Abstract
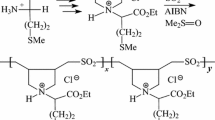
In solvent acetone, ethyl ester hydrochloride of N,N-diallylmethionine I having sulfide motifs underwent alternate cyclopolymerization with SO2 to give copolymer II (i.e. I-alt-SO2), while in DMSO (Me2S = O), it gave terpolymer III containing sulfide and sulfoxide motifs in a 1:1 ratio as a result of oxygen transfer from Me2SO. Remaining sulfide group in III upon oxidation with H2O2 afforded polymer sulfoxide IV and polymer sulfone V. Likewise; copolymerization of hydrochloride salt of N,N-diallylmethionine VI with SO2 in DMSO gave copolymer VII containing sulfide/sulfoxide motifs in a ≈ 1:1 ratio. VII was oxidized to polymer sulfoxide VIII. The chemical and physical properties of these polymers were determined by FT-IR, NMR, TGA and surface tension. The solution properties of these polymers were studied in detail. The critical micelle concentration of the polymers was determined to be ≈ 7 ppm. The presence of polymers III, IV, V, VII and VIII at a very low concentration of ≈ 6 ppm in 1 M HCl imparted superb inhibitions of mild steel corrosion of 89, 94, 87, 96, and 94%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyclopolymerization [1,2,3] of diallylammonium salts or their alternate copolymerization [4,5,6,7] with SO2 have etched an important place in the synthesis of a plethora of industrially significant ionic polymers. Utilization of one such alternate copolymerization, a methionine-based diallylamine salt monomer 3 was synthesized, and copolymerized with SO2 in solvent ethanol or acetone to give water-insoluble copolymer containing sulfide 4 (Scheme 1). Surprisingly, the copolymerization in solvent dimethyl sulfoxide (DMSO) led to the formation of water-soluble polymer as a mixture of sulfide/sulfoxide 5. During the polymerization ≈50% of the sulfide motifs has been oxidized to sulfoxide as a result of oxide exchange from DMSO. The polymer’s water-solubility paved the way to study its inhibitive behavior against corrosion of mild steel in the hostile environment of 1 M HCl. Polymer 5 having so many centers of lone pair of electrons and the unquenched nitrogen and sulfur valences imparted remarkable inhibition of mild steel corrosion in 1 M HCl, as evinced by the preliminary study [8]. At a concentration of 5 ppm, L-Methionine ethyl ester hydrochloride (1), methionine-based monomer 3 and polymer 5 imparted inhibition of mild steel corrosion to the extent of 57, 58 and 96%, respectively. A polymer backbone containing numerous chelation centers associated with the pendants are expected to undergo stronger adsorption onto the metal surface, thereby achieving greater corrosion control than their monomeric analogs [9,10,11]. The effect of sulfoxide motifs in 5 on the corrosion inhibition could not be quantified and compared with that of the sulfide motifs because of water-insolubility of polymer 4. It is worth mentioning that repeated attempts to hydrolyze the ester functionalities in 4 under basic or acidic conditions resulted in failure.
In the current work, the ester functionality in monomer precursor 2 was hydrolyzed to zwitterionic and cationic monomers 8 and 9, respectively with the anticipation that the corresponding polymers 10 and 11, obtained via Butler’s cyclopolymerization protocol [1,2,3,4,5,6,7], would be water-soluble (Scheme 2). The obtainment of the polymers and changing their functional motifs would allow us to study their solution properties, and a preliminary study to compare the corrosion inhibition efficiencies of these polymers imparted by carboxylic acid, ester, sulfide, sulfoxide, and sulfone functional motifs.
Experimental
Physical methods
Perkin Elmer 16F PC FTIR was used to record IR spectra, while 1H and 13C NMR were collected in a JEOL LA 500 MHz spectrometer for the determination of the chemical composition of the synthesized compounds. A Perkin Elmer Elemental analyzer (Carlo-Erba: 2400) was utilized for elemental Analysis. The viscosity values of synthesized compounds were determined in CO2-free water using an Ubbelohde viscometer (Viscometer Constant 0.005317 mm2 s−2) under N2. Thermogravimetric analysis (TGA) was performed under N2 (flow rate 50 mL/min) using an SDT thermogravimetric analyzer (Q600: TA Instruments, New Castle, DE, USA). Surface tension was measured using a surface tensiometer (PHYWE, Germany).
Materials
Ethyl ester hydrochloride of L-Methionine (1) was obtained from Fluka Chemie AG. Hydrogen peroxide (35 w/v %) and potassium carbonate (K2CO3) were purchased from BDH Chemical Ltd. (Pool, England). Azoisobutyronitrile (AIBN) (Fluka Chemie AG) was recrystallized from CHCl3-EtOH. All solvents were of HPLC grade. Dimethylsulfoxide (DMSO), dried over CaH2 overnight, was distilled (bp 4 mmHg 64–65 °C). Diallyl derivative of methionine 2 and its hydrochloride salt 3 were prepared from L-methionine ethyl ester hydrochloride 1 as described [8].
