Abstract
Cellulose grafted with 2-acrylamido-2-methylpropane sulfonic acid (AASO3H) and binary comonomer acrylonitrile (AN) were synthesized and characterized by FTIR, XRD, SEM, TGA/DTA and swelling studies. Adsorption parameters like contact time, temperature, pH and concentration were optimized for maximum adsorption of organic dyes and Pb(II) ions. Dye adsorption behavior studied for cationic malachite green (MG) and crystal violet (CV) dyes followed the order Cell-g-AASO3H > Cell-g-AASO3H-co-AN > cellulose. Order for adsorption of dyes were reported as MG > CV. Pseudo-second-order model is best-fitted with the experimental data and adsorption capacity from Langmuir adsorption isotherm for MG, and CV were 61.46 and 55.71 mg.g−1 respectively, for Cell-g-AASO3H. Percent uptake capacities followed the order Cell-g-AASO3H-co-AN > Cell-g-AASO3H > cellulose for Pb(II) ions. Maximum adsorption capacities for Pb(II) ions from Langmuir adsorption isotherm were 154.32 and 181.49 mg.g−1 for Cell-g-AASO3H and Cell-g-AASO3H-co-AN, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The release of highly toxic and poisonous dyes and heavy metal ions from the textile, dyeing, printing, food, pharmaceutical, cosmetic, and paint industries into the water bodies is of major concern nowadays. Organic dyes are generally non-biodegradable and accumulate in the water bodies. Due to strongly visible light absorption properties, these dyes increase the biological and chemical oxygen demand, change the pH and color of the water, which drastically affect the growth of flora and fauna [1]. It has been reported that these dyes have highly adverse carcinogenic, mutagenic, cytotoxic and genotoxic effect in addition to less drastic skin disease, allergic reactions and respiratory problems [2]. Photo-catalytic degradation, sonochemical degradation, biodegradation, electrochemical degradation, chemical coagulation-flocculation, chemical precipitation, ultrafiltration, adsorption on activated charcoal/carbon have been reported as traditional methods for the removal of toxic dyes from aqueous solution [3]. Among all the reported techniques for the removal of toxic dyes from the industrial effluent, solid phase extraction have been a benchmark due to its low cost, easy applicability, and recyclability [4]. The need of the hour is to thus develop highly efficient biodegradable super-absorbent materials for wastewater treatment applications.
Cellulose is a pre-eminent biopolymer comprising β-D- anhydroglucopyranose units linked through covalent C1-C4 β-glycosidic linkages to form long straight chain fibrous macromolecules. The composition of this incredible biopolymer in different lignocellulosic biomasses varies from species to species. Different physical, chemical, biological, and physicochemical methods for the extraction of pure cellulose, from lignocellulosic biomass, are reported and well reviewed in the literature [5,6,7,8]. Due to the presence of three hydroxyl groups (–OH) at C2, C3 and C6 atoms of each β-D- anhydroglucopyranose units of cellulose, its properties can further be improved, either through chemical modification [9, 10] or through the formation of more significant graft cellulose copolymers [11]. Graft copolymers of cellulose have neen reported for heavy metal ion sorption from industrial effluents [12,13,14], dye adsorption from textile industry [15,16,17], controlled drug delivery [18, 19], enzyme immobilization [20], antimicrobial food packaging materials [21, 22] and, component modification of secondary Li-ion batteries [23]. The performance of graft copolymers of cellulose is highly dependent upon the degree of crystallinity, specific surface area, the degree of polymerization of the grafted monomers onto cellulose, the nature of the functional groups present on the grafted monomers and co-monomers, hydrophilicity and hydrophobicity etc.
Malachite green (MG) and crystal violet (CV) dyes are triarylmethane cationic dyes, widely used in as histological stain. Both the dyes are water-soluble and non-biodegradable in nature which pose serious carcinogenic, mutagenic, and cytotoxic effects on mammalian cells. Many cellulose based adsorbents have been reported in the literature for the adsorption of these toxic dyes. M. Ghaemy et al. reported the preparation of adsorbent nanoparticles based on treated low-value agricultural by-product rice husk and poly(methylmethacrylate-co-maleic anhydride) for the removal of CV dye from water [24]. S. Chowdhury and coworkers developed low-cost adsorbents from NaOH-modified rice husk for removal of CV [25] and MG dyes [26]. Cellulose modified with glycidyl methacrylate and sulfosalicylic acid was reported as a better adsorbent of CV dye than native cellulose [27]. Cellulose modified with maleic and phthalic anhydride were synthesized and reported better adsorbents than pristine cellulose for the removal of MG from aqueous solution by Zhou et al. [28]. Zheng et al. [29] and Ruan et al. [30] removed CR dye using composite thin files and beads, respectively composed of chitosan and 2,3-dialdehyde cellulose. Wang et al. synthesized polyacrylamide grafted quaternized cellulose to remove Congo red and Eriochrome blue SE anionic dyes from aqueous solution [31]. Carboxylated cellulose nanocrystals were also reported for the effective adsorption of cationic dyes like CV, MG and methylene blue [32].
Chauhan and coworkers have developed grafted cellulose extracted from forest waste pine needles with various vinyl monomers to develop hydrogels which were sensitive to change in temperature, pH, ionic strength and selectivity to metal ions [33, 34]. Interpenetrating networks based on extracted cellulose grafted with methacrylamide in presence of N,N-methylene bisacrylamide were synthesized and used in sorption of Fe(II), Cu(II) and Cr(VI) ions [35]. Cellulosic adsorbent were synthesized by γ-initiated grafting of poly(glycidyl methacrylate) onto cellulose filter paper for Cd(II), Pb(II) and Cu(II) removal from aqueous media [36]. Natural cellulosic fibers like cotton grafted with glycidyl methacrylate and methacrylic acid using gamma irradiation were used for sorption of Co(II) ions [37]. Cellulose cotton fibers were also modified through graft copolymerization of polyacrylonitrile for removal and extraction of Au(III), Pd(II) and Ag(I) precious metal ions [38]. Cellulose grafted with 2-hydroxy methacrylate and its binary monomer mixtures with acrylamide, acrylic acid, and acrylonitrile were investigated for sorption of Fe(II), Cu(II) and Cr(VI) ions [39]. Hajeeth and et al. reported the extraction of cellulose from sisal fiber and grafted it with acrylic acid to develop adsorbent for Cu(II) and Ni(II) ions [40] and grafted acrylonitrile for sorption of Cr(VI) ions from aqueous solution [41]. Grafting of acrylic acid and 2-acrylamido-2- methyl propane sulphonic acid onto cellulose in the presence of crosslinking agent of N,N-methylene bisacrylamide were found to be selective for the removal of Pb(II) over Cu(II) and Cd(II) ions [42]. Cellulose was extracted from peanut hulls, soybean shells and grape fruit peels and then copolymerized with epichlotohydrin and ethylenediamine used as potential low-cost and high efficient adsorbents for removal of lead ions from wastewater [43].
2-Acrylamido-2-methylpropane sulfonic acid (AASO3H) is a hydrophilic, sulfonic acid acrylic monomer and consists of geminal dimethyl substituents. The protonation – deprotonation properties (\( -{SO}_3H\to -{SO}_3^{-} \)) of the sulfonic acid (-SO3H) moiety in the medium makes it an attractive site for the adsorption of a variety of adsorbate materials including toxic metal ions and hazardous non-biodegradable dyes. The hydrophilic nature of the monomer also makes it a suitable candidate to perform its grafting on to natural polysaccharides like cellulose, starch, chitin, and chitosan etc. in non toxic aqueous media. Further, acrylonitrile (AN) is also a vinyl monomer with a nitrile (-C ≡ N) pendent group. Un-hydrolyzed -C ≡ N group as well as amide group (-CONH2), formed after the acidic or alkaline hydrolysis act as potential sites for the attraction of different varieties of adsorbates similar to AASO3H.
In context to properties of AASO3H and AN copolymers, herein we reported a fast, facile and low-cost synthesis of graft copolymers of AASO3H and its binary co-monomer mixture with AN onto cellulose extracted from rice husk. >The morphological, structural, compositional, thermal, and vibrational properties of the synthesized materials were characterized by different characterization techniques. Graft copolymers were investigated for their potential applications for adsorption of MG, CV dyes and Pb(II) ions from aqueous solution.
