Abstract
A series of sulfonated poly(ether ether ketone)s were successfully synthesized via nucleophilic displacement condensation. The membranes are accordingly cast from their DMSO solutions, and fully characterized by determining the ion-exchange capacity, water uptake, dimensional stabilities, proton conductivity and mechanical properties. Among membranes, the block-7c-3 was successfully synthesized by the reaction of Block-6c with chlorosulfonic acid, with the feed ratio of repeated unit to chlorosulfonic acid of 1:8. The experimental results show that the membrane from block-7c-3 has good mechanical, oxidative and dimensional stabilities together with high proton conductivity (5.15 × 10−2 Scm−1) at 80 °C under 100 % relative humidity. The membranes also possess excellent thermal and dimensional stabilities, in this respect, they are potential and promising proton conducting membrane material for PEM full cell applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proton exchange membrane fuel cells (PEMFC) are being explored as the technology of choice for clean and efficient energy conversion systems for automobiles, portable applications and power generation [1–8]. Proton-exchange membrane (PEM), as proton conductive material, is a key component of the PEM fuel cells for transferring protons from the anode to cathode as well as providing a barrier to prevent the fuel cross-leaks between the electrodes. poly(perfluoroalkylsulfonic acid) (Nafion) is the most preferable from the standpoint of chemical and thermal stabilities and physical and proton-conducting properties. [7–17] However, its extremely high cost has limited to a large-scale practical use of Nafion. In this regard, several proton-exchange membranes based on sulfonated aromatic polymers have been investigated for fuel cells [17–24].
Recently, there are several reports on the proton-exchange membranes based on sulfonated poly(aryl ether ketone)s. It was reported that the polymers with acidic groups on the pendant chains were more stable against hydrolysis than those with acidic groups directly on the backbone of the polymers [25, 26].
In this paper, herein, we report the synthesis of fluorene-containing sulfonated poly(ether ether ketone) (SPEEK) with sulfonic acid groups only on the fluorenyl rings. fluorene groups can improve the oxidation resistance of polymers [26]. The ion exchange capacity (IEC), sulfonation degree (SD), equivalent weight (EW), water-uptake, oxidative stabilities, and mechanical properties as well as proton conductivities of the synthesized polymers were fully investigated.
Experimental
Materials
9,9-Bis(4-hydroxyphenyl) fluorene(BPF) (purity > 98 %) was purchased from Tokyo Kasei Kogyo Co. Ltd., Japan (TCI). 2,2-Bis(4-hydroxyphenyl) hexafluoropropane (6 F-BPA) and 4, 4′-difluorobenzophenone (DFBP) were purchased from Aldrich Reagent-grade. Dimethyl sulfoxide (DMSO), methanol, toluene, and anhydrous potassium carbonate were obtained from commercial sources. DMSO was dried over 4A molecule sieves and toluene was dried over sodium wire prior to use. Anhydrous potassium carbonate was vacuum dried at 180 °C for 10 h before use. Other reagents and solvents were used as received.
Synthesis of poly (ether ether ketone)(PEEK)
A typical procedure for the synthesis of the copolymers is as follows [26]0.1.4016 g (4 mmol) of BPF,0.9313 g (4.267 mmol) of DFBP and 0.7189 g (5.2 mmol) of potassium carbonate were added to a three-necked round bottom flask equipped with a magnetic stirrer, a nitrogen inlet, and a Dean-Stark trap. DMSO (18 mL) was introduced to afford a 13 % (w/v) solid concentration. Toluene (18 mL, usually DMSO/toluene = 1/1, v/v) was used as an azeotroping agent. Under the protection of nitrogen atmosphere, the reaction mixture was refluxed at 145 °C for 6 h to dehydrate the system. After the removal of toluene, the temperature was slowly increased to 175 °C and maintained for another 6 h. After cooling to the room temperature, 1.2511 g (5.733 mmol) of DFBP, 2.0178 g (6 mmol) of 6 F-BPA and 1.0793 g (7.8 mmol) of potassium carbonate were added with DMSO (18 mL) and toluene (18 mL). The reaction mixture was refluxed at 145 °C for 6 h to dehydrate the system. The temperature was raised slowly to 175 °C by the controlled removal of toluene. The reaction was allowed to proceed for 6 h. After the solution became very viscous, the reaction mixture was cooled to 80 °C, diluted with the addition of 15 mL DMSO and poured into 300 mL stirred methanol/water solution (1/1, v/v) to precipitate the product. The precipitate was filtered and washed several times with water to remove inorganic salts. Finally, it was vacuum dried at 80 °C for 24 h. An amount of 5.0845 g polymer of Block-6c was obtained in high yield of 97 %.