Synthesis of monomers and polymers
Cyclocopolymerization of monomer 3 with SO2
Polymer 5 was prepared as described [8]. Briefly, a mixture of 3 (2.70 g, 9.19 mmol) and sulfur dioxide (SO2) (0.588 g, 9.19 mmol) in DMSO (2.02 g) was polymerized using an initiator (AIBN, 0.066 g, 0.4 mmol) at 60 °C for 24 h. The mole ratio of monomers (3 and SO2) to initiator was thus kept at 46:1. After dialysis against deionized water, the polymer was freeze dried. The isolated polymer sulfide/sulfoxide 5 was obtained as a white powder (2.23 g, 66%). Polymer 4 was synthesized following the same procedure except that ethanol (2.02 g) or acetone (2.02 g) was used as a solvent.
Conversion of polymer sulfide/sulfoxide 5 to polymer sulfoxide 6
Hydrogen peroxide (35 w/v %) (0.888 g, 9.14 mmol) was slowly added to a solution of 5 (0.802 g; 2.20 mmol) in glacial acetic acid (2.37 g) at 20 °C. The reaction mixture was stirred at 20 °C for 5 h or until the completion of oxidation of the sulfide to sulfoxide as indicated by the 1H NMR spectrum. The resultant solution was dialyzed against de-ionized water for 3 h followed by 30 min in 0.2 M HCl then 30 min again in de-ionized water. The freeze-drying of the dialyzed solution afforded polymer 6 as a white solid (0.720 g, 88%). Elemental analysis of C13H24ClNO5S2 found: C, 41.4; H, 6.6; N, 3.6; S, 16.8; requires: C, 41.76; H, 6.47; N, 3.75; S, 17.15%; νmax (KBr) 3427, 2924, 2609 (br), 1741, 1634, 1408, 1378, 1305, 1214, 1129, 1016, 946, 854, 782, 670 and 514 cm−1.
Conversion of polymer sulfide/sulfoxide 5 to polymer sulfone 7
The sulfide to sulfone oxidation was carried out using a slightly modified procedure [12]. Hydrogen peroxide (35 w/v%) (0.586 g, 6.03 mmol) was slowly added to a solution of polymer 5 (0.537 g; 1.47 mmol) in glacial acetic acid (1.50 g) at room temperature. After stirring the reaction mixture at 35 °C for 4 h or until the 1H NMR indicated the completion of oxidation to sulfone 7, the polymer solution was dialyzed against de-ionized water for 4 h followed by 30 min in 0.2 M HCl to reduce the cloudiness of the solution then 30 min again in de-ionized water. The solution was then freeze-dried to obtain polymer 7 as a white solid (0.470 g, 82%). Elemental analysis of C13H24ClNO6S2 found: C, 39.7; H, 6.4; N, 3.4; S, 16.1; requires C, 40.05; H, 6.20; N, 3.59; S, 16.44%; νmax (KBr) 3414, 2926, 2609 (br), 2361, 1742, 1633, 1451, 1409, 1377, 1304, 1216, 1128, 1038, 966, 851, 771, 669 and 510 cm−1.
Synthesis of cationic monomer 9 and its zwitterionic counterpart 8
A heterogeneous mixture of monomer precursor 2 (20.6 g, 80 mmol), CH3OH (50 mL), H2O (20 mL), and sodium hydroxide (3.60 g, 90 mmol) was stirred for 4 h at 40 °C. After concentrating the homogeneous mixture, the residue was diluted with water (15 mL), acidified with concentrated HCl (37%) (8.87 g; 170 mmol) (i.e. two equivalents of HCl), and then freeze-dried to obtain a mixture of cationic acid hydrochloride monomer 9 and NaCl. After trituration with acetone (100 mL) and filtering off the insoluble NaCl, the filtrate was concentrated to obtain monomer 9 as a colorless thick liquid (20.2 g, 95%). Elemental analysis of C11H20ClNO2S found: C, 49.4; H, 7.7; N, 5.2; S, 11.8; requires C, 49.71; H, 7.58; N, 5.27; S, 12.06%; νmax. (neat) 3348, 3088, 2921, 2848, 2360, 2331, 1735, 1635, 1448, 1425, 1388, 1325, 1201, 1157, 1078, 995, 946, 872, 770 and 736 cm−1; δH (D2O) 1.98 (3H, s), 2.06 (1H, m), 2.52 (1H, m), 2.65 (1H, m), 3.70 (2H, m), 3.81 (2H, m), 4.09 (1H, d, J 9.2), 5.48 (4H, m), 5.79 (2H, m), (residual H in D2O at 4.65 ppm); δC (D2O): 15.00 (1C, SCH3), 26.0 (1C, CH2 CH2S), 30.4 (1 C, CH2CH2S), 55.1 (2C, NCH2), 62.9 (1C, NCH), 126.44 (2C, CH = CH2), 127.79 (2C, CH = CH2), 171.3(1C, CO2), (67.4, dioxane). The 13C spectral assignments were confirmed by DEPT- 135 NMR analysis.
