Abstract
A series of phthalonitrile end-capped sulfonated polyarylene ether nitriles are synthesized via K2CO3 mediated nucleophilic aromatic substitution reaction at various molar ratios. The as-prepared polymer structures are confirmed by 1H NMR and FTIR spectroscopy. The properties of membranes cast from the corresponding polymers are investigated with respect to their structures. The membranes exhibit good thermal and mechanical properties, low methanol permeability (0.01 × 10−6–0.58 × 10−6 cm2·s−1 at 20 °C), and high proton conductivity (0.021–0.088 S·cm−1 at 20 °C). The introduction of phthalonitrile is proved to increase intermolecular interaction, mainly contributing to the reduction in water uptake, swelling ratio, and methanol permeability. More importantly, its introduction does not decrease the proton conductivity, but there is a slight increase. Furthermore, the selectivity of SPEN-CN-50 can reach 4.11 × 105 S·s·cm−3, which is about nine times higher than that of Nafion 117. All the data show that the as-prepared membranes may be potential proton exchange membrane for DMFCs applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer electrolyte fuel cells (PEFCs) as a source of renewable energy have attracted wide attention in many applications, such as portable devices, electric vehicles, and remote locations [1–4]. Proton exchange membrane (PEM) is the key component of PEFCs for transporting protons as well as providing a barrier between fuel gas and oxidants [5]. Nafion which is a representative of perfluorosulfonic acid membranes has been commercially available PEM due to high proton conductivity, outstanding thermal, and mechanical properties. However, high cost, low operation temperature, and high methanol permeability limit its use [6–9]. Thus, numerous efforts have focused on the development of new PEM materials with low cost and high performance.
As effective alternatives, a large number of sulfonated aromatic polymer membranes have been investigated, such as sulfonated polyimides [10–12], sulfonated poly (ether ether ketone) [13–15], sulfonated poly (ether sulfone)s [16–19], and sulfonated polybenzimidazole [20–22]. Among these polymers, sulfonated polyarylene ether nitrile (SPEN) has come into our vision owing to its excellent comprehensive properties such as good mechanical properties, high chemical and thermal resistance [23–28]. Besides, polar nitrile groups in SPEN can decrease the swelling of membranes and promote the adhesion of the polymers to the catalyst which is useful for membrane electrode assemblies to decrease electrochemical impedance of fuel cell [23]. However, for poly (aryl ether nitrile)s with comparable conductivities to Nafion, the water swelling is still higher than that of Nafion, particularly in hot water.
As is known, polymer chemical structure has important effect on polymer electrolytes properties. The design and synthesis of new structures have become an active research area [29–31]. In earlier studies, we have synthesized a series of sulfonated polyarylene ether nitrile copolymers and found that sulfonated polyarylene ether nitrile containing biphenyl structures has excellent comprehensive properties [32]. To further understand the relationship between the chemical structure and properties of the polymers, phthalonitrile end-capped sulfonated polyarylene ether nitrile was designed and synthesized. As mentioned above, the introduction of strongly polar nitrile groups can increase the intermolecular interaction between polymer chains and make the polymers less water absorbable. In this work, phthalonitrile end-capped sulfonated polyarylene ether nitrile is synthesized via nucleophilic aromatic substitution reaction and their properties are studied in details, including thermal stability, mechanical properties, ion exchange capacity, water uptake, swelling ratio, proton conductivity, and methanol permeability.
Experimental
Materials
Potassium 2,5-dihydroxybenzenesulfonate (SHQ), 2,6-difluorobenzonitrile (DFBN), 4,4′-biphenol (BP), and 4-nitrophthalonitrile were purchased from Aldrich. Concentrated sulfuric acid and ethanol were supplied by Chengdu Haihong Chemical Co. Dimethyl acetamide (DMAc), toluene, N-methyl pyrrolidone (NMP), and potassium carbonate (K2CO3) were obtained by Tianjin BODI chemicals. All the materials were used without further purification.