A series of poly(ether ether ketone) were successfully synthesized using the same procedure as described for synthesis of polymer block-6c. The molecular feed ratio of monomers and the polymerization results were list in Table 1.
Sulfonation of poly (ether ether ketone)(SPEEK)
The sulfonation of Block-7c was carried out by the reaction of Block-6c with chlorosulfonic acid as shown in Scheme 3. The SD of the Block-7c can be readily controlled by adjusting the molar ratio of the repeated units of BPF to chlorosulfonic acid. The sulfonation procedures are depicted as follows: 75 mL dichloromethane and 0.2582 g (0.2 mmol repeated units of BPF) Block-6c were introduced to a 150 mL round bottom flask. The reaction mixture was stirred vigorously until the polymer was dissolved completely. About 16 mL of 0.1 M solution of chlorosulfonic acid (1.6 mmol) in dichloromethane was then added drop-wise under the vigorously stirring at room temperature for about 3 h, meanwhile the resulting brown product gradually precipitated out from the solution. The reaction mixture was stirred vigorously for another 5 h, then the solvent of the mixture was removed using a rotary evaporator at about 40 °C. The precipitate was washed with hexane for three times (3 × 5 mL) and dissolved in 10 mL DMSO, 10 mL of 3 wt % potassium hydroxide aqueous solution was added to the solution. Reaction for another 5 h, the reaction mixture was acidified with 100 mL of 5 vol % hydrochloric acid for another 5 h. The resulting solution was dialyzed for 3 days to remove all small molecules which molecular weight was less than 8000 Da, the dialyzing water was refreshed everyday. The resulting product Block-7c was recovered by removing the water with rotary evaporator at about 60 °C.
A series of sulfonated block copolymers 7a-c with various SD were synthesized by adjusting the molar ratio of the BPF to chlorosulfonic acid according the above sulfonation procedures. Their relative physical and chemical properties were listed in Table 2.
Preparation of polymer membrane
The membranes were prepared by casting from the corresponding 8 % block polymers in DMSO solution onto a glass plate in the dust-free environment, and then dried at 80 °C for 12 h and at 90 °C under vacuum for 48 h. The thickness of all membrane samples was controlled in the range of 100–200 μm.
Measurement
Nuclear magnetic resonance (NMR) spectra was recorded at 400 MHz in a Bruker NMR instrument (model DRX) and listed in parts per million (ppm) downfield from tetramethylsilane(TMS). FT-IR spectra were recorded on a Nicolet520 Fourier transform infrared spectrometer with film samples. Tensile strength was measured at 25°C by a universal test machine (CMT-4014, SANS corporation, Shenzhen, China). The samples were prepared by cutting them into a dumbbell shape (DIN-53504-S3A) and immersing in water at 80°C for 24 h. The cross-head speed was set at a constant speed of 1 mm/min. For each testing reported, at least three measurements were taken and average value was calculated.
Inherent viscosity
Inherent viscosity was determined in 0.5 g dL−1 solution in CHCl3 at 25 °C using a calibrated Ubbelonhde viscometer.
Water uptake and swelling ratio
The water uptake of the membrane was evaluated by measuring the weight change between dried and hydrated state at 80 °C. The membranes were first dried at 150 °C in vacuum to a constant weight. The dried membranes were then immersed in water at 80 °C for 24 h until equilibrium of water absorption. The water uptake was calculated according to the following equation:
Where Wwet and Wdry are the mass of the wet and dry membranes, respectively. The dimension stability of the membrane was characterized by the swelling ratio, which was calculated from the following equation:
where Lwet and Ldry are the lengths of dry and wet samples respectively.
Ion exchange capacity (IEC)
The ion-exchange capacity (IEC) was determined by titration. A piece of dried and protonized membranes was weighted, immersed and equilibrated in large excess of 2 M NaCl aqueous solution for 24 h to exchange the protons of sulfonic acid groups with sodium ions. The concentration of HCl released from the membrane sample was determined by titration with 0.01 M NaOH aqueous solution using phenolphthalein as an indicator. Ion-exchange capacity was calculated according to the following equation:
Where ΔV NaOH is the consumed volume of NaOH solution, C NaOH is the concentration of NaOH solution and Ws is the weight of the membrane sample.
