Abstract
3-[2-(N-methylacrylamido)-ethyldimethylammonio] propanesulfonate (MAEDAPS), a novel zwitterionic monomer, was designed and synthesized in this study. Then it reacted with acrylamide and N, N-dimethyl acrylamide by free radical polymerization in aqueous solution with ammonium persulfate ((NH4) 2S2O8) and sodium sulfite (NaHSO3) as initiator. The structure of MAEDAPS was characterized by 1H NMR and IR. Thermal stability of the obtained copolymer was tested by DSC, TGA and TG-IR analysis. Glass transition temperature (Tg) and melt temperature (Tm) was observed by DSC, meanwhile, the thermal degradation process was studied via TGA and TG-IR. It turned out that the thermal degradation process can be divided into three stages including removal of physically absorbed water, decomposition of side groups and degradation of polymer main chain. Anti-polyelectrolyte behavior was observed based on the intrinsic viscosity. Solution properties of ternary copolymer were exhibited by reduced viscosity. The result showed that the addition of small molecular electrolytes weakened the coulomp attraction between sulfonic acid group and quaternary ammonium group, and the conformation became extend, which led to the increase of hydrodynamic volume and reduced viscosity. The ability of monovalent and divalent cationic charges influencing the viscosity of zwitterionic copolymer obeyed the following sequence: K+ < Li+ < Na+, Cu2+ < Ca2+ < Mg2+, and anions was in the order: Cl−< I−< Br−.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water-soluble zwitterionics copolymer is a kind of polymer that contains both zwitterionic monomers and ordinary water soluble monomers. Polyzwitterionic are dipolar species, whose cationic and anionic groups are separately bound to the same monomer unit [1]. In order to obtain copolymer that possessed special properties, introducing zwitterionic monomers into polyacrylamide become a mainly and efficiently method that make the polyacrylamide multifunctional in recent years. Maintaining the preeminent properties of polyzwitterionic and polyacrylamide, water-soluble zwitterionic copolymer has attracted increasingly attention and research in the past few years [2].
Zwitterionic copolymers have been paid much attention because of their chemical and thermal stability [3]. On the one hand, many researchers have put their effort into the synthesis, viscosity and structure of polyzwitterionics. Der-jang Liaw et al. studied the microstructure, reactivity ratio and viscometric of a kind of zwitterionic copolymer [4]. Lukas Sonnenschein and Andreas Seubert synthesized a series of zwitterionic monomers using 4-vinylbenzyl as precursors [5]. Michael S. Donovan et al., focused their attention on the synthetic of different type of polyzwitterionics. Nelly Bonte and Andre Laschewsky synthesized a series of polysulfobetaines and polycarbobetaines and paid attention to viscometric studies [6]. In a more practical sense, some researchers devoted themselves to the application of polyzwitterionics and achieved abundant results. Under the effort of many researchers, polyzwitterionics can now be widely used into water treatment [7], enhanced oil recovery [8], smoke filtration [9], drag reduction [10], salt-resistant thickeners [11], biomedical engineering [12–14], surfactants [15], fiber modification [16] and formulation of personal care items. However, the limited research is far from being enough.
Most of water-soluble zwitterionic copolymers are copolymerized with cationic monomers and anionic monomers. For this reason, the control of the number of cationic and anionic becomes a difficult problem. The zwitterionic monomer we obtained contains both cationic and anionic in the same monomer unit. So it can be perfectly copolymerized with AM (acrylamide) and not profoundly reduced the viscosity of the water-soluble copolymer. At the same time, this method removed the deficiency of the two traditional synthesis routes, make the control of positive and negative charges becomes possible [17].
This study aimed at providing an interesting opportunity to examine the synthesis and aqueous solution properties of the novel zwitterionic copolymer which has the same number of anionic and cationic groups. In this paper, through the ring opening reaction of 1,3-propane sultone with tertiary amine we obtained a kind of zwitterionic monomer [18]. Then the zwitterionic monomer copolymerized with AM and DMAM (N, N-dimethyl acrylamide) statistically. The copolymer we obtained contains both quaternary ammonium cations and sulfonate acid anions which are less susceptible to the influence of brine solution ambient. Then, the zwitterionic monomer and copolymer are characterized. In the meantime, thermal stability of the polymer and the dilute solution properties in different electrolyte are studied.
Experimental
Materials
Acrylamide (Chengdu Kelong Chemicals Co., Ltd.) was recrystallized from acetone and dried at 30 °C for 24 h. Methylacryloyl chloride (Shanghai Puzhen chemicals Co., Ltd.), N, N-dimethylethylenediamine (Shanghai Haohua chemicals Co., Ltd.) and 1,3-propane sultone (Shanghai Ziyi chemicals Co., Ltd.) was used directly. NN-dimethylacrylamide was vacuum distilled to remove inhibitor. Ammonium persulfate (Chengdu Kelong Chemicals Co., Ltd.) and sodium hydrogen sulfite (Chengdu Kelong Chemicals Co., Ltd.) were used as initiator. 4-methoxyphenol (Chengdu Kelong Chemicals Co., Ltd.) was used as polymerization inhibitor. Water was purified with a PCS-01 system.