Synthesis of sulfonated polyarylene ether nitriles terminated with phthalonitrile (SPEN-CN)
SPEN-CN was synthesized via nucleophilic aromatic substitution reaction (Scheme 1). A typical synthetic procedure to prepare SPEN-CN-40 (where 40 is the molar percentage of SHQ monomer in the total diphenol monomers) could be described as follows. To a 250-mL three-necked round-bottom flask, BP (11.35 g, 0.06 mol), SHQ (9.12 g, 0.04 mol), DFBN (13.91 g, 0.1 mol), K2CO3 (30.4 g, 0.22 mol), NMP (65 mL), and toluene (25 mL) were added successively with stirring at 140 °C for 3 h. After the water-toluene azeotrope distilled off, the mixture slowly heat up to 190 °C for about 3 h. Then the mixture was continued to be heated at 190 °C for 2 h. When the mixture was cooled to 80 °C, 4-nitrophthalonitrile (2.6 g, 0.015 mol) and NMP (5 mL) were added into the three-necked round-bottom flask and keep the reaction at 80 °C for 5 h. After cooling to room temperature, the mixture was poured into alcohol. The precipitate was collected by filtration and washed with alcohol and deionized water. Finally, acid-form polymers were obtained by soaking the precipitate in 100 mL of 2 mol/L H2SO4 solution for 24 h and washed with water for several times, then dried in vacuum oven at 100 °C overnight.
Preparation of membranes
The membranes were prepared by casting from the solution of SPEN-CN (1 g) in DMAc (15 mL) on clean glass plates and dried in an oven at the temperature of 80, 100, 120, 140, and 160 °C each for 2 h to evaporate the solvent completely. After cooling to room temperature, the membranes were obtained by peeling off.
Membrane characterizations
Instrumentation
The 1H NMR spectra were measured on Bruker AV II-400 spectrometer (400 MHz). FTIR spectra were recorded with Shimadzu FTIR8400S Fourier Transform Infrared spectrometer.
Ion exchange capacity (IEC)
The IEC of the membranes was evaluated via acid–base titration method. The dried membrane was immersed in 1 M NaCl solution for 48 h to liberate the H+ ions to the solution. Then the solution was titrated using 0.01 M NaOH solution with the phenolphthalein as the indicator. The IEC (meq·g−1) was calculated by
where V NaOH is the titrant volume of NaOH, C NaOH is the molar concentration of the titrant, and W dry is the mass of dried sample.
Water uptake and swelling ratio
Before the initial measurements, the membranes were dried in oven at 80 °C overnight. After measuring the weights and lengths of dry membranes, the membranes were soaked in deionized water for 24 h at the desired temperatures. Then the surface water was wiped with paper quickly to measure weight and length measurements of hydrated membranes. The water uptake and swelling ratio of the membrane were calculated by
Proton conductivity and methanol permeability
The proton conductivity of the membranes was carried out via AC impedance spectroscopy using a Model 600E Series electrochemical analyzer in potentiostat mode with 50 mV over frequencies of 0.1 Hz to 100 kHz. The proton conductivity was calculated by
where σ, L, R, and A represent proton conductivity, thickness, measured resistance, and membranes area, respectively.
The methanol permeability of the membrane was conducted by a two-chamber liquid permeability cell. The two cells were separated by a membrane. Then one compartment was filled with 10 M methanol solution (A, 20 mL) and the other was filled with deionized water (B, 20 mL). The concentration of the methanol in cell B was measured by using SHIMADZU GC-8A chromatograph. The methanol permeability is calculated by
Where A is the effective area, L is the thickness of membrane, and V B is the volume of receptor reservoir. C A and C B are the methanol concentration in the donor and receptor reservoirs, respectively.
Thermal properties
Thermogravimetric analysis (TGA) of the membranes was performed by a TA Instruments TGA-Q50 module. The samples were measured in the range of 80–600 °C at 20 °C/min under nitrogen atmosphere.
