzyme-accelerated hydrolysis of polyglycolic acid. J Bioeng 1:231–238
Li S (2016) Synthetic biodegradable medical polyesters. In: Zhang X (ed) science and principles of biodegradable and Bioresorbable medical polymers: materials and properties. Woodhead publishing, pp 37–70
Kumbar S, Laurencin C, Deng M (2014) Natural and synthetic biomedical polymers. Elsevier, USA
Farah S, Anderson DG, Langer R (2016) Physical and mechanical properties of PLA, and their functions in widespread applications — a comprehensive review. Adv Drug Deliv Rev 107:367–392
Lin CC (1983) The rate of crystallization of poly(ethylene terephthalate) by differential scanning Calorimetry. Polym Eng Sci 23:113–116
Engelberg I, Kohn J (1991) Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials 12:292–304
Ueda H, Tabata Y (2003) Polyhydroxyalkanonate derivatives in current clinical applications and trials. Adv Drug Deliv Rev 55:501–518
Ulery BD, Nair LS, Laurencin CT (2011) Biomedical applications of biodegradable polymers. J Polym Sci Part B Polym Phys 49:832–864
Gunatillake P, Mayadunne R, Adhikari R (2006) Recent developments in biodegradable synthetic polymers. Biotechnol Annu Rev 12:301–347
Pätilä T, Jokinen JJ, Salminen J, Kankuri E, Harjula A (2008) Polyglycolic acid glue does not prevent Intrapericardial adhesions in a short-term follow-up. J Surg Res 148:181–184
Aysan E, Bektas H, Ersoz F et al (2010) A novel colonic anastomosis technique involving fixed polyglycolic acid mesh. Int J Clin Exp Med 3:341
Caballé-Serrano J, Munar-Frau A, Delgado L, Pérez R, Hernández –Alfaro F (2019) Physicochemical characterization of barrier membranes for bone regeneration. J Mech Behav Biomed Mater 97:13–20
Wang J, Wang L, Zhou Z, Lai H, Xu P, Liao L, Wei J (2016) Biodegradable polymer membranes applied in guided bone/tissue regeneration: a review. Polymers (Basel) 8:115
Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K (2013) Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res 57:3–14
Scantlebury TV (1993) 1982-1992: a decade of technology development for guided tissue regeneration. J Periodontol 64:1129–1137
Caffesse RG, Mota LF, Quiñones CR, Morrison EC (1997) Clinical comparison of resorbable and non-resorbable barriers for guided periodontal tissue regeneration. J Clin Periodontol 24:747–752
Gentile P, Chiono V, Tonda-Turo C, Ferreira AM, Ciardelli G (2011) Polymeric membranes for guided bone regeneration. Biotechnol J 6:1187–1197
Hutmacher D, Hürzeler MB, Schliephake H (1996) A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int J Oral Maxillofac Implants 11:667–668
Dimitriou R, Mataliotakis GI, Calori GM, Giannoudis PV (2012) The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Med 10:81
Isogai N, Landis W, Kim TH et al (1999) Formation of phalanges and small joints by tissue-engineering. JBJS 81:306–316
McVicar I, Hatton PV, Brook IM (1995) Self-reinforced polyglycolic acid membrane: a bioresorbable material for orbital floor repair. Initial clinical report. Br J Oral Maxillofac Surg 33:220–223
Törmälä P (1992) Biodegradable self-reinforced composite materials; manufacturing structure and mechanical properties. Clin Mater 1:29–34
Semwal R, Semwal RB, Semwal DK (2017) Drug delivery systems: selection criteria and use. Concise Encycl Biomed Polym Polym Biomater 439–450
Mao HQ, Kdaiyala I, Leong KW et al (1999) Biodegradable polymers: polyesters. In: Mathiowitz E (ed) Encyclopedia of controlled drug delivery. John Wiley and Sons, New York, NY, pp 45–60
Langer R (2000) Biomaterials in drug delivery and tissue engineering: one laboratory’s experience. Acc Chem Res 33:94–101