Synthesis
Synthesis of zwitterionic monomer
To a 250-mL three-necked round-bottom flask fitted with a dropping funnel and a reflux condenser was charged with N, N-dimethylethylenediamine (8.82 g, 0.1 mol) was added into methylene chloride (40 mL) and triethylamine (11.11 g, 0.11 mol). The above solution was agitated and placed over an ice bath under nitrogen. The mixture of methacryloyl chloride (11.495 g, 0.11 mol) and anhydrous methylene chloride (20 mL) was slowly added into the flask at a rate of 2–3 drops per minute so that the temperature of suspension was maintained at <5 °C. After complete addition, the reaction was continued over night at 30 °C. The mixture was filtered and liquid and white crystal was obtained. The white crystal was washed twice with acetonitrile. Meanwhile the organic layer was evaporated to remove methylene chloride, and the brown liquid was dissolved in acetonirtile. All collected organic layers were dried with anhydrous MgSO4 overnight. After evaporation of the solvent, brown oil was obtained. The crude product was purified by vacuum distillation in the presence of a small amount of methoxyphenol as an inhibitor. In the end, a slightly yellow oily liquid was collected [19].
N-(2-Dimethylamino-ethyl)-2-methyl-acrylamide, MEHQ (methoxyphenol as the inhibitor), and dry acetonitrile were added into a 250 mL three-necked flask equipped with a stirrer, a cooler and a thermometer at room temperature. 1,3-propyl sulfonic acid lactone was added dropwise under nitrogen at room temperature over a period of 2 h. After complete addition, the mixture was then heated to 50 °C for 24 h. On completion of the reaction, the mixture was cooled to room temperature. Then the solvent was removed by filtrate and a white color crude production was obtained [20–23]. The crystals were collected by filtration, washed with dry acetone several times, and drying in vacuum oven for 24 h, at 40 °C.
Aqueous copolymerization of AM/DMAM/MAEDAPS
To a 250-mL four-necked round-bottom flask equipped with a mechanical stirrer, a condenser, a nitrogen inlet, and a thermometer was charged into AM, DMAM, and MAEDAPS was dissolved completely in deionized water by agitating and then was poured into the round-bottom flask. The quantities of AM, DMAM, and MAEDAPS were in the desired ratio and total monomer concentration was 20 %. The mixture was stirred over a 30 °C water bath. The system was purged with nitrogen at least 30 min, and then initiator (ammonium persulfate and sodium hydrogen sulfite 1 wt %) was added dropwise. Polymerization was conducted continuously at 30°Cfor 6 h. After the reaction, the polymer solution was precipitated by a large quantity of acetone. The precipitate was washed by acetone three times and immersed in acetone for 12 h to remove all traces of water, initiator, and residual monomers. Then the white precipitate was sliced, and freeze-drying under vacuum at −45 °C for 24 h. At last, the slices were shattered, and the white powder was obtained [24].
Characterization
Structure determination
1H spectra were obtained by Varian UNITY INOVA400NMR spectrometer (Varian Co. USA). Monomer structure was characterized by 1H NMR in deuterium oxide (D2O).
Fourier Transform Infrared Spectroscopy (FTIR) was obtained by Nicolet 560 (Nicolet Co. USA).
Thermal Gravity Analysis- Fourier Transform Infrared Spectroscopy (TGA-IR) information was recorded by Nicolet is10 (Thermo Co. USA).
Elemental analysis was performed with a Euro Ea 3,000 instrument (Leeman Labs INC. USA).
Thermal analysis
Differential Scanning Calorimetry (DSC) curves were obtained by Modulated Differential Scanning Calorimety Q2000 (TA Co. USA), in the nitrogen gas environment.
TGA and DTG curves were obtained by Thermogravimetric Analysis TG209F1 (Netzsch Co. Germany), in the nitrogen gas environment.
Intrinsic viscosity
Intrinsic viscosity of polymer solution was determined using a dilution-type Ubbelohde viscometer with 1.0 mol/L NaCl as solvent and the concentration of the polymer was 0.1 g/dL. The measurement was kept at 30.0 ± 0.1 °C. The relation of reduced viscosity and polymer concentration was extrapolated to zero concentration and intrinsic viscosity and Huggins constant were obtained by intercept and slope. The reduced viscosity was calculated by dividing flow time of polymer solution by flow time of solvent obtained by the dilution method.
Salt –resistant properties measurements
Reduced viscosity of the polyzwitterionics was determined utilizing a dilution-type Ubbelohde viscometer in different kinds of small molecule electrolyte solution and different concentration of electrolyte solution, and the concentration of the polymer was 0.1 g/dL. The measurement was kept at 30.0 ± 0.1 °C.
Results and discussion
Synthesis and characterization of zwitterionic monomer
The zwitterionic monomer MAEDAPS was synthesized via the ring-opening reaction of 1,3-Propanesultone with the substitution reaction product of N, N-dimethylethylenediamine and Methacryloyl chloride [25, 17]. The structure and the synthetic route of the MAEDAPS are shown in Scheme 1.