In a separate experiment, the above procedure was followed in one-tenth scale. Here, instead of two equivalents, one equivalent HCl (9.00 mmol; based on NaOH used) was used to neutralize the CO2 −Na+ salt to generate zwitterionic monomer 8 as a white solid (1.74 g; 93%). Mp. 55–59 °C. Elemental analysis of C11H19NO2S found: C, 57.4; H, 8.4; N, 6.0; S, 13.7.; requires C, 57.61; H, 8.35; N, 6.11; S, 13.98%; νmax. (KBr) 3415, 3082, 3024, 2976, 2964, 2918, 2838, 1627,1455, 1426, 1357, 1339, 1302, 1275, 1219, 1154, 1121, 1080, 1060, 1012, 994, 951, 773, 760, 718, 694, 666, 628, and 575 cm−1; δH (D2O) 1.98 (3H, s), 2.02 (2H, m), 2.46 (1H, m), 2.57 (1H, m), 3.65–3.75 (5H, m), 5.45 (4H, m), 5.79 (2H, m); δC (D2O): 15.1 (1C, SCH3), 27.0 (1C, CH2 CH2S), 30.6 (1 C, CH2CH2S), 54.4 (2C, NCH2), 65.6 (1C, NCH), 126.7 (2C, CH = CH2), 127.2 (2C, CH = CH2), 172.9 (1C, CO2), (67.4, dioxane). The 13C spectral assignments were confirmed by DEPT- 135 NMR analysis.
Cyclopolymerization of monomer 9 and SO2 in acetone
As described in Table 1, sulfur dioxide (0.962 g, 15 mmol) was adsorbed onto a solution of monomer 9 (4.0 g; 15 mmol) in acetone (8.0 g) at 0 °C in a 25-mL round bottom flask. After addition of the specified amount of AIBN [150 mg (0.91 mmol) or 200 mg (1.22 mmol)], the closed flask was heated at 60 °C for 48 h. The mole ratio of monomers (9 and SO2) to initiator was thus kept at 33:1 or 24.6:1. Within 2 h, the polymer started to separate as a white precipitate. After the time elapsed, the polymer was filtered, dissolved in NaHCO3 solution, and dialyzed against deionized water for 3 h, 0.1 M HCl for 3 h, followed by de-ionized water for additional 24 h. Phase separation occurs within the dialysis tube. The resultant polymer 10 was thereafter isolated and dried under vacuum at 60 °C. Elemental analysis of C11H19NO4S2 found: C, 44.8; H, 6.7; N, 4.6; S, 21.6; requires C, 45.03; H, 6.53; N, 4.77; S, 21.85%.νmax. (KBr) 3570, 2971, 2921, 2852, 2553 (br), 1622, 1456, 1397, 1303, 1126, 1017, 954, 877, 768, 654, and 514 cm−1.
Cyclopolymerization of monomer 9 and SO2 in DMSO
As described in Table 1, sulfur dioxide (0.962 g, 15 mmol) was adsorbed onto a homogeneous mixture of monomer 9 (4.0 g; 15 mmol) in DMSO (3.5 g) in a 25 mL round bottom flask. After the addition of the specified amount of AIBN, the closed flask was heated at 60 °C for 48 h. Noted that few times the reaction flask was cooled and opened to release N2 gas obtained from the decomposition of the initiator. The resultant reaction mixture was dialyzed against deionized water for 10 h, then freeze-dried to obtain polymer 11. Elemental analysis of polymer 11 having an approximate 1:1 ratio of sulfide (C11H19NO4S2) and sulfoxide motifs (C11H19NO5S2) found: C, 43.4; H, 6.2; N, 4.4; S, 20.8; requires C, 43.87; H, 6.36; N, 4.65; S, 21.29%; νmax. (KBr) 3425, 2922, 1626, 1452, 1400, 1305, 1128, 1021, 947, 876, 766, 653 and 515 cm−1.
Conversion of polymer sulfide-sulfoxide 11 to corresponding sulfoxide 12
Hydrogen peroxide (35 w/v %) (0.237 g; 2.44 mmol) was slowly added to a solution of polymer 11 (0.675 g; 2.24 mmol) in glacial acetic acid (2.24 g) at 20 °C. The reaction mixture was then stirred at 20 °C for 5 h or until the completion of oxidation as indicated by 1H NMR spectrum. After the time elapsed, the resultant mixture was dialyzed against de-ionized water for 8 h, and then freeze-dried to obtain polymer 12 (0.496 g, 72%). Elemental analysis of C11H19NO5S2 found: C, 42.4; H, 6.3; N, 4.4; S, 20.4; requires C, 42.70; H, 6.19; N, 4.53; S, 20.72%; νmax (KBr) 3481, 2923, 2678 (br), 1632, 1456, 1401, 1305, 1128, 1021, 787, 677 and 507 cm−1.
Potentiometric titrations
The protonation constant (K) of basic nitrogen was calculated by potentiometric titration at 23 °C in salt-free water following the published literature procedure described elsewhere [13, 14]. As described in Table 2, a certain amount of zwitterionic polymer 12 (ZH±) in CO2-free water (200 mL) was titrated using step-wise addition of 0.0959 M NaOH solution (0.05–0.15 mL). After each addition, the solution was stirred briefly using a magnetic stir bar under N2 and the pH values were recorded, and the log Ks were calculated using the Henderson-Hasselbalch Eq. (2) (Scheme 2). Where degree of protonation (α) is defined as the ratio [ZH±]eq/[Z]o. The [Z]o and [ZH±]eq are the respective initial analytical concentration of the monomeric units in 12 (ZH±) and its concentration at the equilibrium as given by: [ZH]eq = [Z]o - COH − - [H+] + [OH−]. The COH − is the concentration of the added NaOH, while [H+] and [OH−] at equilibrium were calculated from the pH value.