Oxidative and hydrolytic stabilities
Oxidative Stability was tested by immersing a small piece of membrane sample in Fenton’s reagent (3 % H2O2 + 2 ppm FeSO4) in a shaking bath at 80 °C. The oxidative stability was recorded as the expanded time that the membrane began to break into pieces or fracture is observed, the membrane was observed every 10 min to make sure whether or not any change in appearance.
Proton conductivity
Proton conductivity measurement was performed by a Solartron 1255B frequency response analyzer functioning with an oscillating voltage of 10 mV using two probes with the frequency between 1 mHz and 5 kHz. Before the measurement, the membrane samples were full hydrated in de-ionized water for at least 24 h. The proton conductivity of the membrane was measured at 30 °C and 80 °C respectively. The conductivity (σ) of the samples was calculated from the impedance data, using the following equation:
where d and A are the thickness and face area of the sample, respectively, and R was derived from the low intersect of the high-frequency semicircle on a complex impedance plane with the Re (Z)’ axis.
Results and discussion
Polymer synthesis
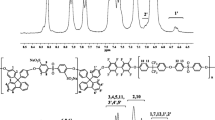
The block PEEK copolymers were successfully synthesized by a two-stage one-pot method. Firstly, the oligomer terminated with fluorine atoms was first prepared from the monomers of BPF and DFBP, and then the monomers of 6 F-BPA and DFBP were in turn added to the reaction mixture in order to form block copolymers. As illustrated in Scheme 1, fluorine-terminated oligomers were first synthesized by condensation copolymerization of 9, 9-bis (4-hydroxyphenyl) fluorine (BPF) and 4, 4′-difluorobenzophenone (DFBP). Excess of DFBP was added to ensure the formation of fluorine-terminated structure. n (5, 10 or 15) equivalents of BPF were reacted with corresponding (n + 1) (5,10 or 15) equivalents of DFBP to give different segment lengths of the fluorine-terminated oligomers. The desired fluorine-terminated oligomers were confirmed using 1H NMR spectrum (Fig. 1). The characteristic peaks for the aromatic proton can be clearly observed at 6.94, 7.00, 7.15, 7.22, 7.28, 7.31, 7.42, 7.74 and 7.79 ppm. In the second stage, the monomers of 6 F-BPA and DFBP were used to continue the chain growth and condensation copolymerization. The block copolymers were prepared as shown in Scheme 2. In this case, the synthesized block PEEKs have the feed ratio of BPF to 6 F-BPA of 4:6. The block copolymers were confirmed by their 1H NMR spectrums and 13C NMR accordingly. The 1H NMR spectrums of block-6a is show in Fig. 2, with proper assignment of all the resonance peaks. The peaks d 7.85 and 7.08 ppm could be assigned to the chemical shifts of the aromatic proton of the 6 F-BPA. All the other peaks were well assigned according to its chemical structure. Figure 3 shows the 13C NMR spectrums of block-6a, with 30 the resonance peaks, representing 30 different carbon atoms. The peaks at 194.16, 194.10, 194.05 ppm assigned to the carbonyl peak, indicating that there are three types of carbonyl molecules.
Sulfonated polymers synthesis
All the polymers were sulfonated using chlorosulfonic acid in methylene chloride. The sulfonation completed at room temperature in a few hours. Because of the insolubility of the resulted products, chlorosulfonic acid must be added as slowly as possible under acutely stirring. Residual sulfonyl chloride was neutralized with aqueous potassium hydroxide in DMSO. Upon treatment with hydrochloric acid followed by dialysis, brownish polymers were obtained after evaporating the water. The sulfonated block copolymers were confirmed by their 1H NMR spectrums accordingly. The 1H NMR spectrums of block-7a is show in Fig. 4, with all the peaks assigned to the molecular structure shown in the figure. FT-IR technique is used to confirm functional groups in the synthesized polymers. As illustration in Fig. 5, sulfonic acid groups can be detected by their corresponding characteristic absorption bands as follows. The absorption bands at 1241, 1020 and 1091 cm−1 can be assigned to symmetric and asymmetric stretching vibrations of O-S-O. The peak at 675 cm−1 is due to the stretching of the S–O. It can be then concluded that the sulfonic acid groups were successfully introduced into the block copolymers as expected (Scheme 3).