Li S, Vert M (1999) Biodegradable polymers: Polyesters. Montpellier, France
Armentano I, Dottori M, Fortunati E, Mattioli S, Kenny JM (2010) Biodegradable polymer matrix nanocomposites for tissue engineering: a review. Polym Degrad Stab 95:2126–2146
Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M (2004) Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release 98:47–56
Pan H, Jiang H, Chen W (2006) Interaction of dermal fibroblasts with electrospun composite polymer scaffolds prepared from dextran and poly lactide-co-glycolide. Biomaterials 27:3209–3220
Joshi J a YR, Patel RP (2012) Role of biodegradable polymers in drug delivery. Int J Curr Pharm Res 4:74–81
Braunecker J, Baba M, Milroy GE, Cameron RE (2004) The effects of molecular weight and porosity on the degradation and drug release from polyglycolide. Int J Pharm 282:19–34
Moll F, Ries R (1991) Biodegradable microtablets made of low molecular weight polyglycolic acid: Bioabbaubare Mikrotabletten aus niedermolekularer Polyglycolsäure. Arch Pharm (Weinheim) 324:939–940
Hurrell S, Cameron RE (2003) The effect of buffer concentration, pH and buffer ions on the degradation and drug release from polyglycolide. Polym Int 52:358–366
Hurrell S, Cameron RE (2002) The effect of initial polymer morphology on the degradation and drug release from polyglycolide. Biomaterials 23:2401–2409
Hurrell S, Milroy GE, Cameron RE (2003) The distribution of water in degrading polyglycolide. Part I: Sample size and drug release J Mater Sci Mater Med 14:457–464
Milroy GE, Cameron RE, Mantle MD, Gladden LF, Huatan H (2003) The distribution of water in degrading polyglycolide. Part II: magnetic resonance imaging and drug release. J Mater Sci Mater Med 14:465–473
Sheikh Z, Najeeb S, Khurshid Z, Verma V, Rashid H, Glogauer M (2015) Biodegradable materials for bone repair and tissue engineering applications. Materials (Basel) 8:5744–5794
Dahlin C, Sennerby L, Lekholm U et al (1989) Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants 4:33–44
Schumann P, Lindhorst D, Wagner MEH, Schramm A, Gellrich NC, Rücker M (2013) Perspectives on resorbable osteosynthesis materials in craniomaxillofacial surgery. Pathobiology 80:211–217
Van Bakelen NB, Buijs GJ, Jansma J et al (2014) Decision-making considerations in application of biodegradable fixation systems in maxillofacial surgery - a retrospective cohort study. J Cranio-Maxillofacial Surg 42:417–422
Kanno T, Sukegawa S, Furuki Y, Nariai Y, Sekine J (2018) Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn Dent Sci Rev 54:127–138
Vasenius J, Vainionpää S, Vihtonen K, Mäkelä A, Rokkanen P, Mero M, Törmälä P (1990) Comparison of in vitro hydrolysis, subcutaneous and intramedullary implantation to evaluate the strength retention of absorbable osteosynthesis implants. Biomaterials 11:501–504
Okuyama K, Yanamoto S, Naruse T, Sakamoto Y, Rokutanda S, Ohba S, Asahina I, Umeda M (2018) Clinical complications in the application of polyglycolic acid sheets with fibrin glue after resection of mucosal lesions in oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol 125:541–546
Cipurković A, Horozić E, Đonlagić N et al (2018) Biodegradable polymers: production, properties and application in medicine. Technol Acta 11:25–35
Chung C, Ki D, Park Y et al (1997) Biological effects of drug-loaded biodegradable membranes for guided bone regeneration. J Periodontal Res 32:172–175
Mooney DJ, Mazzoni CL, Breuer C, et al (1996) Stabilized polyglycolic acid fibre-based tubes for tissue engineering. In: Williams DF (ed) The biomaterials: silver Jubilee Compendium. Elsevier, pp 129–138