Materials and methods
Materials
For the extraction of cellulosic fibers, agricultural residue, rice husk was purchased from a local rice mill. For the extraction and chlorine-free bleaching of the cellulose, sodium peroxide (Na2O2), hydrogen peroxide (H2O2), ethanol, dimethylformamide (DMF), acetone and sodium hydroxide were purchased from CDH fine chemicals, India. 2-acrylamido-2-methylpropane sulfonic acid (AASO3H; HiMedia Lab. Pvt., Ltd. Mumbai, India), potassium persulfate (KPS, K2S2O8; Paskem Fine Chemicals, India), N,N′-Methylenebisacrylamide (N,N′-MBA; HiMedia Lab. Pvt., Ltd. Mumbai, India) were purchased for the synthesis of cellulose grafted copolymer. Co-monomer AN was supplied by SD Fine Chemicals Ltd., Mumbai, India. All the chemicals were of analytical grade and used as received without any further purifications. MG and CV dyes were purchased from Loba Chemicals, India. For sorption studies of Pb(II) ions, Pb(NO3) was purchased from SD Fine, India. All the solutions for batch experiments were prepared in ultrapure water obtained from BIO-AGE Water Purifier System, model Direct Ultra TUVF-5.
Extraction of cellulose from rice husk
A greener, total chlorine free and ultrasonic physicochemical process of extraction and purification of cellulose from rice husk, as explained by Sharma et al. [44], was employed. Briefly, the initial step involves the treatment of the rice husk with a concentrated aqueous alkali solution for the duration of 6 h at 80 °C in round bottom flask in order to completely dissolve lignin and silica. After alkali pretreatment, the product obtained was thoroughly washed with distilled water, filtered and dried. The dried product was subjected to mechanical treatment followed by bleaching. A sonolytic technique was applied for the bleaching of cellulose using Na2O2/H2O2 in alkaline medium, as a chlorine-free bleaching agent.
Synthesis of cellulose based graft copolymers
Optimization of the different grafting reaction conditions for AASO3H onto 1 g of extracted cellulose in the presence of N,N′-MBA cross-linker was carried out by KPS initiated free radical mechanism. The grafting reaction scheme was designed in such a way that one reaction parameter was varied for a set of grafting reactions keeping the other reaction parameters constant (Table 1). Various grafting reaction parameters optimized to obtain maximum graft yield were nature of the solvent, solvent volume, monomer concentration, cross-linker concentration, initiator concentration, grafting temperature and reaction time. Methanol-acetone mixture was used as an extraction solvent for the separation of graft copolymers and homopolymers of AASO3H. The percent grafting (Pg) and percent grafting efficiency (GE) were calculated using Eqs. 1 and 2, respectively [45].
Where, Wo = Weight of ungrafted cellulose, W1 = Weight of monomer, and W2 = Weight of grafted copolymer.
At the optimized grafting reaction conditions for grafting of AASO3H onto cellulose binary comonomer, AN was grafted at five different concentrations (Table 2). Homopolymers poly(AN) were removed by using DMF solvent.
Characterization of graft copolymers
Characterization of pristine cellulose, Cell-g-AASO3H, and Cell-g-AASO3H-co-AN for structural, morphological, crystalline, transmittance and thermal properties was carried out by different techniques. Morphological and structural properties were studied by field emission scanning electron microscopic (SEM) analysis (Jeol, JSM-6100 at an accelerating voltage of 20 kV). X-ray diffraction (XRD; PAN analytical, Netherlands, Rigaku Rota-Flex operating with Cu-Kα radiation, 45 kV) patterns were studied between diffraction angles (2θ) ranging from 10-80o. Fourier transform infrared (FTIR; Thermo Nicolet Model 6700 spectrophotometer) spectra of the samples were analyzed through KBr pelletization in 400–4000 cm−1 range. Thermal-gravimetric (TGA) and differential thermal analysis (DTA) (Hitachi STA7200 Thermal Analyser; simultaneous TG/DT model) were carried out at 30-750 °C temperature. Finally, swelling properties of the cellulose-based copolymers were also studied in buffer solutions with pH values 2.2, 7.0 and 9.4. Accurately weighed (100 mg) polymer sample was placed in 10.00 mL of the buffer solution for different time intervals. After a suitable time interval, surface water was removed from the swollen polymer and polymer was dried by softly pressing it between the folds of filter paper. The increase in weight of the swollen copolymer was recorded. Percent swelling (Ps) was calculated using Eq. 3 [46].
Where, Ws = Weight swollen copolymer, and Wd = Weight of dried copolymer.
Dye adsorption applications
The adsorption behavior of cellulose and graft copolymers for both the cationic dyes was studied. Batch experiments were conducted using 0.05 g of the graft copolymer as adsorbent for 50 mL dye solutions of different concentrations. After specific time intervals, dye solution aliquots were withdrawn and the concentration of the remaining dye was determined at the characteristic wavelength (λmax = 615 and 588 nm, for MG and CV respectively) with the help of UV − Vis spectrophotometer (Systronics−2202, Double beam spectrophotometer) at calibrated curves. This optimized time was used for all the subsequent experiments to evaluate the adsorption performance of Cell-g-AASO3H copolymers. In order to improve the reliability and validity of the adsorption data, all the experiments were performed in triplicate and the average of the results is used for analysis.
Effects of the initial dye concentration, contact time and temperature (20–40 °C), on the adsorption capacities of the synthesized Cell-g-AASO3H and Cell-g-AASO3H-co-AN were studied at optimized conditions, and results were compared with adsorption capacities of extracted cellulose as a reference. The nature of the dyes and the pH of the solution have a significant effect on the adsorption. Thus, the effect of pH (2.2, 7.0 and 9.4) on the adsorption performances of Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers were studied at optimized conditions of time, temperature, and concentration of the dye solutions. Amount of dye adsorbed (qt – mg/g) and percent dye removal (De – %), were calculated as per Eqs. 4 and 5.
Where, Co = Initial dye concentrations (ppm), Ct = Dye concentrations (ppm) in the solution after time ‘t’ (min), V = Volume of the dye solution (L) and, Wd = Weight of adsorbent taken (g).
Metal ions sorption applications
Batch experiments were also conducted to evaluate the sorption capacities of pure cellulose, Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers towards the sorption of Pb(II) metal ions from the medium. Accurately weighed 0.05 g of the sorbent was immersed in 50 mL aqueous solution of Pb(II) ions of known concentration. The concentration of Pb(II) ions after sorption was estimated through back titration using standardized EDTA solution and Eriochrome black T as an indicator [47]. All the batch experiments were repeated thrice to calculate the mean values. The sorption amount ‘q’ and percent uptake (Pu) of the Pb(II) ions sorbed were calculated using Eqs. 6–7, respectively.
Where, Co = Initial concentration of metal ions, Ct = Concentration of the Pb(II) ions left in solution after time ‘t’, Wd = Weight of the copolymer (g), and V = Volume of the of the Pb(II) ion solution taken.
Results and discussion
Optimization of grafting parameters of AASO3H onto extracted cellulose
Effect of nature and amount of solvent
Solvent acts as a medium for the transportation and diffusion of the monomer units to the vicinity of the –OH groups of cellulose backbone. Generation of the free radicals, wetting, and swelling of the cellulose backbone, chain transfer, H-abstractions, control of the chain termination effects, etc. are some of the other important factors which depend upon the nature of the solvent. Thus, the proper selection of the solvent is critical. The solvent effect on Pg and percent GE was studied in 10.00 mL of CCl4, benzene, water, dioxane, and acetone, in the presence of monomer; [AASO3H] = 48.251 × 10−2 mol L−1, initiator; [KPS] = 9.25 × 10−2 mol L−1, cross-linker; [N,N′-MBA] = 0.1 g (10 wt% of monomer, AASO3H), grafting temperature = 60 °C and grafting time = 5 min. Under these grafting conditions, maximum Pg and percent GE were recorded in the water (Table 1). The order for Pg and GE in different solvents was: Water > Acetone > dioxane > CCl4 > Benzene. The results may be attributed to the excellent solubilities of the monomer, free radical initiator, and crosslinker, fast diffusion of the monomer, maximum swelling of the cellulose backbone, negligible chain transfer constant, and minimum wastage of the monomer in water as compared to other solvents under study.
The reasonable change was observed in the Pg and percent GE of the copolymers when the volume of the water was increased from 10 mL to 25 mL. All other parameters were kept constant (Table 1). Pg values of 78, 38, 29, and 11 were observed for 10, 15, 20 and 25 mL of water as a solvent, respectively. A large volume of water makes the reacting species (monomer) less accessible to cellulose backbone which results in homopolymer formation [48]. Volume less than 10 mL was found insufficient for the proper wetting of the cellulose backbone and dissolution of other chemical reagents, hence no proper results could be inferred. As maximum Pg and percent, GE was recorded for 10 mL of water, all the subsequent experiments were performed using the same optimized volume.