Oxidative stabilities
The oxidative stability of the samples is listed in Table 2., the maximum anti-oxidative timing was 150 min for 7b-1 and 142 min for 7c-1, respectively. These results indicated that the membranes from 7a-1 to 7c-3 exhibited much better oxidative stability than those previously reported of fluorene-containing sulfonated poy(arylene ether sulfone) [23]. Presumably, this was resulted from that the fluorine groups can enhance the oxidative stability of the polymer. In general, the oxidative stability decrease with increasing sulfonation degree, because higher sulfonation degree can augment the swelling of the membrane, and then increase the attack opportunity of free radicals to the polymer backbone with absorbed water.
Water uptake and proton conductivity measurements
The water content and water state within the membrane are very important factors that directly affect proton transport across the membranes. Water can act as the carrier and medium for proton as it moves through the membranes. However, excess water-uptake can lead to the decrease in mechanical strength and a dimensional mismatch for fuel cell assembly. As can be seen from Table 2, the water uptakes as well as the linear expansion ratio of the membrane increased with increasing IEC, owing to the increase of the hydrophilicity. As the IEC was increased from 0.83 to 1.16, an increase in water uptake was observed for the block polymer. For example, block-7a-1 and block-7a-3 membranes with IEC 0.95 and 1.16 mequiv.g−1 exhibit the value of water uptake of 17.1 and 22.8 %, respectively. Proton conductivity of fully hydrated block membranes with different IEC was determined at 30 and 80 °C respectively. The results are listed in Table 3. As expected, the proton conductivity of the membranes increases with increasing IEC value and temperature. Block-7a-3 membrane with the highest IEC value of 1.16 mequiv.g−1 exhibits the highest conductivity among these 7a-c membranes. This can be explained that more protons participating in conduction are derived from higher content of sulfonic acid groups. For instance, block-7a-3 membrane exhibits conductivity of 4.09 × 10−2 Scm−1 at 30 °C and 5.15 × 10−2 Scm−1 at 80 °C, which is higher than block-7c-3 membrane, 2.97 × 10−2 Scm−1 at 30 °C and 3.75 × 10−2 Scm−1 at 80 °C, respectively.
Mechanical strength of membranes 1a-6b
The mechanical properties of the membranes were measured at 25 °C and 100 % relative humidity (Table 3). All samples were treated by immersing in deionized water at 25 °C for 24 h prior to test. The membranes samples of block 7a-c showed higher tensile strength (18–32 MPa) and lower strain (28–50 %). These data indicate these membranes are strong and tough enough to be applied as proton exchange membrane in the fuel cell [27].
Conclusions
Fluorene-containing block copolymer can be synthesized by a one-pot two-step methodology. All the polymers were sulfonated using chlorosulfonic acid in methylene chloride. The synthesized membranes exhibit high ion-exchange capacities, excellent mechanical properties in hydrated state, thermal stability, as well as better oxidative stabilities at relatively lower IEC value. Especially the membrane derived from block-7a-3 with IEC value of 1.16 mequiv.g−1 exhibits high conductivities of 4.09 × 10−2S/cm at 30 °C and 5.15 × 10−2 S/cm at 80 °C , respectively. In conclusion, the membranes are suitable for the fuel cell applications as potential proton exchange membrane materials.