Li S (2017) Synthetic biodegradable medical polyesters. In: Zhang X (ed) Science and principles of biodegradable and Bioresorbable medical polymers. Elsevier, pp 37–78
Okamoto T, Rosini KS, Miyahara GI, Gabrielli MF (1994) Healing process of the gingival mucosa and dental alveolus following tooth extraction and suture with polyglycolic acid and polyglactin 910 threads. Comparative histomorphologic study in rats. Braz Dent J 5:35–43
Mizutani T, Nakayama A, Iwasaki H, Miyazawa H (2012) Suitability of polymers as screw post materials in primary teeth: an in vitro study. Eur J Paediatr Dent 13:19
Nagatomi J (2006) Mechanical adaptation of bone: bioreactors for orthopedic tissue engineering applications. In: Salz U (ed) Shalaby SW. CRC Press, Polymers for Dental and Orthopedic Applications, pp 351–367
Frölke JPM, Nulend JK, Semeins CM et al (2004) Viable osteoblastic potential of cortical reamings from intramedullary nailing. J Orthop Res 22:1271–1275
Agrawal CM (2002) Biodegradable polymers for orthopaedic applications. In: Reis RL, Cohn D (eds) Polymer based systems on tissue engineering. Replacement and Regeneration. Springer, Dordrecht, pp 25–36
Borden M, Attawia M, Laurencin CT (2002) The sintered microsphere matrix for bone tissue engineering: in vitro osteoconductivity studies. J Biomed Mater Res 61:421–429
Borden M, Attawia M, Khan Y et al (2004) Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J Bone Jt Surgery Br Vol 86:1200–1208
Mikos AG, Sarakinos G, Leite SM et al (1993) Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomater Silver Jubil Compend 14:323–330
Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, Vacanti JP (1994) Preparation and characterization of poly(l-lactic acid) foams. Polymer (Guildf) 35:1068–1077
Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT (2005) Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials 26:1523–1532
Lu HH, Cooper JA, Manuel S et al (2005) Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials 26:4805–4816
Cohen SB, Meirisch CM, Wilson HA, Diduch DR (2003) The use of absorbable co-polymer pads with alginate and cells for articular cartilage repair in rabbits. Biomaterials 24:2653–2660
Mikos AG, Bao Y, Cima LG, Ingber DE, Vacanti JP, Langer R (1993) Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J Biomed Mater Res 27:183–189
Moran JM, Pazzano D, Bonassar LJ (2003) Characterization of polylactic acid-polyglycolic acid composites for cartilage tissue engineering. Tissue Eng 9:63–70
Ma PX, Langer R (1995) Degradation, structure and properties of fibrous nonwoven poly(glycolic acid) scaffolds for tissue engineering. Mater Res Soc Symp - Proc 394:99–104
Aydin HM (2011) A three-layered osteochondral plug: structural, mechanical, and in vitro biocompatibility analysis. Adv Eng Mater 13(12):511–517
Liu H, Slamovich EB, Webster TJ (2006) Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int J Nanomedicine 1:541–545
Hosseini V, Evrova O, Hoerstrup SP, Vogel V (2018) A simple modification method to obtain anisotropic and porous 3D microfibrillar scaffolds for surgical and biomedical applications. Small 14:1702650
Kodama S, Kojima K, Furuta S, Chambers M, Paz AC, Vacanti CA (2009) Engineering functional islets from cultured cells. Tissue Eng A 15:3321–3329
Pina S, Ferreira JMF (2012) Bioresorbable plates and screws for clinical applications: a review. J Healthc Eng 3:243–260
Vainionpää S, Kilpikari J, Laiho J, Helevirta P, Rokkanen P, Törmälä P (1987) Strength and strength retention vitro, of absorbable, self-reinforced polyglycolide (PGA) rods for fracture fixation. Biomaterials 8:46–48