Effect of monomer and initiator concentration
In order to check the effect of the concentration, grafting experiments were conducted at different concentration of AASO3H varying from 24.126 × 10−2 mol L−1 to 120.627 × 10−2 mol L−1 at KPS concentration of 9.25 × 10−2 mol L−1, N,N′-MBA amount of 0.1 g, temperature 60 °C, grafting reaction time 5 min and at optimum solvent volume (10 mL). The increase in the Pg and percent GE was observed with the initial increase in the monomer concentration up to 96.501 × 10−2 mol L−1, maximum Pg, and percent GE were recorded as 152.0 and 76.0 respectively. At higher AASO3H concentrations, the possibilities of coupling of grown macro-radicals accelerate the mutual chain termination, thereby forming homopolymers rather than grafted copolymers.
Free radical initiators can either increase the number of grafts per cellulose backbone or can add monomer units to the already grafted chains. For these two processes to operate, a proper concentration of the free radicals is required and hence the chemical initiator is necessary for the reaction mixture. Herein, the effect of change in KPS concentration (4.624 × 10−2 mol L−1 to 23.13 × 10−2 mol L−1) on Pg and percent GE was studied. It is apparent from Table 1 that at with increase in KPS concentration to 18.5 × 10−2 mol L−1 maximum Pg, and percent GE were recorded as 249.0 and 124.5 respectively. But with furthur increase in concentration of initiator, it did not increase the Pg and percent GE. It is due to the fact that at higher KPS concentration, either homopolymerization is induced or coupling of the grown polymer chains takes place [49]. These two attributes result in the decreased grafting yield.
Effect of temperature and time
For temperature optimization, grafting of AASO3H onto cellulosic backbone was carried out between 30 and 70 °C, by keeping all other influencing parameters constant. As the grafting temperature was increased from 30 °C to 60 °C, Pg was increased significantly from 10 to 249. After that, a decrease in Pg was reported as the grafting temperature was increased to 70 °C. Low grafting yield, at a temperature lower than the optimum temperature, is due to the lower rate of decomposition of the KPS initiator and retarded diffusion rates of monomers to the active sites, whereas at high temperature, the graft copolymerization occurs with poor selectivity and various other side reactions such as hydrogen abstraction, chain transfer, and homopolymerization reactions become operative [39].
With the increase in the reaction time from 3 min to 5 min the Pg increased from 190.2 to 249.0 and after this, a further increase in the grafting time up to 7 min, a significant decrease was recorded for the Pg as well as GE (Table 1). This may be attributed to the partial decomposition of grown polymer chains over the cellulose backbone [45, 46, 50].
Effect of cross-linker concentration
The amount of N,N′-MBA was varied from 2.5–12.5 wt% with respect to the amount of the cellulose backbone by maintaining all other grafting parameters constant. Maximum Pg and percent GE were 249.0 and 24.5, respectively for 10 wt% of N,N′-MBA. Beyond 10 wt%, both Pg and GE were decreased (Table 1). As the concentration of the N,N′-MBA cross-linker increases, large number of cross-linking points are formed which promote the formation of network structures rather than grafting of the vinyl monomers [51, 52].
Synthesis of Cell-g-AASO3H-co-AN copolymers
In order to increase the density of functional groups, AN as binary comonomer, was grafted onto 1 g cellulose at the optimized grafting conditions evaluated for grafting of AASO3H i.e. [AASO3H] = 96.501 × 10−2 mol L−1, initiator; [KPS] = 18.5 × 10−2 mol L−1, cross-linker; [N,N′-MBA] = 0.1 g (5 wt% of monomer AASO3H), grafting temperature = 60 °C and grafting time = 5 min in 10 mL of water as solvent. Only the concentrations co-monomer AN was varied from 0.5 to 2.5 M ratios with respect to the molar concentration of AASO3H (Table 2). The grafting parameters like Pg and percent GE were continuously increased with increase in the concentration of AN. Maximum Pg and GE values of 267.0 and 133.5, respectively were calculated for Cell-g-AASO3H-co-AN at 2.5 M ratios. However, for initial concentrations of AN i.e. 0.5–2.0 M ratios w.r.t. the molar concentration of AASO3H, both Pg and GE for Cell-g-AASO3H-co-AN copolymer were low as compared to Cell-g-AASO3H copolymer. The fact may be attributed to the hydrophobic nature of the AN vinyl monomer. The simultaneous presence of the AN and AASO3H monomers in the reaction medium increases the monomer molecules relative to the available graft free radical sites, which results in competition for the active sites, thereby reducing Pg and GE. This further favors homopolymerization over graft copolymerization [53].
Characterization of the pure cellulose and grafted copolymers
SEM analysis
The morphological and structural properties of the pure cellulose, Cell-g-AASO3H, and Cell-g-AASO3H-co-AN were evaluated through SEM analysis. Long and individual bundled cellulose fibers with high aspect ratio and an average diameter of 6 ± 1 μm were observed. Additionally, the raw cellulosic fibers have relatively smooth surfaces as compared to the cellulose grafted with unitary and binary monomers. Cell-g-AASO3H displayed a thick flaky appearance, whereas the binary Cell-g-AASO3H-co-AN copolymer displayed highly flaky, porous and lumpish appearance due to the grafting of AN monomer units (Fig. 1).
XRD analysis
The XRD spectrum for pure cellulose revealed diffraction peaks at diffraction angles (2θ) = 15.36o, 22.33o and 34.55o which correspond to the (101), (002) and (004) diffraction planes, respectively. These are the characteristic peaks for Iβ form of cellulose polymorphs [54]. The intensities for the peaks, corresponding to all the diffractions planes were decreased for Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers (Fig. 2). The crystallinity index (CI) values for all the samples were calculated using the maximum intensities corresponding to the crystalline region and the intensities at the minimum for the amorphous fraction according to the Segal method (Eq. 8) [55].
Cell-g-AASO3H and Cell-g-AASO3H-co-AN showed lower crystallinity index values of 44.03 and 40.11%, respectively as compared to 68.33% crystallinity index for pure cellulose fibers (Table 3).
It is interesting to note that, the crystallinity indexes were decreased as the percent grafting of the copolymers was increased. Lower crystallinity index values for all the grafted cellulose fibers further indicate that there may be the disorientation of the cellulose crystals upon grafting of different monomer units onto cellulose fibers.
FTIR analysis
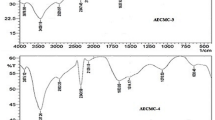
The FTIR spectra for the pure cellulose, Cell-g-AASO3H, and Cell-g-AASO3H-co-AN copolymer were analyzed to ascertain the composition and grafting of the monomer units onto the cellulosic fibers. The FTIR peaks at 1637.8, 1430.8, 1320.6, 1163.7, 1057.8 and 897.0 cm−1 are the characteristic peaks of the native cellulose (Fig. 3a) [44]. The peak at 1637.8 cm−1 may be assigned to the bending vibrational modes for the inter and intra-molecular hydrogen bonded hydroxyl (–OH) groups and adsorbed water molecules. Weak peaks at 1430.8 and 1320.6 cm−1 were attributed to the bending vibrational modes of the methylene group (δ–CH2). An additional set of strong peaks present at 1163.7, 1057.8 cm−1 corresponds to the glycosidic bond (C–O–C) of the cellulose backbone and O–H bending vibrational modes for the primary alcoholic groups. The peak around 897.8 cm−1 corresponds to the C–O stretching vibrational mode of the saccharide units.
The FTIR spectrum of Cell-g-AASO3H showed characteristics peaks for the AASO3H moiety in addition to the normal cellulose backbone peaks. This confirms the grafting of the AASO3H monomer units on to the cellulose backbone. Sharp and well-defined peak at 1640.3 cm−1 is attributed to the >C=O moiety of the amide group, relatively low-intensity peak at 1549.1 cm−1 is due to the N-H bending vibrational mode of amide group, very low-intensity peak at ~3000 cm−1 is due to the stretching band of –OH moiety of the sulfonic acid (–SO3H) group. Additional prominent peaks at 1153.6 and 1115.2 cm−1 are also the characteristic peaks of the –SO3H) group (Fig. 3b). The peaks at 1369.4, 1031.7 and 619.0 cm−1 may be attributed to C–N stretching vibration of the amide group as well as C-H bending modes of the methyl (-CH3) group, S–O stretching vibrational modes of the –SO3H, and C–S stretching vibrations, respectively. The presence of a low intensity but sharp peak at 2251.2 due to the C ≡ N stretching affirms the grafting of the AN monomer units in Cell-g-AASO3H-co-AN grafted copolymer (Fig. 3c).