The Eq. 2 (Scheme 2) describes the apparent basicity constants of typical polyelectrolytes; in the case of sharp basicity constants log K o = pH at α = 0.5 and n = 1. The pH vs. log [(1-α)/α)] plot gave log K o and ‘n’ as the intercept and slope, respectively.
The modified Henderson-Hasselbalch Eq. (3) (Scheme 2) is obtained by inserting the value of pH from Eq. 2 into Eq. 1 whereby (n – 1) gives a measure of the deviation of the studied polymers from the behavior of small molecules which show sharp basicity constants with an n value of 1.
Surface tension
A surface tensiometer (PHYWE, Germany) as described [15] equipped with torsion dynamometer (0.01 N) and platinum iridium ring (diameter = 1.88 cm) was utilized to determine the surface tension of 1 M HCl containing various concentrations of the synthesized polymers at 60 °C.
The standard free energy of micelle formation (ΔG°mic)
The ΔG° mic of the synthesized polymers were determined by Eq. (4) [16]:
where C cmc represents the polymer concentration at the CMC.
Inhibition efficiency by gravimetric measurements
The inhibition study of synthesized polymers were performed by gravimetric weight loss method following published literature procedure [15], using steel coupons having the dimensions 2.5 × 2.0 × 0.1 cm3 in 1 M HCl (250 mL) for an immersion time of 6 h.
Results and discussion
Monomer and polymer syntheses
Cationic monomer 3 underwent cyclopolymerization with SO2 in ethanol or acetone solvent afforded cyclocopolymer 4, while in DMSO solvent to give alternate copolymer 5 in which the sulfide and sulfoxide were formed in an approximate ratio of 1:1 (Scheme 1) [7]. Copolymer 5 upon oxidation using H2O2/HOAc at 20 and 35 °C afforded polymer sulfoxide 6 and polymer sulfone 7, respectively, in excellent yields.
Upon hydrolysis of the ester group in trivalent amine 2 [8] with NaOH followed by acidification with one and two equivalents of HCl led to zwitterionic 8 and cationic acid hydrochloride monomer 9, respectively (Scheme 2). Monomer 9 underwent AIBN-initiated copolymerization with SO2: while in acetone medium it gave copolymer 10 after depletion of HCl during dialysis, the polymerization in DMSO afforded polymer 11. The formation of polymer 11 with a ≈ 1:1 ratio of the sulfide/sulfoxide moieties is a result of oxygen exchange between the sulfide in the polymer and sulfoxide in DMSO (Me2S = O) [17]. The polymers are obtained in moderate to good yields (Table 1) despite possible degradative chain transfer owing to the presence of allylic motifs [18] and the ability of sulfide functionality to act as chain transfer agent. The sulfide groups in copolymer 11 was oxidized to polymer 12 having fully oxidized sulfoxide motifs.
Solubility behavior
Like polymer sulfide 4 containing ester functionalities (CO2Et), copolymer (±) 10 having zwitterionic motifs [−NH+ − CO2 −] was found to be insoluble in water. However, (+) 6, (+) 7, (+) 9, (±) 11 and (±) 12 were water-soluble owing to the greater polarity of the sulfoxide motifs which dominates over the zwitterionic motifs in dictating the solubility behavior. While overall hydrophobicity of polymer sulfide (+) 4 makes it water-insoluble, the electroneutral polymer sulfide/sulfoxide (±) 11 and sulfoxide (±) 12, like the majority of known as polyzwitterions [19,20,21], was expected to be insoluble in water owing to intragroup, intra- and interchain attractive interactions leading to the formation of ionic crosslinks. Their water solubility, therefore, is attributed mainly to the presence of polar sulfoxide motifs. The presence of salts of smaller masses is known to impart water-solubility causing disruption of the attractive forces [22]; however polyzwitterion (±) 10 having sulfide groups remained insoluble in the presence of NaCl (0–5 M) or HCl (1–12 M). A 1 wt.% solution of copolymer 10 in 2 M NaI was found to be partially soluble. Being softer (more polarizable), iodide ions effectively neutralizes the ionic crosslinks so as to disrupt attractive interactions [22]. Note that the treatment of polymer (±) 10 with 1.0 equivalent NaOH changes the zwitterionic motifs [−NH+………CO2 −] to anionic motifs [−N………CO2 −Na+] thereby imparting water-solubility.
TGA curves, FT-IR, NMR spectra, and molar mass by end group analysis
The TGA curves of 6, 7, 10, and 12 are shown in Fig. 1; a loss of ≈2–7% up to 180–200 °C was accounted for the removal of moisture. An accelerated loss of ≈ 40% for the polymers in the range ≈ 200–250 °C resulted from the release of CO2 and SO2 whose combined masses are calculated to be ≈ 36%. A further loss of ≈ 30% may be linked to the removal of the methionine pendants occurred in the range 250–400 °C. Overall, all these polymers were asserted to be stable up to ≈200 °C.