Mechanical property
Before testing, the samples have been prepared through immersion in deionized water for 24 h at room temperature. The mechanical properties of the membranes were investigated on a SANS CMT6104 series desktop electromechanical universal testing machine at a strain rate of 5 mm/min.
Results and discussion
Synthesis of SPEN-CN
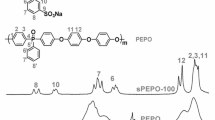
A series of SPEN-CN were synthesized via nucleophilic aromatic substitution reaction, as shown in Scheme 1. The structure of SPEN-CN is characterized by 1H NMR spectrum (Fig. S1). It is obviously seen that the end-capped phenyl ring protons Hi,j are emerged at high frequency (Hi,j: 7.97–8.04 ppm) due to the electron-withdrawing effect of the cyano groups and other protons of the SPEN-CN are well assigned, confirming successful preparation of the end-capped polymer SPEN-CN. Meanwhile, the FTIR spectra of copolymers (Fig. S2) also characterize the formation of SPEN-CN.
Thermal properties
The thermal stabilities of SPEN-CN membranes are investigated by TGA under N2 atmosphere in Fig. 1. There are three degradation steps which can be observed from the curves of SPEN-CN membranes. The TGA curves show a small weight loss about 200 °C due to the solvent loss which may be caused by the interaction between the polymer and the solvent. When the temperature rises to around 320–400 °C, the sulfonic acid groups begin to fall from the polymer chain. The last weight loss step at 430–600 °C is ascribed to the degradation of polymer main chain. The results show the SPEN-CN membranes exhibit excellent thermal stability, which are good enough for PEM application.
Mechanical properties
The mechanical properties of the SPEN-CN membranes are evaluated in the full wet state at room temperature and the results are listed in Table 1. It could be seen that the Young’s modulus, tensile strengths, and elongation at break of the SPEN-CN membranes are 1.31–2.15 GPa, 42.99–72.63 MPa, and 11.45–18.64%, respectively. In addition, the tensile strength decreases with the increasing sulfonic acid groups. It is ascribed to water plasticization in membranes. The hydrogen bonds between sulfonic acid groups and H2O exist in wet membranes. With the increasing sulfonic acid groups, the membranes are growing in size due to imbibing more water, causing the rigid network structure of the membranes to weaken, which results in a decrease in its tensile strength [24]. Moreover, the tensile strength of SPEN-CN membranes is higher than that of Nafion 117 (22 MPa) [33], suggesting that the membranes have excellent mechanical property.
IEC, water uptake, and swelling ratio
IEC plays an important role in determining the water uptake and proton conductivity. With the increase of sulfonic acid groups, IEC shows a significant increase. The IEC values of SPEN-CN membranes are in the range of 1.16–2.2 mmol/g, as shown in Table 2.
In general, water uptake is related to the number of sulfonic acid groups and molecular structure, which can evaluate the performance of polymer electrolytes fuel cells. The water uptake of SPEN-CN as a function of temperature is illustrated in Fig. 2 and the main data are shown in Table 3. All the SPEN-CN membranes show the increase of water absorption with the increase of temperatures. Among of them, water uptake of SPEN-CN-70 shows larger variations than the others above 50 °C, which is attributed to the increase of sulfonic acid group. In addition, the water uptake of SPEN-CN-70 membrane reaches 34.58 and 173.99% of at 20 and 80 °C, respectively. In the previous reports, the SPEN-70 without end caps swollen excessively at 80 °C [25]. This change is explained by the introduction of phthalonitrile. The strongly polar nitrile groups of phthalonitrile may increase the intermolecular interaction of the polymer, making the polymers less water absorbable. Therefore, phthalonitrile as end-capped groups could decrease the water uptake of membrane. Besides, the swelling ratio is measured as a function of temperature in the range of 20–80 °C, as shown in Fig. 3. The swelling ratio exhibits similar trends with water uptake. For example, the swelling ratio of SPEN-CN-70 membrane increases from 3.4 to 37.57% as temperature increases from 20 to 80 °C. Moreover, it is worth noting that the water uptake and swelling ratio of all these membranes are far lower than that of the previous reported SPEN membrane with the similar structure (Table 3). In addition, their water uptake and swelling ratio are also superior to Nafion 117, except SPEN-CN-70 (Table 3). All these show the introduction of phthalonitrile can effectively enhance dimensional stability as expected.