Thermal analysis
Cellulose thermogram shows single stage degradation up to 199.5 °C with 7.9% weight loss (Fig. 4a). From 199.5 to 368 °C sharp loss in weight of 71.4% is observed which can be attributed to the processes like depolymerization, decomposition of the glycosidic bonds (C–O–C) and dehydration [44]. Final decomposition temperature (FDT) is observed at 499.5 °C leaving 0.7% residue. In addition to a sharp exothermic peak at 338.4 °C, two low-intensity exothermic peaks at 377.6 and 439.5 °C can be seen in DTG thermogram of cellulose.
DTA thermogram for pure cellulose extracted from rice husk exhibits two prominent exothermic peaks at 390.4 °C and 442 °C. For Cell-g-AASO3H graft copolymer, TGA thermogram shows a steep and continuous weight loss of 71.4% up to 500.8 °C which can be due to the degradation of sulfonic acid (−SO3H) groups, decomposition of cellulosic backbone resulting in the evolution of CO2 molecules and the disintegration of the charred residue (Fig. 4b). FDT was recorded at 543.7 °C at which 16.6% residue is left. Higher FDT and more amount of residue left at FDT for Cell-g-AASO3H copolymer confirm its greater stability than pure cellulose. DTG showed three exothermic peaks at 233.1 °C, 303.2 °C and 534.5 °C whereas the DTA thermograph of the Cell-g-AASO3H graft copolymer exhibited only a single exothermic peak at 540.7 °C which can be assigned to the degradation −SO3H groups of Cell-g-AASO3H graft copolymer.
In the case of Cell-g-AASO3H-co-AN, the TGA thermogram represents a marginal decrease of 10.5% weight up to 199.4 °C. A significant weight loss of 34.4% can be seen from 199.4 °C to 367.1 °C. After that 14.8% weight loss was recorded between 367.1 °C to 500.1 °C (Fig. 4c). FDT was even higher than the Cell-g-AASO3H copolymer and was marked at 658.6 °C with 5.4% residue left. Thus it can be concluded that the introduction of AN comonomer in the grafting process increases the thermal stability of the graft copolymer. Exothermic peaks at 582.7 °C in DTA of Cell-g-AASO3H-co-AN may be because of decomposition of (−SO3H) groups of AASO3H and –CN groups of AN comonomers. Three prominent exothermic peaks at 309.7 °C, 577.9 °C and 612.4 °C are observed in DTA of Cell-g-AASO3H-co-AN which may also be because of the decomposition of –C ≡ N groups of AN.
Swelling properties
Swelling properties of bare cellulose and its graft copolymers Cell-g-AASO3H and Cell-g-AASO3H-co-AN with maximum Pg (all 100 mg), under different pH conditions (2.2, 7.0 and 9.4) were compared. Under different pH conditions, Cell-g-AASO3H showed maximum swelling as compared to the cellulose and Cell-g-AASO3H-co-AN. All the samples attained maximum swelling within 2 h of the contact time (Fig. 5a–c). After that marginal change in swelling was observed. Figure 5d represents the Ps values for different adsorbents at 2.2, 7.0 and 9.4 pH after 24 h of the contact time. Un-grafted cellulose showed minimum Ps as compared to all the grafted copolymers. With the increase in the pH of the medium, however, the Ps values were increased.
Ungrafted cellulose showed maximum Ps of 29.4, 54.4 and 128 at 2.2, 7.0 and 9.4 pH values, respectively. Ps values for Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers of cellulose followed the order 9.4 > 7.0 > 2.2. At pH 9.4 maximum Ps of 1942.9, 1339.3 and 128 was recorded for Cell-g-AASO3H, Cell-g-AASO3H-co-AN and cellulose respectively. The maximum rate of swelling at pH 9.4 is attributed to the sodium salt formation and hydrolysis of the nitrile group for Cell-g-AASO3H, and Cell-g-AASO3H-co-AN, respectively.
Applications of the grafted cellulosic copolymer for dye adsorption
Effect of contact time
Batch experiments were conducted using 50 mL of the 20 ppm dye solutions and 0.05 g of the pure cellulose and grafted copolymers with maximum Pg. The variations of percent dye removal (De) was studied with respect to contact time ranging from 10 to 120 min for cationic MG and CV dyes with polymeric samples at 25 °C temperature and at pH 7.0. For all the batch experiments, the De values were found to increase with time and the equilibrium was established after about 120 min. For pure cellulose, Cell-g-AASO3H and Cell-g-AASO3H-co-AN the initial rapid rates of adsorption of the dyes onto the surface of the copolymers are due to the availability of sufficient active sites on the adsorbent surface initially which gradually decreases with time. Further increase in the contact time practically exhibited no effect on the De value of dye molecules. Further, for cationic dyes, the De values are much higher for Cell-g-AASO3H (MG = 95.63%, CV = 98.93%) graft copolymer as compared to bare cellulose (MG = 10.72%, CV = 8.3%) and Cell-g-AASO3H-co-AN (MG = 30.12%, CV = 55.85%) grafted copolymer (Fig. 6a and b). The results are in good agreement with the swelling studies conducted at pH 7.0. This may be also be attributed to the incorporation of the negatively charged sulphonic acid (\( -{SO}_3^{-} \)) groups after grafting of the cellulose backbone which can easily attract the cationic dyes such as MG and CV. Due to less swelling behavior of Cell-g-AASO3H-co-AN grafted copolymer in contrast to Cell-g-AASO3H, De of the former was lower than the latter. In case of cellulose minimum De was also recorded as least Ps was observed at pH 7.0 for cellulose. The order of adsorption of cationic dyes by polymeric samples is observed as Cell-g-AASO3H > Cell-g-AASO3H-co-AN > Cellulose. Grafting of the AASO3H monomers onto the cellulose backbone improved swelling and De drastically for cationic dyes. It can also be inferred from the results that the hydrophobic nature of AN in Cell-g-AASO3H-co-AN lowered the extent of adsorption of cationic dyes as compared to the Cell-g-AASO3H copolymer.
It is important to mention here is that, the adsorption studies of the grafted copolymers were also evaluated for anionic congo red (CR) dye. However, as compared to cationic dyes, the rate of adsorption for anionic CR dye was quite low, though De values were observed to increase with time and the equilibrium was established after 8 h of contact time. Pure cellulose, Cell-g-AASO3H, and Cell-g-AASO3H-co-AN copolymers adsorbed 3.33%, 6.046% and 4.85% CR dye after 8 h of contact time at pH 7.0, which is very low as compared to adsorption of cationic dyes. The lower rate of the adsorption for CR dye may be due to the electrostatic repulsions between the anionic dye and negatively charged functional groups on cellulose and its graft copolymers. In contrast, MG and CV dyes have positively charged amine moieties that are strongly attracted towards negatively charged functional groups on cellulose and its graft copolymers. Hence, adsorption data for the anionic CR dye is not discussed in this manuscript.
Effect of temperature
Effect of temperature on dye adsorption batch experiments was studied within the temperature range of 20–40 °C with an interval of 5 °C for optimum contact time evaluated in the earlier step. Maximum De for cationic MG and CV dyes was observed for all polymeric samples at 30 °C and 25 °C respectively in a contact time of 2 h (Fig.7a). The De values followed the order Cell-g-AASO3H > Cell-g-AASO3H-co-AN > pure cellulose at optimized temperatures.
For MG dye the De values for Cell-g-AASO3H, Cell-g-AASO3H-co-AN, and pure cellulose were found to be 99.88%, 32.44%, and 11.33%, respectively at 30 °C. Cell-g-AASO3H showed the almost complete removal of for CV dye (99.93%) at 25 °C from 50 mL of the 20 ppm dye solutions. Low adsorption of the dyes at a higher temperature may be attributed to the faster rates of desorption of the dyes molecules as compared to the adsorption.
Effect of pH
Another important external parameter, which affects the chemistry of the functional moieties and hence the De of adsorbents, is the pH of the medium. Dye adsorption studies for cellulose and graft copolymers were performed at three pH 2.2, 7.0 and 9.4. For cationic dyes the De of pure cellulose was reported to be very low at all the pH values as compared to Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers. With the rise in pH of the medium from 2.2 to 9.4, the De of the pure cellulose towards cationic dyes was marginally increased. For adsorption of MG dye, the graft copolymers of cellulose followed the order 7.0 > 2.2 > 9.4, while for CV dye the observed order was 7.0 > 9.4 > 2.2. Cell-g-AASO3H exhibited maximum De of 99.88% and 98.93% for MG and CV dyes, respectively and Cell-g-AASO3H-co-AN showed maximum De of 32.44% and 55.85% for MG and CV dyes, respectively at pH = 7.0 after 2 h of contact time (Fig. 7b).