Thompson DE, Agrawal CM, Athanasiou K (1996) The effects of dynamic compressive loading on biodegradable implants of 50–50% polylactic acid–polyglycolic acid. Tissue Eng 2:61–74
Eberhart RC, Su S-H, Nguyen KT, Zilberman M, Tang L, Nelson KD, Frenkel P (2003) Bioresorbable polymeric stents: current status and future promise. J Biomater Sci Polym Ed 14:299–312
Labinaz M, Zidar JP, Stack RS, Phillips HR (1995) Biodegradable stents: the future of interventional cardiology? J Interv Cardiol 8:395–405
Colombo A, Karvouni E (2000) Biodegradable Stents: “Fulfilling the Mission and Stepping Away.” Circulation 102:371–374
Tanguay JF, Zidar JP, Phillips HR, Stack RS (1994) Current status of biodegradable stents. Cardiol Clin 12:699–713
Van der Giessen WJ, Lincoff AM, Schwartz RS et al (1996) Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation 94:1690–1697
Maurus PB, Kaeding CC (2004) Bioabsorbable implant material review. Oper Tech Sports Med 12:158–160
Pillai CKS, Sharma CP (2010) Absorbable polymeric surgical sutures: chemistry, production, properties, biodegradability, and performance. J Biomater Appl 25:291–366
Tomihata K, Suzuki M, Ikada Y (2001) The pH dependence of monofilament sutures on hydrolytic degradation. J Biomed Mater Res 58:511–518
Nakamura T, Shimizu Y, Watanabe S, Hitomi S, Kitano M, Tamada J, Matsunobe S (1990) New bioabsorbable pledgets and non-woven fabrics made from polyglycolide (PGA) for pulmonary surgery: clinical experience. Thorac Cardiovasc Surg 38:81–85
Munteanu R, Eva L, Dobrovăţ B et al (2017) Longer survival of a patient with glioblastoma resected with 5-aminolevulinic acid (5-ALA)-guided surgery and foreign body reaction to polyglycolic acid (PGA) suture. Romanian J Morphol Embryol 58:671–680
Chu CC, Campbell ND (1982) Scanning electron microscopic study of the hydrolytic degradation of poly (glycolic acid) suture. J Biomed Mater Res 16:417–430
Chu CC, Williams DF (1983) The effect of gamma irradiation on the enzymatic degradation of polyglycolic acid absorbable sutures. J Biomed Mater Res 17:1029–1040
Jang J-Y, Shin YC, Han Y, Park JS, Han HS, Hwang HK, Yoon DS, Kim JK, Yoon YS, Hwang DW, Kang CM, Lee WJ, Heo JS, Kang MJ, Chang YR, Chang J, Jung W, Kim SW (2017) Effect of polyglycolic acid mesh for prevention of pancreatic fistula following distal pancreatectomy: a randomized clinical trial. JAMA Surg 152:150–155
Knecht S, Erggelet C, Endres M et al (2007) Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J Biomed Mater Res Part B Appl Biomater 83:50–57
Wang L, Dormer NH, Bonewald LF, Detamore MS (2010) Osteogenic differentiation of human umbilical cord mesenchymal stromal cells in polyglycolic acid scaffolds. Tissue Eng - Part A 16:1936–1948
Dunne N, Jack V, O’Hara R, Farrar D, Buchanan F (2010) Performance of calcium deficient hydroxyapatite–polyglycolic acid composites: an in vitro study. J Mater Sci Mater Med 21:2263–2270
Pihlajamäki HK, Salminen ST, Tynninen O, Böstman OM, Laitinen O (2010) Tissue restoration after implantation of polyglycolide, polydioxanone, polylevolactide, and metallic pins in cortical bone: an experimental study in rabbits. Calcif Tissue Int 87:90–98
Pihlajamäki H, Tynninen O, Karjalainen P, Rokkanen P (2007) The impact of polyglycolide membrane on a tendon after surgical rejoining. A histological and histomorphometric analysis in rabbits J Biomed Mater Res - Part A 81:987–993
Xu L, Cao D, Liu W, Zhou G, Zhang WJ, Cao Y (2010) In vivo engineering of a functional tendon sheath in a hen model. Biomaterials 31:3894–3902