The IR spectrum of monomer (+) 9 revealed a strong absorption band at 1735 cm−1 attributed to C = O stretch of CO2H group, while the band was absent in the spectrum of zwitterionic monomer (±) 8. Likewise, this band was absent in the spectra of polymers (±) 10–12; instead peaks at ≈1400 (symmetric stretching) and ≈1427 cm−1 (anti-symmetric stretching) were assigned to the COO− motifs in the dipolar zwitterionic form of the polymers [23]. The strong bands at ≈ 1305 cm−1 and ≈1128 cm−1 in the IR spectra of all the as-synthesized polymers are due to the SO2 stretching vibrations, while a band at 1021 cm−1 can be assigned to the S = O stretching absorption in 5, 6, 11 and 12.
Elemental analysis of C13H24ClNO6S2 found: C, 39.7; H, 6.4; N, 3.4; S, 16.1; requires C, 40.05; H, 6.20; N, 3.59; S, 16.44%; νmax (KBr) 3414, 2926, 2609 (br), 2361, 1742, 1633, 1451, 1409, 1377, 1304, 1216, 1128, 1038, 966, 851, 771, 669 and 510 cm−1.
Figures 2, 3, and 4 display some representative 1H and 13C NMR spectra of monomer 9 and several polymers. As can be seen, the methyl protons marked ‘c’ and ‘c′’ of 5 appeared at δ2.0 and 2.6 ppm, respectively (Fig. 2a). The downfield signal at δ2.6 was attributed to the sulfoxide motifs as a result of greater electron withdrawing ability of S = O as compared to sulfide group. Upon oxidation, the sulfide group in 5 is converted into sulfoxide 6 as evident by disappearance of the signal marked ‘c’ (Fig. 2b). Further oxidation resulted in the formation of sulfone motifs in 7; the methyl protons marked ‘c′’ appeared at a downfield shift of δ3.0 ppm because of greater electronegativity of sulfone groups in 7 (Fig. 2c).
Polymers 10–12 even after extended dialysis contain ≈ 3–4% residual alkene as revealed by alkene protons and carbons in the range δ5.4–5.8 (Fig. 3) and ≈125 ppm (Fig. 4), respectively. The presence of pendant double bonds [13] could be resulting either from chain propagation without cyclization or transfer to the monomer [4]. The SMe protons of polymer 10 marked ‘g’ appeared at δ 2.0 ppm (Fig. 3b), while partial oxidation of SMe to O = SMe is confirmed by the presence of additional signal of methyl protons of polymer 11 marked ‘g′’ at ≈ δ2.6 ppm (Fig. 3c). Complete oxidation of sulfide to sulfoxide polymer 12 is assured by the missing SMe signal both in the 1H and 13C spectra of polymer 12; carbon signal of SMe (marked g) and O = SMe (marked g′) appeared at ≈ δ15 and 37 ppm, respectively.
As reported earlier [24] and found in the current work, the molar masses of the polymers could not be obtained by GPC presumably owing to the strong adsorption of amine and carboxyl motifs to the column materials. End group analysis, however, helped [25] us to calculate the approximate number average molar masses (M n ) of some of the polymers 10–12. The (CH3)2C(CN) group (originating from the AIBN initiator) attached to the chain end is displayed as a broad singlet at δ1.31 ppm and was not interfered with any competing signal (Fig. 3). However, the calculation is complicated by the lack of understanding of the mode of chain termination. In the absence of chain transfer, while the coupling mechanism would lead to two initiator fragments at two chain ends, termination by disproportionation on the other hand would have only one terminal with the initiator fragment. Low molar masses (vide infra) are suggestive of the chain termination by a degradative chain transfer process [18] involving transfer of an allylic hydrogen from monomer 9 to a chain radical. The resultant stable allylic radical cannot reinitiate polymerization and as such the transfer would amount to a termination process. However, chain transfer to the sulfide motifs may give a radical which may able to reinitiate a new chain which may not have the initiator fragment (CH3)2C(CN) at either end of the dead polymer. Even though sulfide group may act as a chain transfer agent, its chain transfer constant, Cs (i.e. the ratio of rate constant of the chain transfer of a propagating radical to the rate constant for propagation of the radical) value of ≈ 0.0022 does make it an insignificant process. Keeping in view the degradative chain transfer as the main process, it can be assumed that each polymer chain on average will have one initiator fragment. The integrated area (A) at δ1.31 is attributed to the 6 H of the end group (CH3)2C(CN), while the area B covering signals in the range δ1.5–4.3 ppm belongs to all the 18 H of each repeating unit (RU). The degree of polymerization (DP), calculated by taking the area ratio of 1 H of the RU and the end group, thus equals to (B/18)/(A/6). The average DP is thus found to be 40.9 and 42.1 for polymer 11 and 12, respectively. The similar values are expected since polymer 11 was transformed to 12. The DP value of 40.9 was translated into a number average molar mass of 12,300 g mol−1 for polymer 11. Likewise, the DP of polymer 10 was calculated to be 95 with a molar mass of 27,900 g mol−1 suggesting that polymerization in solvent acetone led to polymer 10 having higher molar mass than polymer 11 obtained in DMSO solvent. End group analysis cannot be carried out for polymers 5–7 since the signal for the initiator fragment (CH3)2C(CN) is expected to overlap with that of the methyl protons marked ‘a’ (Fig. 2).