This behavior can be explained on the basis of protonation-deprotonation of the \( -{SO}_3^{-} \) groups of Cell-g-AASO3H and hydrolysis of –CN groups of Cell-g-AASO3H-co-AN at different pH conditions. Under acidic conditions (pH = 2.2), the protonation of the \( -{SO}_3^{-} \) groups increases the magnitude of the electrostatic repulsions for the cationic dyes. Under neutral or alkaline pH conditions, deprotonation is resulted favoring reverse trends for cationic dyes. In spite of the higher degree of deprotonation the \( -{SO}_3^{-} \), −CONH2 and −COO−groups in alkaline medium with pH 9.4, as compared to neutral pH of 7.0, low extent of adsorption capacity of grafted copolymers for cationic dyes may be attributed to the strong electrostatic attractions between the OH− ions present in the medium and the cationic sites of the dye molecules blocking the later for the adsorption onto the deprotonated \( -{SO}_3^{-} \) groups.
Kinetics studies for dye adsorption applications
The kinetic behavior of Cell-g-AASO3H and Cell-g-AASO3H-co-AN towards the adsorption of different dyes was evaluated using pseudo-first-order, pseudo-second order, Elovich’s kinetic and Intra-particle diffusion models (Fig. S1 and Fig. S2). The equation along with different parameters, their meanings and units are reported elsewhere in the literature [56, 57]. The various adsorption parameters like rate constants, adsorption capacities (qe) and correlation regression coefficients (R2) calculated from these kinetics model plots are given in Table 4. The adsorption measurements were carried out at optimized temperatures and pH values using 50 mL of 20 ppm dye solutions, accurately weighed adsorbent dose of 0.05 g and contact time of 0–120 min for cationic dyes. The pseudo-first-order rate constants (k1) were higher for Cell-g-AASO3H as compared to a Cell-g-AASO3H-co-AN copolymer for all the model dyes. However, for both the cationic dyes, the pseudo-second-order model seems to be best-fitted with the experimental data as compared to other models. This confirms the fact that the rate-determining step for the dye adsorption involves physicochemical interactions between the dye molecules and the adsorbent. The view is further supported by the higher values of correlation regression coefficients (R2) for pseudo-second-order kinetics models as compared to pseudo-first order and Elovich’s kinetics models. Further, the qe values calculated from the pseudo-second-order model for both the cationic dyes are closer to the experimental qexp values as compared to pseudo-first order kinetics models (Table 4).
Thus, the initial physical interaction between the dye molecules and the copolymer chains are followed by strong physicochemical interactions in case of for Cell-g-AASO3H copolymer. For MG and CV higher values of α as compared to β values in Elovich’s kinetic confirm the higher rate of the adsorption of the dyes than desorption. The regression coefficient constants were 0.92438 and 0.9740 for MG and CV dyes, respectively which further reflect the applicability of the Elovich’s kinetic model for Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers (Table 4). A larger value of boundary layer thickness parameter (C) for Cell-g-AASO3H as compared to a Cell-g-AASO3H-co-AN copolymer for MG and CV dyes in intra-particle diffusion model revealed a greater boundary layer diffusion effect for the former. Such type of dye adsorption behavior has also been reported earlier [58].
Adsorption isotherms models for dye adsorption applications
Freundlich, Langmuir and Temkin isotherms were studied for the adsorption of cationic dyes from the 50 mL aqueous solutions with concentrations ranging from 5.0–70 ppm at an optimized contact time, temperature and pH conditions (Fig. S3 and Fig. S4). The linear forms for these adsorption models along with the meaning and units of different parameters are reported elsewhere in the literature [56, 57, 59]. Adsorption parameters, constants and linear correlation coefficients (R2) obtained from different adsorption isotherms for various dyes are listed in Table 5. For all the dyes the values of \( \frac{1}{n} \) are between 0 → 1 which indicate a favorable physisorption of the dyes onto the surface of the copolymers [60]. The data also support the poor adsorption behavior of the Cell-g-AASO3H-co-AN copolymer than Cell-g-AASO3H copolymer. Greater R2 values for Langmuir adsorption isotherm, as compared to the Freundlich adsorption isotherm confirm the better suitability of the former for explaining the adsorption of the dye molecules onto grafted copolymers. The maximum adsorption capacity, qm obtained from the Langmuir adsorption isotherm are 61.46 and 55.71 mg.g−1 for Cell-g-AASO3H and 44.09 and 54.71 mg.g−1 for Cell-g-AASO3H-co-AN copolymer against MG and CV, dyes, respectively.
For all the dye concentrations, the values of the dimensionless quantity, RL ranged between 0 → 1 which further supports the better fitting of the adsorption data into Langmuir adsorption isotherm model as compared to Freundlich adsorption isotherm model (Table 5). A higher value of Temkin isotherm equilibrium binding constant (AT) for CV dye as compared to MG dye suggests a better preference of the Cell-g-AASO3H copolymer for CV dye than MG. Very low values of AT for Cell-g-AASO3H-co-AN copolymer as compared to Cell-g-AASO3H copolymer against all the two model dyes confirm its poor adsorption capacities (Table 5). However, the regression correlation coefficient values for both the dyes obtained from the Temkin adsorption isotherm model were found to be less as compared to the Langmuir adsorption isotherms.
The results for the different adsorption isotherm models confirm that for cationic dyes, the adsorption was mono-layered and took place at certain specific, energetically identical and homogeneous sites i.e. appended \( -{SO}_3^{-} \) groups on the surface of the Cell-g-AASO3H copolymer and \( -{SO}_3^{-} \) and −CONH2 groups (generated by hydrolysis of –CN groups of AN comonomers) in Cell-g-AASO3H-co-AN copolymer. The adsorption results, in terms of ease of synthesis of adsorbent and other adsorption parameters for Cell-g-AASO3H graft copolymer for cationic dyes are better as compared to the other adsorbent materials reported in literature (Table 6).
Applications of the grafted cellulosic copolymer for metal ion sorption
Effect of contact time
Sorption behavior of 0.05 g pure cellulose, Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers for Pb(II) ions with 100 ppm concentration at 7.0 pH, and 25 °C temperature was studied as a function of contact time (Fig. 8a). All the samples showed significant sorption of Pb(II) ions up to 120 min, but after that, no significant sorption was recorded. Thus, after a contact time of 120 min, the sorption sites of the sorbents were saturated. Further, both the grafted copolymers exhibited remarkably improved sorption behavior as compared to pure cellulose. Deprotonation of the sulfonic acid groups of the AASO3H groups in Cell-g-AASO3H copolymer into the \( -{SO}_3^{-} \) groups provides the favorable active sites for the sorption of positively charged Pb(II) ions. Introduction of AN as binary comonomer in Cell-g-AASO3H-co-AN, further provides –CN groups as active sites for better sorption of Pb(II) ions as compared to Cell-g-AASO3H though marginal. Very small increase in metal ion sorption capacity of Cell-g-AASO3H-co-AN as compared to Cell-g-AASO3H, may be attributed to the hydrophobic nature of the AN comonomer which results in a slight increase in percent grafting (Table 2). For pure cellulose, Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers Pu of 13.5, 41.8 and 43.3% were observed, respectively for Pb(II) ions.
Effect of temperature
Pb(II) metal ion sorption efficiencies of pure cellulose and grafted copolymers were evaluated as a function of temperature ranging from 20 to 40 °C at pH 7.0, 100 ppm concentration of Pb(II), and contact time of 120 min (Fig. 8b). With the initial increase in temperature, the sorption capacities for Pb(II) ions were increased for all the samples up to 30 °C. The increased swelling, weakening of intermolecular polymer-polymer interactions leading to exposure of the active sites, and faster diffusion of the metal ions into the polymer matrix with an increase in temperature improved the metal ions sorption capacities and Pu of the polymeric samples [16]. Further, increased temperature also provides the required activation energy for the chemisorption of the metal ions onto the surface of the copolymers. With a further rise in temperature, the desorption rates which lowered the Pu capacities for pure cellulose and grafted copolymers. At an optimized temperature of 30 °C for pure cellulose, Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers observed Pu values were 15.7, 46.3 and 48.4%, respectively.