Proton conductivity, methanol permeability, and relative selectivity
Proton conductivity is an important factor for fuel cell applications. The proton conductivity of SPEN-CN membranes at different temperatures in the full wet state is shown in Fig. 4 and the main data are shown in Table 2. The proton conductivity of SPEN-CN ranges from 0.021 to 0.088 S·cm−1 at 20 °C, whereas the values ranged from 0.040 to 0.24 S·cm−1 at 80 °C. As expected, the proton conductivity of all membranes increased with the increase of sulfonic acid groups. In addition, for all SPEN-CN membranes, the proton conductivity increased as the elevation of temperature. The highest proton conductivity can reach 0.24 S·cm−1 at 80 °C. Besides, it is worth mentioning that the proton conductivity of SPEN-CN-70 membrane has a slight increase compared to that of SPEN-70. This may be caused by the introduction of phthalonitrile groups. The introduction of phthalonitrile groups may affect the aggregations of each domain and further affect the properties of the resulting membranes. Nitrile is one of the most polar functional groups which has a high dipole moment of ∼3.9 D. Based on Kim’s model [34], strong polar nitrile-nitrile interactions between polymer chains can lead to an ideal combination of high proton conductivity and low water swelling. Overall, the SPEN-CN membranes show good proton conductivity, which is comparable to Nafion 117.
The methanol permeability and selectivity of SPEN-CN membranes at 20 °C are shown in Table 2. The variation tendency of methanol permeability is related to proton conductivity. It is clearly observed that the methanol permeability of SPEN-CN-70 membrane becomes lower than that of SPEN-70 membrane, which is due to the introduction of phthalonitrile groups. It creates strong interactions or hydrogen bonds with other polar groups that makes the molecular chains more close [30], preventing membranes from over-swelling, mainly contributing to the reduction in water uptake, swelling ratio, and methanol permeability. Moreover, all the composite membranes show lower methanol permeability than that of Nafion.
In general, the low water uptake may reduce the proton conductivity and lower the performance of the fuel cell. Fortunately, SPEN-CN membranes reduce methanol permeability without significantly decreasing conductivity. As a result, high selectivity of SPEN-CN-50 is obtained, which reaches 4.11 × 105 S·s·cm−3, while Nafion 117 membrane is 0.45 × 105 S·s·cm−3 (Table 2).
Conclusions
Phthalonitrile end-capped sulfonated polyarylene ether nitriles are synthesized via nucleophilic aromatic substitution reaction. The introduction of phthalonitrile has a strong influence on the properties of the membranes. The resulting membranes display lower water uptake, methanol permeability and swelling, and higher proton conductivity compared to that of SPEN and Nafion 117. SPEN-CN-50 membrane shows high proton conductivity (0.058 and 0.072 S·cm−1 at 20 and 80 °C) and low methanol permeability (0.14 × 10−6 cm2·s−1 at 20 °C) simultaneously. Moreover, it exhibits high selectivity (4.11 × 105 S·s·cm−3), which is about nine times higher than that of Nafion 117. The results suggest that the membranes show great potential in DMFC applications.