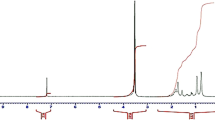
The FTIR spectrum and 1H NMR spectrum of MAEDAPS are shown in Fig. 1 and Fig. 2, respectively.
According to Fig. 1 the peak at 3416.7 cm−1 is the asymmetric stretching vibration of N-H bond, and 1657.5 cm−1 is the stretching vibration of carbonyl group. The peaks at 3040.9 cm−1 and 2963.5 cm−1 is stretching vibration of methyl and methylene, with the corresponding bending vibration can be find at 1474.8 cm−1. The stretching vibration of C = C in the vinyl is shown at 1620.6 cm−1, at the same time, the peak 923.5 cm−1 is vinyl hydrogen bending vibration. The absorption bands at 1202.7 cm−1 and 1042.8 cm−1 can be attributed to asymmetric and symmetric O = S = O stretching vibrations of sulfonic acid groups. And the peak at 1319.9 cm−1 can be stretching vibrations of C-N in quaternary ammonium group. These observations can confirm the successful synthesis of the zwitterionic functional monomer [26, 2].
In the 1H-NMR spectrum of MAEDAPS (shown in Fig. 2), the peak d 3.26 ppm is the absorption of -CH3 linking with N in quaternary ammonium group, the peak a 2.00 ppm is the absorption of –CH3 connecting with carbon-carbon double bond. The peaks b 2.34 ppm, c 3.06 ppm, e 3.61 ppm and f 3.83 ppm are attributed to the protons belongs to methylene group, respectively. Finally the peaks g 5.58 ppm and h 5.82 ppm is the protons directly connecting to the carbon-carbon double bond. The 1H-NMR spectrum of MAEDAPS further proves the successfully synthesis of the zwitterionic monomer [27].
Synthesis and characterization of copolymer
AM, DMAM and MAEDAPS were copolymerized statistically in water. The synthesis route and the structure of the ADM series are shown in Scheme 2.
The reaction parameters of ADM series are shown in table 1. Sample code is made of ADM and a number which represent the feed ratio of MAEDAPS. The yields of the samples are all above 93 % by weighing method.
As shown in Table 2, dissolving the ADM series in different kinds of solvents, we can observe that ADM series can only dissolve in redistilled water.
With respect to ADM-1, in Fig. 3 the peak at 3431.2 cm−1 is the asymmetric stretching vibration of N-H bond, and 1633.6 cm−1 is the stretching vibration of carbonyl group. The absorption bands at 1179.5 cm−1 and 1038.5 cm−1 can be ascribed to be asymmetric and symmetric O = S = O stretching vibrations of sulfonic acid groups [28]. The peaks at 2925.2 cm−1 and 1452.9 cm−1 represent the asymmetrical stretch vibration and curve vibration of methyl,and 1318.1 cm−1 can be stretching vibrations of C-N in -N-(CH3)3, respectively. The peak at 1452.9 cm−1 is mixed in-plane bending vibration of C-N and N-H, from Fig. 3, it can be seen that characteristic absorption peak of acrylamide, 2787.9 cm−1 is the stretching vibration of methylene and the peak of 2943.3 cm−1 disappeared indicate that the copolymerization happened in the chain of ADM-1 [29, 26].
As shown in Table 3, sulphur can be found in ADM-1 and ADM-2. This phenomenon can indicate that copolymerization happened in the ADM series.
In a word, these observations can confirm the successful copolymerization of MAEDAPS, DMAM and AM.
Thermal analysis
Thermal stability of the copolymers was illustrated by the Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA) and TGA-IR analyses, and the corresponding measuring curves were presented from Fig. 3 to Fig. 7.
DSC study
Realizing the glass transition temperature (Tg) of the polymer, which relates to the structural properties of the polymer, is of fatal importance and conduces to recognize the transformation of a polymer chain segment from a rigid material to a flexible one. At the same time, that only one Tg is the symbol that it is a kind of copolymer. Thus, DSC study was carried out and the tested curves were presented in Fig. 3.
In general, in DSC curves, the endothermic platforms are the glass transition temperature (Tg), meanwhile the single or multiple endothermic peaks are the crystal melting [22].
As shown in Fig. 4, there exist several endothermic for respective copolymer DSC curve. Tg of ADM series decrease from 67.45 °C, 61.89 °C to 57.67 °C, the regular above is opposite with the content of MAEDAPS in copolymer. Furthermore, we can deduce that the melting point temperature (Tm) of the copolymers ADM-0, ADM-1, and ADM-2 was at near 131.44 °C, 129.14 °C and 145.81 °C, respectively. Moreover, the phenomenon that ADM series copolymer only have one Tg demonstrate that the functional monomer MAEDAPS can copolymerized to copolymer with acrylamide.