Effect of pH
The protonation-deprotonation equilibrium of the \( -{SO}_3^{-} \) groups of Cell-g-AASO3H and hydrolysis –CN groups of Cell-g-AASO3H-co-AN at different pH conditions, significantly affect the Pb(II) metal ions sorptions by the grafted copolymers. Deprotonation of the –SO3H with increased pH fabricates the \( -{SO}_3^{-} \) groups which strongly attracts the positively charged Pb(II) metal ions. The uptake studies of the Pb(II) ions by pure cellulose and grafted copolymers were studied in the pH range of 2.0–7.0 with the initial concentrations of 100 ppm at optimized conditions of temperature and contact time. For all the sorbent samples maximum metal ions uptake was reported at pH 5.0 (Fig. 8c). Below and above this optimized pH, metal ions uptake capacities were low which is in good agreement with the surface complexation theory, according to which at low pH conditions, metal ions and H+ ions of the medium compete for the negatively charged active sites of the sorbent material [14, 71]. Precipitation of the metal ions beyond pH 7.0, lowers the metal ion uptake capacities of the grafted copolymers.
Effect of metal ion concentration
Under optimum conditions of contact time, temperature, and pH, the sorption behavior of Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers was evaluated for different concentrations of Pb(II) metal ions ranging from 10 to 200 ppm. Figure 8d represents the variations of the equilibrium sorption amount ‘qe’, percent uptake (Pu) for Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers. For Cell-g-AASO3H-co-AN copolymers, Pu values were higher than Cell-g-AASO3H copolymer for low concentrations (20–100 ppm), but for higher Pb(II) concentrations (150 and 200 ppm) Pu values were higher for Cell-g-AASO3H than Cell-g-AASO3H-co-AN copolymer. The qe values followed opposite trends as compared to Pu values for the Cell-g-AASO3H and Cell-g-AASO3H-co-AN.
Kinetics studies for metal ion sorption applications
Similar to dyes, various kinetic models viz. pseudo first order, pseudo-second order, Elovich’s and intraparticle diffusion kinetic models were applied to the sorption data obtained for the sorption of Pb(II) using Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers (Fig. S5). From coefficient of determinant (R2), the pseudo-second-order kinetic models were best fitted for Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers. This indicates that the chemisorption is the controlling force for the sorption of Pb(II) ions. The highly fitted plot, as obtained for Elovich kinetic model, suggests that the binding mechanisms such as ion exchange, chelation, and ion entrapment etc. control the sorption of Pb(II) ions onto Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers [72]. Further, significantly higher values of initial adsorption rate ‘α’ as compared to desorption constant ‘β’ indicate that adsorption is more favorable than desorption for Pb(II) ions onto Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers (Table 7). Comparable values of boundary layer parameter (C) calculated from intraparticle diffusion kinetic model for sorption of Pb(II) ions indicate that the boundary layer effects are controlling the diffusion rate of metal ions through the surface of the grafted copolymer [73].
Adsorption isotherms for metal ion sorption applications
The sorption capacities of the Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers were studied for different Pb(II) ion concentrations ranging from 20 to 200 ppm at the optimized conditions and the data obtained were used to explore sorption parameters for different adsorption isotherm models similar to the dye adsorption applications (Fig. S6). From the value of the determinant coefficient, Freundlich and Langmuir adsorption isotherm plots were found to best fitted for Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers, respectively for the sorption of Pb(II) ions (Table 8). This indicates that for Cell-g-AASO3H-co-AN copolymer, monolayered sorption of the Pb(II) ions occurs onto the active sites [74]. However, values of dimensionless quantity RL for different concentrations ranging from 0 → 1 for both the copolymers which support the monolayered sorption of the metal ions. Maximum adsorption capacities for Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers observed from Langmuir adsorption isotherm were 154.32 and 79.94 mg.g−1 respectively. Comparative higher R2 values for Cell-g-AASO3H-co-AN as compared to Cell-g-AASO3H copolymer suggest a more uniform distribution of the binding energies for Cell-g-AASO3H-co-AN as compared to Cell-g-AASO3H copolymer during adsorption. The comparative analysis of the sorption efficiencies of Cell-g-AASO3H and Cell-g-AASO3H-co-AN copolymers with those of the already reported copolymers/sorbents shows that as-synthesized copolymers are excellent sorbents for Pb(II) metal ions (Table 9).
Conclusion
Cellulose-based biosorbents were synthesized from agricultural residue, rice husk. At optimized conditions i.e. [AASO3H] = 96.501 × 10−2 mol L−1, initiator; [KPS] = 18.5 × 10−2 mol L−1, cross-linker; [N,N′-MBA] = 0.1 g (5 wt% of monomer AASO3H), temperature = 60 °C and time = 5 min in 10 mL of water as solvent, maximum Pg of 249.0 was observed for Cell-g-AASO3H. For Cell-g-AASO3H-co-AN binary copolymer, maximum Pg of 267.0 was recorded at 2.5 M ratio of AN w.r.t. AASO3H concentration. Dye adsorption behavior studied for cationic dyes followed the order Cell-g-AASO3H > Cell-g-AASO3H-co-AN > cellulose, whereas, for Pb(II) metal ions, percent uptake by Cell-g-AASO3H-co-AN (62.0%) was slightly higher than Cell-g-AASO3H (59.6%) at optimized conditions of contact time, temperature and pH. Almost complete adsorption was reported for MG and CV dyes at pH 7.0 and contact time 2 h from 50 mL of 20 ppm dye solutions. For dye adsorption study, the pseudo-second-order model seems to be best-fitted with the experimental data as compared to pseudo-first order and Elovich’s kinetics models. The maximum adsorption capacity, obtained from the Langmuir adsorption isotherm for MG and CV dyes were 61.46 and 55.71 mg.g−1 respectively, for Cell-g-AASO3H. For Pb(II) ions maximum adsorption capacities from Langmuir adsorption isotherm were 154.32 and 181.49 mg.g−1 for Cell-g-AASO3H and Cell-g-AASO3H-co-AN, respectively.
References
Carneiro PA, Umbuzeiro GA, Oliveira DP, Zanoni MVB (2010) Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J Hazard Mater 174:694–699. https://doi.org/10.1016/j.jhazmat.2009.09.106
Tsuboy MS, Angeli JPF, Mantovani MS, Knasmüller S, Umbuzeiro GA, Ribeiro LR (2007) Genotoxic, mutagenic and cytotoxic effects of the commercial dye CI disperse blue 291 in the human hepatic cell line HepG2. Toxicol in Vitro 21:1650–1655. https://doi.org/10.1016/j.tiv.2007.06.020
Bharathi KS, Ramesh ST (2013) Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 3:773–790. https://doi.org/10.1007/s13201-013-0117-y
Okieimen FE, Sogbaike CE, Ebhoaye JE (2005) Removal of cadmium and copper ions from aqueous solution with cellulose graft copolymers. Sep Purif Technol 44:85–89. https://doi.org/10.1016/j.seppur.2004.11.003
Badiei M, Asim N, Jahim JM, Sopian K (2014) Comparison of chemical pretreatment methods for cellulosic biomass. APCBEE Proc 9:170–174. https://doi.org/10.1016/j.apcbee.2014.01.030
Akhtar N, Gupta K, Goyal D, Goyal A (2016) Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Prog Sustain Energy 35:489–511. https://doi.org/10.1002/ep.12257
Chauhan GS, Dhiman SK, Guleria LK, Kaur I (2002) Polymers from renewable resources: kinetics studies of the radiochemical graft copolymerization of styrene onto cellulose extracted from pine needles and the effect of some additives on the grafting parameters in an aqueous medium. J Appl Polym Sci 83:1490–1500. https://doi.org/10.1002/app.10142
Chauhan GS, Lal H, Mahajan S (2004) Synthesis, characterization, and swelling responses of poly(N-isopropylacrylamide)- and hydroxypropyl cellulose-based environmentally sensitive biphasic hydrogels. J Appl Polym Sci 91:479–488. https://doi.org/10.1002/app.13135