Ohara T, Itaya T, Usami K et al (2010) Evaluation of scaffold materials for tooth tissue engineering. J Biomed Mater Res - Part A 94:800–805
Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW (2009) In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med 37:71–80
Mahmoudifar N, Doran PM (2010) Chondrogenic differentiation of human adipose-derived stem cells in polyglycolic acid mesh scaffolds under dynamic culture conditions. Biomaterials 31:3858–3867
Sayasneh A, Johnson H (2010) Risk factors for mesh erosion complicating vaginal reconstructive surgery. J Obstet Gynaecol (Lahore) 30:721–724
Dai T ting, Jiang Z hua, Li S li, et al (2010) Reconstruction of lymph vessel by lymphatic endothelial cells combined with polyglycolic acid scaffolds: a pilot study. J Biotechnol 150:182–189
Abbushi A, Endres M, Cabraja M, Kroppenstedt SN, Thomale UW, Sittinger M, Hegewald AA, Morawietz L, Lemke AJ, Bansemer VG, Kaps C, Woiciechowsky C (2008) Regeneration of intervertebral disc tissue by resorbable cell-free polyglycolic acid-based implants in a rabbit model of disc degeneration. Spine (Phila Pa 1976) 33:1527–1532
Vacanti CA, Vacanti JP, Langer R (1993) Tissue engineering using synthetic biodegradable polymers. In: Ikada Y, Langer R, Williams J (eds) Shalaby. WS. ACS Publications, Polymers of Biological and Biomedical Significance, pp 16–34
Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, Vacanti JP, Mayer Jr JE (1998) Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg 115:536–546
Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, Stamp A, Taylor G, Moran AM, Landis W, Langer R, Vacanti JP, Mayer Jr JE (1999) Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg 68:2298–2304
Niklason LE, Gao J, Abbott WM, et al (1999) Functional arteries grown in vitro. Science (80- ) 284:489–493
Nikolovski J, Mooney DJ (2000) Smooth muscle cell adhesion to tissue engineering scaffolds. Biomaterials 21:2025–2032
Kim B-S, Nikolovski J, Bonadio J, Smiley E, Mooney DJ (1999) Engineered smooth muscle tissues: regulating cell phenotype with the scaffold. Exp Cell Res 251:318–328
Kim B-S, Nikolovski J, Bonadio J, Mooney DJ (1999) Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol 17:979–983
Agrawal CM, Athanasiou KA (1997) Technique to control pH in vicinity of biodegrading PLA-PGA implants. J Biomed Mater Res 38:105–114
Wang Y, Wang W, Wang X, Wang Y, Wang J, Fu Q, Shi G (2017) Tissue-engineered sling with adipose-derived stem cells under static mechanical strain. Exp Ther Med 14:1337–1342
Aghdam RM, Najarian S, Shakhesi S, Khanlari S, Shaabani K, Sharifi S (2012) Investigating the effect of PGA on physical and mechanical properties of electrospun PCL/PGA blend nanofibers. J Appl Polym Sci 124:123–131
Bailey MM, Wang L, Bode CJ, Mitchell KE, Detamore MS (2007) A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng 13:2003–2010
Weiser B, Prantl L, Schubert TEO, Zellner J, Fischbach-Teschl C, Spruss T, Seitz AK, Tessmar J, Goepferich A, Blunk T (2008) In vivo development and long-term survival of engineered adipose tissue depend on in vitro precultivation strategy. Tissue Eng - Part A 14:275–284
Acknowledgments
We gratefully acknowledge the financial support by Suleyman Demirel University BAP (TSG-2018-6749 Project).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Budak, K., Sogut, O. & Aydemir Sezer, U. A review on synthesis and biomedical applications of polyglycolic acid. J Polym Res 27, 208 (2020). https://doi.org/10.1007/s10965-020-02187-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02187-1