With respect to the glass transition temperature on behalf of the flexibility of polymer chain segment, the lower Tg presents the more flexible the polymer chain segment is. The trend of Tg can be ascribed to the introduction of zwitterionic groups onto the main chain of the acrylamide derivates. The introduction of functional monomer MAEDAPS enlarges the distance between macromolecular chains, and results in the motion of polymer chain segment becomes increasingly easier with the increasing content of MAEDAPS [30, 31].
While, regarding the trend of Tm can be illustrated by the ionic bonds conforming between the zwitterionics pendant groups [32]. Introduction of MAEDAPS, not only results in the distance between polymer chains enlarged, but also leads to the decline of crystallization capacity of polymer. However, along with the increase of the content of zwitterionics monomer, an increasing number of ionic bonds were form between the polymer chains [33]. So the variation tendency of Tm is ADM-1 < ADM-0 < ADM-2.
Hereinafter, the melting endothermic peaks area also demonstrated that the more flexible the copolymer chain segments are the more inclined to crystal formation they are.
TGA study
As shown in Fig. 5, it is interesting to discover that for different copolymers, their change trends in weight loss are similar that they all contain three main degradation stages. Corresponding to the degradation process, several markedly endothermic peaks can be found in DTG curves as presented in Fig. 6, hereinafter. At the temperature below 214 °C there is a slightly weight loss primarily caused by the removal of physically absorbed water in these copolymers. The gradually decline of weight loss over the temperature ranging from 200 °C to 345 °C was ascribed to the decomposition of the side group of polymer chain, mainly the breakage of the pendent groups. Whereas the sharp weight loss beyond 345 °C was attributed to the further degradation of the copolymer chain residues into the black carbon.
Moreover, considering the TGA curves for different copolymers in Fig. 5, it can be noted that the weight loss exhibited a little increase with an increase in zwitterionic content in the copolymers (as shown in Fig. 2 ADM-1 and ADM-2), suggesting an increase of pendant groups in the copolymers.
Figure 6 presented the DTG curves of the investigated copolymers. It can be seen that for various copolymers, there exist different weight loss peak, which corresponds to the weight loss stages as demonstrated in TGA curves (Fig. 5). These weight loss peaks displayed that when the zwitterionic extent of the copolymers increased, the second weight loss peak enhanced slowly from 270, 275 to 282 °C for the respective copolymer, whereas the third weight loss peak shifted to a lower temperature.
These trends above can be attributed to the introduction of ion pairs into the copolymer chains, which will lead to an increase in the Coulombic attraction between the molecules of the prepared copolymer. As a result, the weight loss of the produced copolymer is reduces accordingly [34].
The first weight loss peak was related to the removal of physically absorbed water in these copolymers. The second weight loss peak might be attributed to the decomposition of the side group, accompanied by polymerization of structural relaxation. Meanwhile, this kind of upward trend implied an increase in thermal stability of these copolymers. After the introduction of ion pairs into the copolymer chains, the relative content of acryliamide was lowered with an increase in zwitterionic content in the copolymers; meanwhile, the coulombic attraction between the molecular chains also increases, which will favor the formation of network structure, as a result their thermal stability increases a small extent.
Compared with other polyelectrolyte, polyzwitterionics owned preferable thermostability. In our observation, there are two distinct decomposition peaks of the TGA-DTG curve, and both are above 200 °C, which indicates that the introduction of more stable sulfonic acid groups and quaternary ammonium groups to the molecular chain can improve the stability of the polyzwitterionics.
TG-FTIR study
Taking ADM-1 as example, we minutely further research the thermal decomposition process of ADM-1 by TG-FTIR, and meanwhile, illustrate that the ADM series is a kind of copolymer of MAEDAPS, AM and DMAA.
As shown in the Fig. 7 degradation process, several markedly endothermic peaks can be found in TGA and DTG curves, hereinafter. Stage I, lower than 200.77 °C, with a weight loss 11.02 % was primarily caused by the removal of physically absorbed water in these copolymers. A weight loss of 19.66 % in stage II, from 200.77 °C to 328.79 °C, can be attributed to the decomposition of side groups of polymer chain. A significant weight loss of 43.51 % in stage III from 328.79 °C to 443.28 °C, may be the degradation of the groups connecting directly to polymer main chains. The speculation above would be demonstrated by the infrared spectroscopy of the released gas. And the polymer was weight loss to 25.24 % at the temperature of 600 °C.
As shown in Fig. 8 is the infrared spectroscopy of released gas in different time. Fig. 8 (a) and (d) is the spectroscopy before and after the copolymer degradation, respectively. While Fig. 8 (b) and (c) are spectroscopy corresponding to the degradation stage II and III. Observing from Fig. 8 (b), we can speculate that ammonia gas and sulfur dioxide are released in stage II, because the absorption peaks 1,514,7 cm−1 is the stretching vibration of SO2, and 960.2 and 933.2 cm−1 are the stretching vibration and bending vibration of NH3. Amide groups and sulfonic acid groups in pendant groups mainly degraded in this stage. Similar method can be used to analysis the Fig. 8 (c), the stage III, we found the stretching vibration absorption peaks of 2354.0, 2066.2 and 1742.7 cm−1 belongs to CO2, CO and NO, respectively. We think this stage is the degradation of methyl and acyl groups connecting to the polymer main chains.[35] (Fig. 9).