Hokkanen S, Bhatnagar A, Sillanpa M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173. https://doi.org/10.1016/j.watres.2016.01.008
Sahoo PK, Jena DK (2018) Synthesis and study of mechanical and fire retardant properties of (carboxymethyl cellulose -g-polyacrylonitrile)/montmorillonite biodegradable nanocomposite. J Polym Res 25:260. https://doi.org/10.1007/s10965-018-1659-3
Kang H, Liu R, Huang Y (2015) Graft modification of cellulose: methods, properties and applications. Polym (United Kingdom) 70:A1–A16. https://doi.org/10.1016/j.polymer.2015.05.041
Chauhan GS, Guleria L, Sharma R (2005) Synthesis, characterization and metal ion sorption studies of graft copolymers of cellulose with glycidyl methacrylate and some comonomers. Cellulose 12:97–110. https://doi.org/10.1023/B:CELL.0000049349.66326.5e
Kumar R, Sharma RK, Singh AP (2019) Grafting of cellulose with N-isopropylacrylamide and glycidyl methacrylate for efficient removal of Ni(II), Cu(II) and Pd(II) ions from aqueous solution. Sep Purif Technol 219:249–259. https://doi.org/10.1016/j.seppur.2019.03.035
Sharma RK, Kumar R, Singh AP (2019) Metal ions and organic dyes sorption applications of cellulose grafted with binary vinyl monomers. Sep Purif Technol 209:684–697. https://doi.org/10.1016/j.seppur.2018.09.011
Goel NK, Kumar V, Misra N, Varshney L (2015) Cellulose based cationic adsorbent fabricated via radiation grafting process for treatment of dyes waste water. Carbohydr Polym 132:444–451. https://doi.org/10.1016/j.carbpol.2015.06.054
Kumar R, Sharma RK, Singh AP (2018) Removal of organic dyes and metal ions by cross-linked graft copolymers of cellulose obtained from the agricultural residue. J Environ Chem Eng 6:6037–6048. https://doi.org/10.1016/j.jece.2018.09.021
Bello K, Sarojini BK, Narayana B (2019) Design and fabrication of environmentally benign cellulose based hydrogel matrix for selective adsorption of toxic dyes from industrial effluvia. J Polym Res 26:62. https://doi.org/10.1007/s10965-019-1724-6
Zhao J, Lu C, He X, Zhang X, Zhang W, Zhang X (2015) Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl Mater Interfaces 7:2607–2615. https://doi.org/10.1021/am507601m
Du H, Han R, Tang E et al (2018) Synthesis of pH-responsive cellulose-g-P4VP by atom transfer radical polymerization in ionic liquid, loading, and controlled release of aspirin. J Polym Res 25:205. https://doi.org/10.1007/s10965-018-1601-8
Sharma RK, Singh J, Chauhan GS (2010) Study of immobilization of protease and sorption of bsa on cellulose, cellulose derivatives, and graft copolymers. BioResources 5:2547–2555
Wu Y, Luo X, Li W, Song R, Li J, Li Y, Li B, Liu S (2016) Green and biodegradable composite films with novel antimicrobial performance based on cellulose. Food Chem 197:250–256. https://doi.org/10.1016/j.foodchem.2015.10.127
Kale RD, Gorade VG (2018) Preparation of acylated microcrystalline cellulose using olive oil and its reinforcing effect on poly(lactic acid) films for packaging application. J Polym Res 25:81. https://doi.org/10.1007/s10965-018-1470-1
Chiappone A, Nair J, Gerbaldi C, Zeno E, Bongiovanni R (2014) Flexible and high performing polymer electrolytes obtained by UV-induced polymer–cellulose grafting. RSC Adv 4:40873–40881. https://doi.org/10.1039/C4RA07299E
Masoumi A, Hemmati K, Ghaemy M (2016) Low-cost nanoparticles sorbent from modified rice husk and a copolymer for efficient removal of Pb(II) and crystal violet from water. Chemosphere 146:253–262. https://doi.org/10.1016/j.chemosphere.2015.12.017
Chakraborty S, Chowdhury S, Das Saha P (2011) Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr Polym 86:1533–1541. https://doi.org/10.1016/j.carbpol.2011.06.058
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168. https://doi.org/10.1016/j.desal.2010.07.047
Zhou Y, Zhang M, Wang X, Huang Q, Min Y, Ma T, Niu J (2014) Removal of crystal violet by a novel cellulose-based adsorbent: comparison with native cellulose. Ind Eng Chem Res 53:5498–5506. https://doi.org/10.1021/ie404135y
Zhou Y, Min Y, Qiao H, Huang Q, Wang E, Ma T (2015) Improved removal of malachite green from aqueous solution using chemically modified cellulose by anhydride. Int J Biol Macromol 74:271–277. https://doi.org/10.1016/j.ijbiomac.2014.12.020
Zheng X, Li X, Li J, Wang L, Jin W, liu J, Pei Y, Tang K (2018) Efficient removal of anionic dye (Congo red) by dialdehyde microfibrillated cellulose/chitosan composite film with significantly improved stability in dye solution. Int J Biol Macromol 107:283–289. https://doi.org/10.1016/j.ijbiomac.2017.08.169
Ruan C-Q, Strømme M, Lindh J (2018) Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr Polym 181:200–207. https://doi.org/10.1016/j.carbpol.2017.10.072
Wang Y, Zhao L, Peng H, Wu J, Liu Z, Guo X (2016) Removal of anionic dyes from aqueous solutions by cellulose-based adsorbents: equilibrium, kinetics, and thermodynamics. J Chem Eng Data 61:3266–3276. https://doi.org/10.1021/acs.jced.6b00340
Qiao H, Zhou Y, Yu F, Wang E, Min Y, Huang Q, Pang L, Ma T (2015) Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 141:297–303. https://doi.org/10.1016/j.chemosphere.2015.07.078
Chauhan GS, Chauhan K, Chauhan S, Kumar S, Kumari A (2007) Functionalization of pine needles by carboxymethylation and network formation for use as supports in the adsorption of Cr6+. Carbohydr Polym 70:415–421. https://doi.org/10.1016/j.carbpol.2007.04.020
Chauhan GS, Chauhan S, Kumar S, Kumari A (2008) A study in the adsorption of Fe(2+) and NO(3)(−) on pine needles based hydrogels. Bioresour Technol 99:6464–6470. https://doi.org/10.1016/j.biortech.2007.11.044
Chauhan GS, Mahajan S (2002) Use of novel hydrogels based on modified cellulosics and methacrylamide for separation of metal ions from water systems. J Appl Polym Sci 86:667–671. https://doi.org/10.1002/App.10943
Barsbay M, Kavaklı PA, Tilki S, Kavaklı C, Güven O (2018) Porous cellulosic adsorbent for the removal of Cd (II), Pb(II) and Cu(II) ions from aqueous media. Radiat Phys Chem 142:70–76. https://doi.org/10.1016/j.radphyschem.2017.03.037
Sokker HH, Gad YH, Ismail SA (2012) Synthesis of bifunctional cellulosic adsorbent by radiation induced graft polymerization of glycidyl methacrylate-co-methacrylic acids. J Appl Polym Sci 126:E54–E62. https://doi.org/10.1002/app.34220
Monier M, Akl MA, Ali WM (2014) Modification and characterization of cellulose cotton fibers for fast extraction of some precious metal ions. Int J Biol Macromol 66:125–134. https://doi.org/10.1016/j.ijbiomac.2014.01.068
Sharma RK, Chauhan GS (2009) Synthesis and characterization of graft copolymers of 2-hydroxyethyl methacrylate and some comonomers onto extracted cellulose for use in separation technologies. BioResources 4:986–1005
Hajeeth T, Vijayalakshmi K, Gomathi T, Sudha PN (2013) Removal of Cu(II) and Ni(II) using cellulose extracted from sisal fiber and cellulose-g-acrylic acid copolymer. Int J Biol Macromol 62:59–65. https://doi.org/10.1016/j.ijbiomac.2013.08.029
Hajeeth T, Sudha PN, Vijayalakshmi K, Gomathi T (2014) Sorption studies on Cr (VI) removal from aqueous solution using cellulose grafted with acrylonitrile monomer. Int J Biol Macromol 66:295–301. https://doi.org/10.1016/j.ijbiomac.2014.02.027
Güçlü G, Gürdaǧ G, Özgümüş S (2003) Competitive removal of heavy metal ions by cellulose graft copolymers. J Appl Polym Sci 90:2034–2039. https://doi.org/10.1002/app.12728
Ding Z, Yu R, Hu X, Chen Y, Zhang Y (2014) Graft copolymerization of epichlorohydrin and ethylenediamine onto cellulose derived from agricultural by-products for adsorption of Pb(II) in aqueous solution. Cellulose. 21:1459–1469. https://doi.org/10.1007/s10570-014-0246-y
Sharma RK, Kumar R, Singh AP (2018) Extraction of cellulose micro-whiskers from rice husk: a greener approach. J Nanosci Nanotechnol 18:3702–3708
Sharma RK, Lalita SAP, Chauhan GS (2014) Grafting of GMA and some comonomers onto chitosan for controlled release of diclofenac sodium. Int J Biol Macromol 64:368–376. https://doi.org/10.1016/j.ijbiomac.2013.12.028