Viscosity measurements
The viscosity plots for polymers 5–7 are shown in Fig. 5. In salt-free water, reduced viscosity increases with decrease in concentrations of 6 as expected of a polyelectrolyte. In 0.1 M NaCl, the viscosity plots become normal. The polymers have very low intrinsic viscosity [η] values thereby suggesting lower molar masses for the polymers.
The viscosity plots (Fig. 6) of 10 and 11 (both treated with 1 equivalent of NaOH) in 0.1 M NaCl revealed that the former, obtained from polymerization carried out in acetone, have higher [η] (0.0928 vs. 0.0552 dL g−1) (cf. Fig. 6: a vs. f). The data are in line with the end group analysis (vide supra) which revealed higher molar mass for polymer 10 as compared to that of polymer 11. As expected, polymers 11 and 12 have similar [η] since the latter was derived from the former by oxidation (cf. Fig. 6: f vs. g). The anti-polyelectrolyte behavior of the zwitterionic motifs in (±) 12 is demonstrated by the increasing viscosity values with increasing salt concentrations (cf. Fig. 6: b,c,d) [26]. The Cl− ions bind more strongly to the positive nitrogens as compared to the binding of Na+ to the CO2 − thereby leaving an excess of negative charge on the zwitterionic dipoles of polymer 12, the repulsion among which leads to an increase in the hydrodynamic volume hence viscosity with increasing NaCl concentration [20, 27, 28]. Polyzwitterion, PZ (±) 11 on treatment with 1 equivalent NaOH is converted to an anionic polyelectrolyte as a result of the change of the zwitterionic motifs (NH+...CO2 −) to anionic motifs (N...CO2 −). As such the viscosity curve of PZ (±) 11 in the presence of NaOH became concave upwards in salt-free water (Fig. 6e). The viscosity values of PZ (±) 12 in the presence of NaOH is found to be like that of (±) 11 and almost overlaps with the plot in Fig. 6e; as such it is not shown in the Figure. In 0.1 M NaCl, the viscosity decreases and the plot becomes linear demonstrating its polyelectrolyte behaviour (Fig. 6f).
Using an Ubbelohde Viscometer at 30 °C: the viscosity behavior of (a) ♦10 in 0.1 M NaCl (+ 1 equiv. NaOH), (b) □ 12 in 0.5 M NaCl, (c) ∆ 12 in 0.1 M NaCl, (d) 12 in salt-free water, (e) ■ 11 in salt-free water (+ 1 equiv. NaOH), (f) ● 11 in 0.1 M NaCl (+ 1 equiv. NaOH), (g) ○ 12 in 0.1 M NaCl (+ 1 equiv. NaOH)
Lower viscosity values as revealed in Fig. 6 are indicative of lower molar masses of the polymers. This is expected since monomer 9 have degradative chain transfer allylic groups [18], as well as the sulfide functionality capable of initiating chain transfer.
Basicity constant
The apparent basicity constant of amine nitrogens in polymer 13 is described by Eq. (3) (Scheme 2) where log K o = pH at α = 0.5 and n = 1 observed for basicity constant of small molecules. The slope and intercept of the straight line plot of pH vs. log [(1-α)/α)] gave the values of ‘n’ and log K o, respectively (Fig. 7a). In salt-free water, basicity constant log K o was determined to be 7.30 (Table 2), which is found to be less than the log K o value of 9.93 for a similar polymer having a glutamic acid residue [29]. Lower basicity constant of nitrogens in polymer 13 may be attributed to the higher electronegativity of the sulfoxide motifs. Since log K of a base (B−) is the pK a of its conjugate acid (HB), the presence of sulfoxide motifs in polymer 12 makes it a stronger acid.
The n value of 2.51 reflects the “apparent” [30] nature of the basicity constant. A measure of the polyelectrolyte index n is shown in Fig. 7b displaying a greater variation of K with α signifying a strong polyelectrolyte effect (because of n being greater than 1). With increasing α, a gradual decrease of log K [involving (Z−) 13 + H+ ⇋ (ZH±) 12] is a result of decreasing overall negative charges that induces protonation. The greater n value confirms the consequence of entropy effects [30, 31]. Since anionic polymer (−) 13 is expected to be more hydrated than the zwitterionic (±) polymer 12, water molecules from the repeating unit of anionic polymer 13 is released as it is transformed to zwitterionic polymer 12. With each protonation, the excess average negative charge in the polymer backbone of polymer 12 or 13 decreases as does the average number of water molecules associated with each repeating unit. Therefore, there will be lesser and lesser number of water molecules to be released from the polymer backbone of polymer 12 or 13 with increasing α, and associated entropy change dictates the decrease of K with increasing α.
Surface tension
The plots of surface tension γ versus concentration of the polymers to determine the CMC of 5–7 and 11 were measured in 0.1 M HCl at 60 °C are shown in Fig. 8. The polymers may be considered as cationic surfactants because of the presence of ionic hydrophilic head and moderate hydrophobic pendants of (CH2)2SMe. The polymer backbone consisting of -CH2-CH-CH-CH2-may as well be considered as hydrophobic. Polymers are found to show moderate surface active property having CMC values in the range 18.1–22.0 μM (i.e. 6.3–8.58 ppm) (Table 3). The molarity of polymers is calculated on the basis of molar mass of the repeating unit having a unit each of the monomer (average of S and S = O) and SO2. ΔG o mic of micellization was calculated to be ≈ − 30 kJ mol−1.