Dilute solution properties
The structure of the polyzwitterionic determine its features, the features of the polyzwitterionic determine its application value. Meanwhile, the structure of MAEDAPS, containing sulfonic acid group and quaternary ammonium group, determine the mainly structure of polyzwitterionic we obtained. Because of the special structure of MAEDAPS, so that ADM series possess a lot of excellent properties that general polyelectricities do not has, such as salt-resistance properties [36], temperature-resistance properties and pH stability and so on.
Viscosity behavior of zwitterionic copolymer
In this work, the reduced viscosity of copolymer ADM-0, ADM-1, and ADM-2 were contrasted in a series of NaCl solution concentration as shown in Fig. 8.
It can be seen that the reduced viscosity of the copolymer ADM-1 and ADM-2 increases with the increase of NaCl solution concentration. On the contrast, the reduced viscosity of ADM-0 sharply decreases in the NaCl solution. On account of the introduction of MAEDAPS in ADM-1 and ADM-2, zwitterionic pendant group can form electrostatic interaction with Na+ and Cl− in solution, makes the hydrodynamic volume of the polymer chain enlarged and the polymer chain keep extended, rod-like conformation. On the contrary, the hydrodynamic volume of ADM-0 reduced and polymer chains take collapsed, coil-like conformation [37]. Furthermore, the reduced viscosity of the copolymer ADM-1 is higher than that of ADM-2, and the ADM-1 and ADM-2 shows the similar trend in NaCl solution. These phenomena above were all attributed to the different content of MAEDAPS in polymer. Although the increase of MAEDAPS in polymer chain can enhance the electrostatic interaction, the decrease of AM content may reduce the hydration of polymer and viscosity in solution [38]. Hereinafter, we choose the copolymer ADM-1 as the sample in the following test.
Intrinsic viscosity
The aqueous solution properties of polyelectrolytes and polyzwitterionic are profoundly different and can be dictated primarily by the intra- and intermolecular electrostatic interactions that occur among the charged groups in aqueous media. In dilute, salt-free aqueous solutions, coulombic repulsion between like charges along the polyelectrolyte chain leads to an expansion in the hydrodynamic volume of the polyelectrolyte coil; however, addition of small molecule electrolytes result in coulombic shielding and a decrease in hydrodynamic volume and thus solution viscosity. This solution behavior is the well-known the polyelectrolyte effect [39].
Conversely, because of the markedly different structure of the polyzwitterionics, the positive and negative charges belong to the same pendant group. Coulombic interactions between positively and negatively charges of polyzwitterionics reduce hydrodynamic volume, and the polymer adopts a collapsed or globular conformation in dilute, salt-free aqueous media. In some instances, the electrostatic interactions are so strong that the polymer may become insoluble. Upon addition of simple electrolytes to polyzwitterionics solution in the dilute regime, an increase in hydrodynamic volume of the polymer coil is observed due to the decreasing of the intramolecular charge–charge attractions, allowing the transition from a globuleto a random coil conformation (shown in scheme 3). Such solution behavior is known as the anti-polyelectrolyte effect and is evidenced by increased polymer hydrodynamic volume and solution viscosity.
As shown in Fig. 10, the intrinsic viscosity of the ADM-1 is sharply increased as the salt addition at first, when the concentration of salt solution increases to a certain degree the change trend of intrinsic viscosity turn to be gentled, and finally flatten out.
Salt-resistance study
In this part, we mainly focused on the reduced viscosities in different kinds of small molecular electrolyte solution. This kind of properties can be illustrated by the shielding effect of small molecule electrolytes and complexation between the small molecule electrolytes with the pendant groups of the polyzwitterionics.
As shown in the Fig. 11, the reduced viscosity of ADM-1 in salt solution increase as the concentration of salt solution increase, and this phenomenon can be illustrated by the electrostatic screening [40]. On account of the electrostatic screening of valence metal ions and Cl−, the conformation of the macromolecule chain transformed from a collapsed, coil-like conformation to an extended, rod-like one. Apparently, the sequence of reduced viscosity is different with the ion order. We owe this phenomenon to the ion volume. The extra nuclear electron of O2−is similar to that of Na+, so the electrostatic screening of Na+ is the best, resulting that the reduced viscosity of NaCl solution is higher than that of LiCl and KCl. The difference between Li + and K + is aroused by the electrostatic repulsion between quarternary ammonium ion, as the electrostatic repulsion of K + is stronger than that of Li + result in the polymer chain is more expansion than in LiCl solution [41]. The sequence of reduced viscosity in valence metal ions is Na+ >Li+ >K+.
As shown in Fig. 12, the influence of monovalent anion is similar to that of valence metal ion that the reduced viscosity of ADM-1 increased with the increasing of concentration of salt solution. And the sequence of the reduced viscosity is Br−> I−> Cl−.