Lalita SAP, Sharma RK (2017) Synthesis and characterization of graft copolymers of chitosan with {NIPAM} and binary monomers for removal of Cr(VI), Cu(II) and Fe(II) metal ions from aqueous solutions. Int J Biol Macromol 99:409–426. https://doi.org/10.1016/j.ijbiomac.2017.02.091
Jeffery GH, Bassett J, Mendham J, Denney RC (1989) Vogel’s textbook of quantitative chemical analysis, 5th edn. New York 696. https://doi.org/10.1016/0160-9327(90)90087-8
Sharma RK, Lalita (2011) Synthesis and characterization of graft copolymers of N-Vinyl-2-Pyrrolidone onto guar gum for sorption of Fe2+ and Cr6+ ions. Carbohydr Polym 83:1929–1936. https://doi.org/10.1016/j.carbpol.2010.10.068
Lalita SAP, Sharma RK (2017) Selective sorption of Fe(II) ions over Cu(II) and Cr(VI) ions by cross-linked graft copolymers of chitosan with acrylic acid and binary vinyl monomer mixtures. Int J Biol Macromol 105:1202–1212. https://doi.org/10.1016/j.ijbiomac.2017.07.163
Singha AS, Shama A, Thakur VK (2008) Pressure induced graft-co-polymerization of acrylonitrile onto Saccharum cilliare fibre and evaluation of some properties of grafted fibre. Bull Mater Sci 31:7–13. https://doi.org/10.1007/s12034-008-0002-8
Luo MT, Li HL, Huang C, Zhang HR, Xiong L, Chen XF, Chen XD (2018) Cellulose-based absorbent production from bacterial cellulose and acrylic acid: synthesis and performance. Polymers (Basel). https://doi.org/10.3390/polym10070702
Huacai G, Wan P, Dengke L (2006) Graft copolymerization of chitosan with acrylic acid under microwave irradiation and its water absorbency. Carbohydr Polym 66:372–378. https://doi.org/10.1016/j.carbpol.2006.03.017
Mukherjee A, Halder S, Datta D, Anupam K, Hazra B, Kanti Mandal M, Halder G (2017) Free radical induced grafting of acrylonitrile on pre-treated rice straw for enhancing its durability and flame retardancy. J Adv Res 8:73–83. https://doi.org/10.1016/j.jare.2016.12.003
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10. https://doi.org/10.1186/1754-6834-3-10
Kumar R, Sharma RK, Singh AP (2018) Grafted cellulose: a bio-based polymer for durable applications. Polym Bull 75:2213–2242. https://doi.org/10.1007/s00289-017-2136-6
Kumar R, Sharma RK, Singh AP (2017) Cellulose based grafted biosorbents-journey from lignocellulose biomass to toxic metal ions sorption applications-a review. J Mol Liq 232:62–93
Konicki W, Aleksandrzak M, Mijowska E (2017) Equilibrium, kinetic and thermodynamic studies on adsorption of cationic dyes from aqueous solutions using graphene oxide. Chem Eng Res Des 123:35–49. https://doi.org/10.1016/j.cherd.2017.03.036
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Rahman N, Haseen U (2014) Equilibrium modeling, kinetic, and thermodynamic studies on adsorption of Pb(II) by a hybrid inorganic–organic material: polyacrylamide zirconium(IV) iodate. Ind Eng Chem Res 53:8198–8207. https://doi.org/10.1021/ie500139k
Kumar V, Rehani V, Kaith BS, Saruchi (2018) Synthesis of a biodegradable interpenetrating polymer network of Av-cl-poly(AA-ipn-AAm) for malachite green dye removal: kinetics and thermodynamic studies. RSC Adv 8:41920–41937. https://doi.org/10.1039/c8ra07759b
Crini G, Peindy HN, Gimbert F, Robert C (2007) Removal of C.I. basic green 4 (malachite green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol 53:97–110. https://doi.org/10.1016/j.seppur.2006.06.018
Saruchi KV, Rehani V, Kaith BS (2018) Microwave-assisted synthesis of biodegradable interpenetrating polymer network of aloe vera–poly(acrylic acid-co-acrylamide) for removal of malachite green dye: equilibrium, kinetics and thermodynamic studies. Iran Polym J (English Ed) 27:913–926. https://doi.org/10.1007/s13726-018-0665-y
Naseeruteen F, Hamid NSA, Suah FBM, Ngah WSW, Mehamod FS (2018) Adsorption of malachite green from aqueous solution by using novel chitosan ionic liquid beads. Int J Biol Macromol 107:1270–1277. https://doi.org/10.1016/j.ijbiomac.2017.09.111
Sharma AK, Priya KBS et al (2019) Selective removal of cationic dyes using response surface methodology optimized gum acacia-sodium alginate blended superadsorbent. Int J Biol Macromol 124:331–345. https://doi.org/10.1016/j.ijbiomac.2018.11.213
Gündüz F, Bayrak B (2017) Biosorption of malachite green from an aqueous solution using pomegranate peel: equilibrium modelling, kinetic and thermodynamic studies. J Mol Liq 243:790–798. https://doi.org/10.1016/j.molliq.2017.08.095
Gopi S, Pius A, Thomas S (2016) Enhanced adsorption of crystal violet by synthesized and characterized chitin nano whiskers from shrimp shell. J Water Process Eng 14:1–8. https://doi.org/10.1016/j.jwpe.2016.07.010
Sharma AK, Priya KBS et al (2019) Efficient capture of eosin yellow and crystal violet with high performance xanthan-acacia hybrid super-adsorbent optimized using response surface methodology. Colloids Surf B Biointerfaces 175:314–323. https://doi.org/10.1016/j.colsurfb.2018.12.017
Baghdadi M, Jafari A, Pardakhti A (2016) Removal of crystal violet from aqueous solutions using functionalized cellulose microfibers: a beneficial use of cellulosic healthcare waste. RSC Adv 6:61423–61433. https://doi.org/10.1039/C6RA08901A
Ahmad R (2009) Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J Hazard Mater 171:767–773. https://doi.org/10.1016/j.jhazmat.2009.06.060
Krishnani KK, Meng X, Christodoulatos C, Boddu VM (2008) Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J Hazard Mater 153:1222–1234. https://doi.org/10.1016/j.jhazmat.2007.09.113
Asuquo ED, Martin AD (2016) Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: characterisation, kinetic and isotherm studies. J Environ Chem Eng 4:4207–4228. https://doi.org/10.1016/j.jece.2016.09.024
Li Y, Du Q, Liu T et al (2012) Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene. Mater Res Bull 47:1898–1904. https://doi.org/10.1016/j.materresbull.2012.04.021
Wang C, Wang H, Gu G (2018) Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(II) sorption. Carbohydr Polym 182:21–28. https://doi.org/10.1016/j.carbpol.2017.11.004
Wang C, Wang H (2018) Pb(II) sorption from aqueous solution by novel biochar loaded with nano-particles. Chemosphere 192:1–4. https://doi.org/10.1016/j.chemosphere.2017.10.125
Wang C, Wang H, Cao Y (2018) Pb(II) sorption by biochar derived from Cinnamomum camphora and its improvement with ultrasound-assisted alkali activation. Colloids Surf A Physicochem Eng Asp 556:177–184. https://doi.org/10.1016/j.colsurfa.2018.08.036
Abdelhafez AA, Li J (2016) Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J Taiwan Inst Chem Eng 61:367–375. https://doi.org/10.1016/j.jtice.2016.01.005
Elgueta E, Sánchez J, Dax D, Xu C, Willför S, Rivas BL, González M (2016) Functionalized galactoglucomannan-based hydrogels for the removal of metal cations from aqueous solutions. J Appl Polym Sci 133:44093. https://doi.org/10.1002/app.44093
Zhang M, Li Y, Yang Q, Huang L, Chen L, Ni Y, Xiao H (2018) Temperature and pH responsive cellulose filament/poly (NIPAM-co-AAc) hybrids as novel adsorbent towards Pb(II) removal. Carbohydr Polym 195:495–504. https://doi.org/10.1016/j.carbpol.2018.04.082
Acknowledgments
Authors are thankful to Department of Science & Technology (DST), Ministry of Science & Technology, New Delhi for providing instrumentation facility under DST-FIST scheme to DAV College, Jalandhar. One of the authors, Rajesh Kumar, acknowledges I.K Gujral Punjab Technical University, Kapurthala, Punjab for providing research support to him. Dr. Rajeev Kr. Sharma acknowledges UGC, New Delhi for sanctioning MRP no. F.8-4(92)/2015 (MRP/NRCB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1968 kb)
Rights and permissions
About this article
Cite this article
Kumar, R., Sharma, R.K. & Singh, A.P. Synthesis and characterization of cellulose based graft copolymers with binary vinyl monomers for efficient removal of cationic dyes and Pb(II) ions. J Polym Res 26, 135 (2019). https://doi.org/10.1007/s10965-019-1790-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1790-9