Inhibition of mild steel corrosion
Gravimetric weight loss method [15, 32], the most reliable protocol, was utilized to determine the inhibition efficiency (IE) of the synthesized polymers on mitigating mild steel corrosion in a hostile environment of 1 M HCl for 6 h at 60 °C. The IEs were determined as described [32] after immersing steel coupons into 1 M HCl (250 mL) in the absence (blank) and presence of the synthesized polymers. The %IE was then calculated using Eq. (5):
where W b and W i represent weight loss in the absence and presence of the polymers, respectively. At a meager concentration of 17.5 μM (in terms of repeating unit) (≈ 6 ppm) of polymers 5, 6, 7, 11, and 12, IEs of 89, 94, 87, 96, and 94% are achieved, respectively. Usually, the %IEs are expected to increase with increasing inhibitor concentration. In the ester (−CO2Et) series, the increase of IE of the polymers in the order: 6 > 5 > 7 suggests the greater effectiveness of the sulfoxide (S = O) motifs as compared to sulfide (S) and sulfone (O = S = O) in mitigating the mild steel corrosion. Polymers 11 and 12 having carboxy groups (CO2 −) performed even better, presumably owing to the protection of the metal surface via the formation of coordinate type of bond involving the chelating motifs of amino carboxylate (N….. CO2 −) with the vacant d-orbitals of iron [33,34,35]. Note that amino ester motifs (N….. CO2Et) in 5–7 cannot provide such a chelating motifs to cover and safe guard the metal surface.
Conclusions
Cationic and zwitterionic polymers 4–7 and 10–12 based on amino acid methionine and its derivative methionine sulfoxide and sulfone have been synthesized via cyclopolymerization of diallylamine salts. The TGA indicated that the synthesized polyelectrolytes were stable up to 200 °C. The solubility of the polymers is greatly influenced by the oxidation state of the sulfur; while the sulfide pendant imparts water-insolubility, polymers bearing polar sulfoxide and sulfone pendants were found to be water-soluble. Water-soluble polyzwitterion (±) 12 bearing sulfoxide pendants showed anti-polyelectrolyte behavior as demonstrated by increasing viscosity with increasing NaCl concentrations. Surface tension measurement indicated the polymers (+) 5, (+) 6, (+) 7, and (±) 11 as moderately surface active agents to aid the formation of surface film on the metal surface to assist in the inhibition of metal corrosion in hostile environments. A preliminary investigation has demonstrated that the synthesized polymers containing unquenched nitrogen and sulfur valences acted superbly in mitigating mild steel corrosion in 1 M HCl. The presence of nonbonding electrons in nitrogen as well as in the more polarizable sulfur motifs, leads to the formation of coordinate type of bond with the vacant d-orbitals of iron or accumulated Fe2+ especially on anodic sites of the metal surface thereby imparting corrosion inhibition. The molar masses, surface tension and protonation constant of the nitrogens in these polymers are expected to affect corrosion inhibition efficiency. We are currently examining the corrosion inhibition of these amazing and apparently green polymers in detail using various electrochemical techniques. The study would focus on the long-term inhibition activity especially near CMC of the polymers [36].
References
Butler GB (1992) Cyclopolymerization and cyclocopolymerization. Marcel Dekker, New York,
Singh PK, Singh VK, Singh M (2007) Zwitterionic polyelectrolytes: a review. E-Polymers 030:1–34
Ali SA, Haladu SA, El-Sharif AMZ (2016) Synthesis and application of a cyclopolymer bearing a propylphosphonic acid and a propylcarboxylic acid pendants in the same repeating unit. J Polym Res 23:167–176
Jaeger W, Bohrisch J, Laschewsky A (2010) Synthetic polymers with quaternary nitrogen atoms- synthesis and structure of the most used type of cationic polyelectrolytes. Prog Polym Sci 35:511–577
Ali SA, Al-Hamouz OCS (2012) Comparative solution properties of cyclocopolymers having cationic, anionic, zwitterionic and zwitterionic/anionic backbones of similar degree of polymerization. Polymer 53:3368–3377
Abu-Thabit NY, Kazi IW, Al-Muallem HA, Ali SA (2011) Phosphonobetaine/sulfur dioxide copolymer by Butler's cyclopolymerization process. Eur Polym J 47:1113–1123
Ali SA, Umar Y, Abu-Sharkh BF, Al-Muallem HA (2006) Synthesis and comparative solution properties of single-, twin-, and triple-tailed associating ionic polymers based on diallylammonium salts. J Polym Sci Part A Polym Chem 44:5480–5494
Al-Muallem HA, Mazumder MAJ, Estaitie MK, Ali SA (2015) A novel cyclopolymer containing residues of essential amino acid methionine: synthesis and application. Iran Polym J 24:541–547
Ulman R (1964) In: Mark HF, Gaylord NG, Bikales NM (eds) Encyclopedia of polymer science and technology, vol 1. New York, Interscience,