References
Prater KB (1994) Polymer electrolyte fuel cells: a review of recent developments. J Power Sources 51:129–144
Thomas K (1996) Fuel cells for transport: can the promise be fulfilled technical requirements and demands from customers. J Power Sources 61:61–69
Di Noto V, Lavina S, Giffin GA (2011) Polymer electrolytes: present, past and future. Electrochim Acta 57:4–13
Borroni-Bird CE (1996) Fuel cell commercialization issues for light-duty vehicle applications. J Power Sources 61:33–48
Rikukawa M, Sanui K (2000) Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog Polym Sci 25:1463
Di Noto V, Zawodzinski TA, Ml HA (2012) Polymer electrolytes for a hydrogen economy. Int J Hydrog Energy 37(7):6120–6131
Jacques R, Deborah JJ (2003) Non-fluorinated polymer materials for proton exchange membrane fuel cell. Annu Rev Mater Res 33:503–555
Gupta B, Buchi FN, Scherer GG (1993) Materials research aspects of organic solid proton conductors. Solid State Ionics 61:213–218
Flint SD, Slade RCT (1997) Investigation of radiation-grafted PVDF-g-polystyrene- sulfonic-acid ion exchange membranes for use in hydrogen oxygen fuel cells. Solid State Ionics 97:299–307
Jones JD, Roziere J (2001) Recent advances in the functionalisation of polybenzimidazole and polyetherketone for fuel cell applications. J Membr Sci 185:41–58
Bae JM, Honma I, Murata M (2002) Properties of selected sulfonated polymers as proton-conducting electrolytes for polymer electrolyte fuel cells. Solid State Ionics 147:189–194
Roziere J, Jones DJ, Marrony M (2001) On the doping of sulfonated polybenzimidazole with strong bases. Solid State Ionics 145:61–68
Giffin GA, Piga M, Lavina S (2012) Characterization of sulfated-zirconia/Nafion composite membranes for proton exchange membrane fuel cells. J Power Sources 198:66–75
Tezuka T, Tadanaga K, Hayashi A, Tatsumisago M (2006) Inorganic–organic hybrid membranes with anhydrous proton conduction prepared from 3-aminopropyltriethoxysilane and sulfuric acid by the sol–gel method. J Am Chem Soc 128:16470–16471
Di Noto V, Piga M, Giffin GA (2011) New sulfonated poly (p-phenylenesulfone)/poly (1-oxotrimethylene) nanocomposite proton-conducting membranes for PEMFCs. Chem Mater 23(20):4452–4458
Asano N, Miyatake K, Watanabe M (2004) Hydrolytically stable polyimide inomomer for fuel cell applilcations. Chem Mater 16:2841–2843
Asano N, Aoki M, Suzuki S, Miyatake K, Uchida H, Watanabe M (2006) Aliphatic/aromatic polyimide ionomers as a proton conductive membrane for fuel cell applications. J Am Chem Soc 128:1762–1769
Roy A, Hickner MA, Yu X (2006) Influence of chemical composition and sequence length on the transport properties of proton exchange membranes. J Polym Sci B Polym Phys 44:2226–2239
Ghassemi H, McGrath JE, Zawodzinski J (2006) Multiblock sulfonated–fluorinated poly(arylene ether)s for a proton exchange membrane fuel cell. Polymer 47:4132–4139
Matsumura S, Hlil R, Lepiller C, Gaudet J, Guay D, Hay AS (2008) Ionomers for proton exchange membrane fuel cells with sulfonic acid groups on the end groups: novel linear aromatic poly(sulfide − ketone)s. Macromolecules 41:277–280
Matsumura S, Hlil AR, Lepiller C, Gaudet J, Guay D, Shi Z-Q, Holdcroft S, Hay AS (2008) Macromolecules 41:281–284
Tian SH, Meng YZ, Hay AS (2009) Membranes from poly(aryl ether)-based ionomers containing randomly distritbuted nanoclusters of 6 or 12 sulfonic acid groups. Macromolecules 42:1153–1160
Pang J, Zhang H, Li XF, Jiang ZH (2007) Novel wholly aromatic sulfonated poly(arylene ether) copolymers containing sulfonic acid groups on the pendants for proton exchange membrane materials. Macromolecules 40:9435–9442
Yasuda T, Li Y, Miyatake K, Hirai M, Nanasawa M, Watanabe M (2006) Synthesis and properties of polyimides bearing acid groups on long pendant aliphatic chains. J Polym Sci A Polym Chem 44:3995–4005
Hu H, Xiao M, Wang SJ, Meng YZ (2010) Poly(fluorenyl ether ketone)ionomers containing separated hydrophilic multiblocks usedin fuel cells as proton exchange membranes. Int J Hydrog Energy 35:682–9
Shang XY, Tian SH, Kong LH, Meng YZ (2005) Synthesis and characterization of sulfonated fluorene-containing poly(arylene ether ketone) for proton exchange membrane. J Membr Sci 266:94–101
Wang SJ, Luo JJ, Meng YZ (2012) Design, synthesis and properties of polyaromatics with hydrophobic and hydrophilic long blocks as proton exchange membrane for PEM fuel cell application. Int J Hydrog Energy 37:4545–4552
Acknowledgments
The authors would like to thank for the Natural Science Foundation of China (21306124) financial support of this work and Taiyuan University of Technology College Students’ Innovative Entrepreneurial Training Plan (Grant 13060).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, J.J., Song, Y.H., Wang, Y.L. et al. Synthesis and properties of fluorene-containing sulfonated poly (ether ether ketone) as proton-exchange membrane for PEM fuel cell application. J Polym Res 22, 41 (2015). https://doi.org/10.1007/s10965-015-0683-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0683-9