It happens that there is a similar cases, the reduced viscosity of KBr solution is higher than that of KCl and KI. Because of quaternary ammonium groups possessing a nitrogen-atom centered electron deficiency system, quaternary ammonium groups exhibit coulombie attraction with the electronegative ionic. The polarization ability of Cl−, Br−and I−is Cl−< Br−< I−, meanwhile, hydration capacity decreases as the ionic radius increase, the coulombie attraction between I−with quaternary ammonium group is strongest in theory. However, for the reason that the charge volumic of I−is too large, makes I−difficult to access the quaternary ammonium groups [28]. Therefore, the sequence of reduced viscosity is Br−> I−> Cl−.
Among divalent metal ions solution, the reduced viscosity consequence of ADM-1 is a bit complicated. As shown in Fig. 13, there are exist an optimal divalent metal ions concentration, 2 mol/L, at the optimal concentration the reduced viscosity of ADM-1 are highest. And, before the optimal concentration, the reduced viscosity of ADM-1 increase as the increase of concentration, while after the optimal concentration, the reduced viscosity of ADM-1 is decrease as increase of concentration. This phenomenon can be illustrated by Stern-Gouy-Chapman theory. The charged water-soluble copolymer can form double electro-deposited coating the substantial core and the unconsolidated outer sphere in the electrolyte solution. The outer sphere would overlap as the transformative of the ambient electrolyte concentration. With the increasing of the divalent metal ions, the overlapping expand of the unconsolidated outer sphere between charged particles lead to reduce of van der Waals attraction causing the macromolecular chain expansion and reduced viscosity increase. However, with the concentration of divalent metal ions further increase, the charged particles are shielded by the divalent metal ions causes the molecular chain shrinkage and the reduced viscosity decrease [42–44].
According to the experiment, we find that the optimal concentration of divelent metal ions is 2 mol/L, and the reduced viscosity sequence of ADM-1 in different divalent metal ions solution is Cu2+ < Ca2+ < Mg2+.
Conclusions
A kind of zwitterionic monomer with carbon-carbon double bond has been synthesized via the ring opening reaction, detected by 1H NMR and IR. Afterwards, a series of ternary polyzwitterionics with AM, DMAM, and MAEDAPS have been prepared by the free radical polymerization in aqueous solution. At the same time, if the content of MAEDAPS in polyzwitterionics reaches 15 %, the polyzwitterionics produced cannot resolve in water.
The thermal properties of the polyzwitterionics were tested according to the DSC, TGA and TG-IR. DSC curves suggested that the Tg of ADM series decrease with the increase of content of MAEDAPS in polyzwitterionic. TGA curves shows that there are three degradation stages, and compared with general polyeletrolytes are more stable. The merely Tg in DSC curves and the SO2 absorption peaks in TG-IR analysis illustrated that MAEDAPS is copolymerized with AM and DMAM.
Intrinsic viscosity indicated that the polyzwitterionic is more stability comparable with originally polyelectrolytes. Apparent viscosities of ADM-1 in different electrolyte show that the reduced viscosities increases as the increasing of small molecular electrolytes concentration. But the different is the reduced viscosities in varieties of small molecular electrolytes: K+ < Li+ < Na+, Cu2+ < Ca2+ < Mg2+, and anions is in the order: Cl−< I−< Br−.
References
Tan BH, Ravi P, Tam KC (2006) Synthesis and characterization of novel pH-responsive polyampholyte microgels. Macromol Rapid Commun 27(7):522–528. doi:10.1002/marc.200500830
He L, Wang S, Liu X, Wang C, Chao D (2013) Synthesis and properties of a novel multifunctional hyperbranched polyamide. J Polym Res 20(8):1–8. doi:10.1007/s10965-013-0214-5
Kathmann EEL, Davis DD, McCormick CL (1994) Water-soluble polymers. 60. Synthesis and solution behavior of terpolymers of acrylic acid, acrylamide, and the zwitterionic monomer 3-[(2-acrylamido-2-methylpropyl) dimethylammonio]-1-propanesulfonate. Macromolecules 27(12):3156–3161. doi:10.1021/ma00090a007
Liaw D-J, Huang C-C, Sang H-C, Wu P-L (2000) Macromolecular microstructure, reactivity ratio and viscometric studies of water-soluble cationic and/or zwitterionic copolymers. Polymer 41 (16):6123–6131. doi:http://dx.doi.org/10.1016/S0032-3861(99)00824-1