References
Zhang H, Shen PK (2012) Chem Rev 112:2780–2832
Mishra AK, Bose S, Kuila T, Kim NH, Lee JH (2012) Prog Polym Sci 37:842–869
Subianto S, Pica M, Casciola M, Cojocaru P, Merlo L, Hards G, Jones DJ (2013) J Power Sources 233:216–230
Kraytsberg A, Ein-Eli Y (2014) Energy Fuel 28:7303–7330
Wang Y, Chen KS, Mishler J, Cho SC, Adroher XC (2011) Appl Energy 88:981–1007
Peighambardoust SJ, Rowshanzamir S, Amjadi M (2010) Int J Hydrog Energy 35:9349–9384
Park CH, Lee CH, Guiver MD, Lee YM (2011) Prog Polym Sci 36:1443–1498
Zhang HW, Shen PK (2012) Chem Soc Rev 41:2382–2394
Devrim Y, Erkan S, Bac N, Eroglu I (2012) Int J Hydrog Energy 37:16748–16758
Wang T, Sun F, Wang H, Yang S, Fan L (2012) Polymer 53:3154–3162
Ganeshkumar A, Bera D, Mistri EA, Banerjee S (2014) Eur Polym J 60:235–246
Li W, Guo X, Aili D, Martin S, Li Q, Fang J (2015) J Membr Sci 481:44–53
Zhang L, Qi D, Zhao C, Na H (2016) J Membr Sci 508:15–21
Oh K, Ketpang K, Kim H, Shanmugam S (2016) J Membr Sci 507:135–142
Luo JJ, Song YH, Wang YL, Cui PP (2015) J Polym Res 22:41
Kim DJ, Lee HJ, Nam SY (2014) Int J Hydrog Energy 39:17524–17532
Chen R, Li G (2016) New J Chem 40:3755–3762
Lawrence J, Yamashita K, Yamaguchi T (2015) J Power Sources 279:48–54
Falciola L, Checchia S, Pifferi V, Farina H, Ortenzi MA, Sabatini V (2016) Electrochim Acta 194:405–412
Chen J-C, Wu J-A, Lee C-Y, Tsai M-C, Chen K-H (2015) J Membr Sci 483:144–154
Diaz LA, Abuin GC, Corti HR (2009) J Power Sources 188:45–50
Ngamsantivongsa P, Lin H-L, Yu TL (2015) J Membr Sci 491:10–21
Gao Y, Roberson GP, Guiver MD, Mikhailenko SD, Li X, Kaliaguine S (2005) Macromolecules 38:3237–3245
Chen L, Pu Z, Yang J, Yang X, Liu X (2013) J Polym Res 20:45
Feng M, You Y, Zheng P, Liu J, Jia K, Huang Y, Liu X (2016) Int J Hydrog Energy 41:5113–5122
Gao Y, Robertson GP, Kim D-S, Guiver MD, Mikhailenko SD, Li X, Kaliaguine S (2007) Macromolecules 40:1512–1520
Liang YF, Zhu XL, Jian XG (2008) Solid State Ionics 179:1940–1945
Seong Y-H, Won J, Kim S-K, Nam K, Kim S-K, Kim D-W (2011) Int J Hydrog Energy 36:8492–8498
Gao Y, Robertson GP, Guiver MD, Mikhailenko SD, Li X, Kaliaguine S (2006) Polymer 47:808–816
Zheng J, He Q, Gao N, Yuan T, Zhang S, Yang H (2014) J Power Sources 261:38–45
Wang G, Lee KH, Lee WH, Kang NR, Shin DW, Zhuang Y, Lee YM, Guiver MD (2015) J Membr Sci 490:346–353
Pu Z, Chen L, Long Y, Tong L, Huang X, Liu X (2013) J Polym Res 20:281
Mandal AK, Bera D, Banerjee S (2016) Mater Chem Phys 181:265–276
Kim DS, Kim YS, Guiver MD, Pivovar BS (2008) J Membr Sci 321:199–208
Acknowledgments
The authors wish to thank for financial support of this work from the National Natural Science Foundation (Nos. 51173021, 51373028, 51403029).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yumin Huang and Jingchun Liu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 927 kb)
Rights and permissions
About this article
Cite this article
Huang, Y., Liu, J., Zheng, P. et al. Phthalonitrile end-capped sulfonated polyarylene ether nitriles for low-swelling proton exchange membranes. J Polym Res 23, 256 (2016). https://doi.org/10.1007/s10965-016-1150-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-1150-y