Annand RR, Hurd RM, Hackerman N (1965) Adsorption of monomeric and polymeric amino corrosion inhibitors on steel. J Electrochem Soc 112:138–144
Bacskai R, Schroeder AH, Young DC (1991) Hydrocarbon-soluble alkaline/formaldehyde oligomers as corrosion inhibitors. J Appl Polym Sci 42:2435–2441
Golchoubian H, Hosseinpoor F (2007) Effective oxidation of sulfides to sulfoxides with hydrogen peroxide under transition-metal-free Conditions. Molecules 12:304–311
Ali SA, Haladu SA, Al-Muallem HA (2014) Bis[3-(diethoxyphosphoryl)propyl]diallyl ammonium chloride: synthesis and use of its copolymer as an antiscalant. J Appl Polym Sci 131:40615–40625
Ali SA, Abu-Thabit NY, Al-Muallem HA (2010) Synthesis and solution properties of a pH-responsive cyclopolymer of zwitterionic ethyl 3-(N,N-diallylammonio)propanephosphonate. J Polym Sci Part A Polym Chem 48:5693–5703
Mazumder MAJ, Al-Muallem HA, Ali SA (2015) The effects of N-pendents and electron-rich amidine motifs in 2-(p-alkoxyphenyl)-2-imidazolines on mild steel corrosion in CO2-saturated 0.5 M NaCl. Corros Sci 90:54–68
Butt HJ, Graf K, Kappl M (2003) Physics and Chemistry of Interfaces. Wiley-VCH, Weinheim,
Shechter Y (1986) Selective oxidation and reduction of methionine residues in peptides and proteins by oxygen exchange between sulfoxide and sulfide. J Biol Chem 261:66–70
Butler GB, Angelo RJ (1957) Preparation and polymerizationof unsaturated quaternary ammonium compounds. A proposed alternating intramolecular-intermolecular chain propagation. J Am Chem Soc 79:3128–3131
Yoshizawa M, Ohno H (1999) Molecular brush having molten salt domain for fast ion conduction. Chem Lett 28:889–890
Wielema TA, Engberts JBFN (1987) Zwitterionic polymers—I. Synthesis of a novel series of poly (vinylsulphobetaines). Effect of structure of polymer on solubility in water. Eur Polym J 23:947–950
Salamone JC, Volksen W, Olson AP, Israel SC (1978) Aqueous solution properties of a poly(vinyl imidazolium sulphobetaine). Polymer 19:1157–1162
Kudaibergenov S, Jaeger W, Laschewsky A (2006) Polymeric betaines: synthesis, characterization, and application. Adv Polym Sci 201:157–224
Pearson JF, Slifkin MA (1972) The infrared spectra of amino acids and dipeptides. Spectrochim Acta A 28:2403–2417
Rullens F, Devillers M, Laschewsky A (2004) New regular, amphiphilic poly(ampholyte)s: synthesis and characterization. Macromol Chem Phys 205:1155–1166
Al-Muallem HA, Wazeer MIM, Ali SA (2002) Synthesis and solution properties of a new pH-responsive polymer containing amino acid residues. Polymer 43:4285–4295
Liu C, Gu X, Cui M, Xu Q, Li R (2014) A novel ternary copolymerized polyzwitterionic of poly (AM/DMAM/MAEDAPS): synthesis and solution properties. J Polym Res 21:620–631
Nishida K, Kaji K, Kanaya T, Fanjat N (2002) Determination of intrinsic viscosity of polyelectrolyte solutions. Polymer 43:1295–1300
Higgs PG, Joanny JF (1991) Theory of polyampholyte solutions. J Chem Phys 94:1543–1554
Jamiu ZA, Ali SA (2016) Alternate cyclopolymer of diallylglutamic acid and sulfur dioxide. RSC Adv 6:31019–31030
Barbucci R, Casolaro M, Danzo N, Barone V, Ferruti P, Angeloni A (1983) Effect of different shielding groups on the polyelectrolyte behavior of polyamines. Macromolecules 16:456–462
Barbucci R, Casolaro M, Ferruti P, Nocentini M (1986) Acid-base and metal ion complex formation properties of polymers containing amino acid residues. Macromolecules 19:1856–1861
Ali SA, Al-Muallem HA, Saeed MT, Rahman SU (2008) Hydrophobic-tailed bicycloisoxazolidines: a comparative study of the newly synthesized compounds on the inhibition of mild steel corrosion in hydrochloric and sulfuric acid media. Corros Sci 50:664–675
Ju H, Kai Z-P, Li Y (2008) Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic media: a quantum chemical calculation. Corros Sci 50:865–871
Fang J, Li J (2002) Quantum chemistry study on the relationship between molecular structure and corrosion inhibition efficiency of amides. J Mol Struct (THEOCHEM) 593:179–185
Daoud D, Douadi T, Hamani H, Chafaa S, Al-Noaimi M (2015) Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: experimental and computational study. Corros Sci 94:21–37
Esumi K, Ueno M (2003) Structure performance relationships in surfactants. Marcel Dekker, New York,
Acknowledgements
The authors gratefully acknowledge Deanship of Scientific Research, King Fahd University of Petroleum and Minerals (KFUPM), Saudi Arabia for providing the research facilities and financial assistance to carry out this research through internal grant project No. IN131047.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ali, S.A., Goni, L.K.M.O. & Mazumder, M.A.J. Butler’s cyclopolymerizaton protocol in the synthesis of diallylamine salts/sulfur dioxide alternate polymers containing amino acid residues. J Polym Res 24, 184 (2017). https://doi.org/10.1007/s10965-017-1334-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1334-0