Sonnenschein L, Seubert A (2011) Synthesis of a series of monomeric styrene sulfobetaine precursors. Tetrahedron Letters 52 (10):1101–1104. doi:http://dx.doi.org/10.1016/j.tetlet.2010.12.100
Bonte N, Laschewsky A (1996) Zwitterionic polymers with carbobetaine moieties. Polymer 37 (10):2011–2019. doi:http://dx.doi.org/10.1016/0032-3861(96)87319-8
Kazantsev O, Shirshin K, Sivokhin A, Igolkin A, Goncharova O, Kamorin D (2012) Copolymerization of sodium 2-acrylamido-2-methylpropane sulfonate with acrylamide and acrylonitrile in water: an effect of conditions on the compositional heterogeneity. J Polym Res 19(6):1–10. doi:10.1007/s10965-012-9886-5
McCormick CL (1985) Water-soluble random and graft copolymers for utilization in enhanced Oil recovery. J Macromol Sci A Chem 22(5–7):955–982. doi:10.1080/00222338508056647
Wang H, Wei J, Li S, Chen Y, Ren Z, Qiu S (2012) Preparation and characterization of AA/AMPS grafted polypropylene by two-steps electron beam irradiation for filtration of cigarette smoke. J Polym Res 20(1):1–6. doi:10.1007/s10965-012-0044-x
Kostova B, Kamenska E, Rachev D, Simeonova S, Georgiev G, Balashev K (2013) Polyzwitterionic copolymer nanoparticles loaded in situ with metoprolol tartrate: synthesis, morphology and drug release properties. J Polym Res 20(2):1–8. doi:10.1007/s10965-012-0060-x
Zhang Y, Wang L, Li X, He P (2011) Salt-resistant superabsorbents from inverse-suspension polymerization of PEG methacrylate, acryamide and partially neutralized acrylic acid. J Polym Res 18(2):157–161. doi:10.1007/s10965-010-9402-8
Kim G, Yong Y, Kang HJ, Park K, Kim SI, Lee M, Huh N (2014) Zwitterionic polymer-coated immunobeads for blood-based cancer diagnostics. Biomaterials 35(1):294–303. doi:10.1016/j.biomaterials.2013.09.101
Lin W, Ma G, Chen S (2013) Evasion of the accelerated blood clearance phenomenon of colloidal carriers by using zwitterionic star polymers as shell materials. J Control Release Off J Control Release Soc 172(1):e73–e73. doi:10.1016/j.jconrel.2013.08.150
Mi L, Jiang S (2014) Integrated antimicrobial and nonfouling zwitterionic polymers. Angew Chem Int Ed 53(7):1746–1754. doi:10.1002/anie.201304060
Johnson KM, Poe GD, Lochhead RY, McCormick CL (2004) The synthesis of hydrophobically modified water‐soluble polyzwitterionic copolymers and responsiveness to surfactants in aqueous solution. J Macromol Sci A 41(6):587–611. doi:10.1081/MA-120034192
Lalani R, Liu L (2011) Synthesis, characterization, and electrospinning of zwitterionic poly (sulfobetaine methacrylate). Polymer 52(23):5344–5354
Kudaibergenov S, Jaeger W, Laschewsky A (2006) Polymeric Betaines: Synthesis, Characterization, and Application. In: Supramolecular Polymers Polymeric Betains Oligomers, vol 201. Advances in Polymer Science. Springer Berlin Heidelberg, pp 157–224. doi:10.1007/12_078
Lee W-F, Tsai C-C (1994) Synthesis and solubility of the poly (sulfobetaine) s and the corresponding cationic polymers: 1. Synthesis and characterization of sulfobetaines and the corresponding cationic monomers by nuclear magnetic resonance spectra. Polymer 35(10):2210–2217
An H, Lu C, Wang P, Li W, Tan Y, Xu K, Liu C (2011) A novel hydrophobically associating polyampholytes of poly (AM/AA/AMQC12): preparation, characterization, and solution properties. Polym Bull 67(1):141–158. doi:10.1007/s00289-011-0465-4
Ning J, Kubota K, Li G, Haraguchi K (2013) Characteristics of zwitterionic sulfobetaine acrylamide polymer and the hydrogels prepared by free-radical polymerization and effects of physical and chemical crosslinks on the UCST. Reactive and Functional Polymers 73 (7):969–978. doi:http://dx.doi.org/10.1016/j.reactfunctpolym.2012.11.005
Han D, Letteri R, Chan-Seng D, Emrick T, Tu H (2013) Examination of zwitterionic polymers and gels subjected to mechanical constraints. Polymer 54 (12):2887–2894. doi:http://dx.doi.org/10.1016/j.polymer.2013.04.003
Kitano H, Imai M, Sudo K, Ide M (2002) Hydrogen-bonded network structure of water in aqueous solution of sulfobetaine polymers†. J Phys Chem B 106(43):11391–11396. doi:10.1021/jp020185r
Mary P, Bendejacq DD, Labeau M-P, Dupuis P (2007) Reconciling Low- and high-salt solution behavior of sulfobetaine polyzwitterions. J Phys Chem B 111(27):7767–7777. doi:10.1021/jp071995b
Gui Z, Qian J, An Q, Xu H, Zhao Q (2009) Synthesis, characterization and flocculation performance of zwitterionic copolymer of acrylamide and 4-vinylpyridine propylsulfobetaine. European Polymer Journal 45 (5):1403–1411. doi:http://dx.doi.org/10.1016/j.eurpolymj.2009.02.010
Lowe AB, McCormick CL (2002) Synthesis and solution properties of zwitterionic polymers. Chem Rev 102(11):4177–4190
Pretsch E, Bühlmann P, Badertscher M (2009) IR Spectroscopy. Structure Determination of Organic Compounds. Springer Berlin Heidelberg, In, pp 1–67. doi:10.1007/978-3-540-93810-1_7
Pretsch E, Bühlmann P, Badertscher M (2009) 1H NMR Spectroscopy. Structure Determination of Organic Compounds. Springer Berlin Heidelberg, In, pp 1–86. doi:10.1007/978-3-540-93810-1_5
Benesi HA, Hildebrand J (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71(8):2703–2707
Coates J (2006) Interpretation of infrared Spectra, a practical approach. Encyclopedia of Analytical Chemistry. John Wiley & Sons, Ltd., In. doi:10.1002/9780470027318.a5606
Richardson MJ, Savill NG (1975) Derivation of accurate glass transition temperatures by differential scanning calorimetry. Polymer 16 (10):753–757. doi:http://dx.doi.org/10.1016/0032-3861(75)90194-9
Her L-M, Nail S (1994) Measurement of glass transition temperatures of freeze-concentrated solutes by differential scanning calorimetry. Pharm Res 11(1):54–59. doi:10.1023/A:1018989509893
Okazaki I, Wunderlich B (1997) Reversible melting in polymer crystals detected by temperature-modulated differential scanning calorimetry. Macromolecules 30(6):1758–1764. doi:10.1021/ma961539d
Freire E (1995) Differential Scanning Calorimetry. In: Shirley B (ed) Protein Stability and Folding, vol 40. Methods in Molecular Biology™. Humana Press, pp 191–218. doi:10.1385/0-89603-301-5:191
Liu J, Xu T, Han X, Fu Y (2006) Synthesis and characterizations of a novel zwitterionic hybrid copolymer containing both sulfonic and carboxylic groups via sulfonation and zwitterionic process. European Polymer Journal 42 (10):2755–2764. doi:http://dx.doi.org/10.1016/j.eurpolymj.2006.04.006
Hirose S, Kobashigawa K, Izuta Y, Hatakeyama H (1998) Thermal degradation of polyurethanes containing lignin studied by TG-FTIR. Polym Int 47(3):247–256. doi:10.1002/(SICI)1097-0126(199811)47:3<247::AID-PI966>3.0.CO;2-F
Armentrout RS, McCormick CL (1999) Water soluble polymers. 76. Electrolyte responsive cyclocopolymers with sulfobetaine units exhibiting polyelectrolyte or polyampholyte behavior in aqueous media. Macromolecules 33(2):419–424. doi:10.1021/ma991133b
Popescu I, Airinei A, Suflet D, Popa M (2011) Maleic acid–2-vinylnaphthalene copolymer in aqueous solution: investigation of the dissociation and fluorescence quenching. J Polym Res 18(6):2195–2203. doi:10.1007/s10965-011-9630-6
Jie C, Yebang T, Yuju C, Qiang M (2011) Synthesis of copolymer of acrylamide with sodium vinylsulfonate and its thermal stability in solution. J Polym Res 18(2):171–178. doi:10.1007/s10965-010-9404-6
Ezell Ryan G, Lowe Andrew B, McCormick Charles L (2006) Synthetic Polyzwitterions: Water-Soluble Copolymers and Terpolymers. In: Polyelectrolytes and Polyzwitterions, vol 937. ACS Symposium Series, vol 937. American Chemical Society, pp 47–63. doi:doi:10.1021/bk-2006-0937.ch003 10.1021/bk-2006-0937.ch003
Berlinova IV, Dimitrov IV, Kalinova RG, Vladimirov NG (2000) Synthesis and aqueous solution behaviour of copolymers containing sulfobetaine moieties in side chains. Polymer 41 (3):831–837. doi:http://dx.doi.org/10.1016/S0032-3861(99)00264-5
Chan LL, Wong KH, Smid J (1970) Complexation of lithium, sodium, and potassium carbanion pairs with polyglycol dimethyl ethers (glymes). Effect of chain length and temperature. J Am Chem Soc 92(7):1955–1963. doi:10.1021/ja00710a029
Cantwell FF, Puon S (1979) Mechanism of chromatographic retention of organic ions on a nonionic adsorbent. Anal Chem 51(6):623–632. doi:10.1021/ac50042a009
Valleau JP, Torrie GM (1982) The electrical double layer. III. Modified Gouy − Chapman theory with unequal ion sizes. The Journal of Chemical Physics 76 (9):4623–4630. doi:doi:http://dx.doi.org/10.1063/1.443542
Attard P (2001) Recent advances in the electric double layer in colloid science. Current Opinion in Colloid & Interface Science 6 (4):366–371. doi:http://dx.doi.org/10.1016/S1359-0294(01)00102-9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, C., Gu, X., Cui, M. et al. A novel ternary copolymerized polyzwitterionic of poly (AM/DMAM/MAEDAPS): synthesis and solution properties. J Polym Res 21, 620 (2014). https://doi.org/10.1007/s10965-014-0620-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0620-3