Abstract
Four donor-acceptor type polymers based on quinoline and biquinoline have been synthesized by Pd catalyzed direct C-H (hetero)arylation reaction. Polymers P1 and P2 are alternate copolymers of thiophene-benzothiadiazole-thiophene (TBTT) unit with quinoline and biquinoline unit, respectively. P3 is a random copolymer containing cyclopentadithiophene (CPDT), benzothiadiazole and quinoline moieties in the backbone whereas P4 contains CPDT unit with randomly distributed benzothiadiazole and biquinoline units. All the polymers show good thermal stability and solubility in common organic solvents. CPDT based polymers P3 and P4 exhibit higher absorbance maxima, higher lying Highest Occupied Molecular Orbital (HOMO) energy levels and smaller band gap as compared to thiophene based polymers P1 and P2 as a result of better electron-donating ability of the former leading to stronger intramolecular charge transfer. Also, quinoline based polymers P1 and P3 show a red-shift in the absorbance maxima compared to biquinoline based polymers P2 and P4, respectively due to non-planar transoid conformation of the two quinoline rings in the biquinoline unit. It is found that the use of N-heterocycle based comonomers allows the tuning of the HOMO level over a remarkably wide range (~0.8 eV). Additionally, the use of quinoline or biquinoline along the conjugated chain leads to deeper lying HOMO levels suggesting good oxidative stability for this class of materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few decades, fabrication of a variety of optoelectronic devices such as organic photovoltaic devices (OPVs), organic light emitting diodes (OLEDs), and organic field effect transistors (OFETs) have attracted considerable interest. [1–4] The active layer in all these devices is a conjugated organic material. In recent years, low band gap donor-acceptor (DA) polymers consisting of alternating electron-donating and electron-withdrawing building blocks along the polymer backbone has been widely used as an active layer in OPVs. [5–7] Apart from providing low band gap to maximize light harvesting, DA concept leads to tuning of the HOMO and Lowest Unoccupied Molecular Orbital (LUMO) energy levels by varying the electron-donating ability of the donor and electron-accepting ability of the acceptor. [8, 9] The internal charge transfer (ICT) between the donor and acceptor moiety leads to enhanced double bond character between the repeating units leading to more planar configuration of the polymer backbone thus facilitating π-electron delocalization which subsequently leads to smaller band gap and promotes high charge mobility. Therefore, a large number of DA polymers with various donor and acceptor units have been designed, synthesized and applied in the fabrication of photovoltaic devices and has played an important role in improving the device performance. [10–17].
Due to the presence of electron rich sulphur atom both thiophene and CPDT act as efficient electron-donating moieties and numerous conjugated polymers containing thiophene and CPDT as donor with various acceptor units with improved device performance have been designed and synthesized. [18–21] 2,1,3-Benzothiadiazole is the most widely investigated electron-accepting unit and its copolymers with various electron-donating species such as fluorene, thiophene, carbazole, CPDT etc. have been synthesized and applied in the fabrication of photovoltaic devices. [22–24] Polymers containing thiophene-benzothiadiazole-thiophene (TBTT) segment with various aromatic moieties such as fluorene, carbazole and thiophene exhibit broad absorption in the region of 300–700 nm. [25–29].
Quinoline based copolymers show n-type electrically conducting properties due to the presence of electron-withdrawing aromatic ring containing imine-nitrogen. [30–32] Polyquinolines possess outstanding oxidative and thermal stability, high glass transition temperature, low moisture absorption and good film forming properties. [33–36] In our previous work, it was shown that the introduction of electron-deficient quinoline unit in polyfluorene backbone results in low lying LUMO energy levels in the resulting polymers. [37].
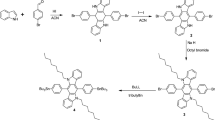
In the present study, four novel DA polymers poly[(quinoline-5,8-diyl)-alt-(4,7-bis-(4-octylthiophene-2-yl)-2,1,3-benzothiadiazole-5ʹ,5ʹʹ-diyl)] P1, poly[(8ʹ,8-biquinolin-5,5ʹ-diyl)-alt-(4,7-bis-(4-octylthiophene-2-yl)-2,1,3-benzothiadiazole-5ʹ,5ʹʹ-diyl)] P2, poly{[2ʹ-(quinoline-5-yl)-4ʹ,4ʹ-dioctylcyclopenta[2,1-b:3,4-bʹʹ]dithiophene-8,6ʹ-diyl)]-co-[2ʹ-(2,1,3-benzothiadiazole-4-yl)-4ʹ,4ʹ-dioctylcyclopenta[2,1-b:3,4-bʹʹ]dithiophene-7,6ʹ-diyl]} P3 and poly{[2ʹ-(8,8ʹʹ-biquinoline-5-yl)-4ʹ,4ʹ-dioctylcyclopenta[2,1-b:3,4-bʹʹʹ]dithiophene-5ʹʹ,6ʹ-diyl)]-co-[2ʹ-(2,1,3-benzothiadiazole-4-yl)-4ʹ,4ʹ-dioctylcyclopenta[2,1-b:3,4-bʹʹʹ]dithiophene-7,6ʹ-diyl]} P4 have been synthesized (Fig. 1). P1 and P2 are alternating copolymers where P1 contains alternate TBTT and quinoline unit in the polymer backbone and P2 is a copolymer containing TBTT and biquinoline. P3 and P4 are random polymers having CPDT moiety as donor and randomly distributed 2,1,3-benzothiadiazole and either quinoline (P3) or biquinoline (P4) unit as acceptor in the polymer chain. All the four polymers have been synthesized by Pd catalyzed direct-arylation polymerization reaction and have been analyzed for their optical, thermal, electrochemical properties.
Experimental
Materials
The precursors 4,7-bis(4-octylthiophen-2-yl)-benzothiadiazole [23] (1), 5,8-dibromoquinoline [37] (2), 5,5ʹdibromo-8,8ʹ-biquinoline [38] (3), 4,4-dioctylcyclopenta[2,1-b:3,4-b′]dithiophene [39] (4) and 4,7-dibromobenzothiadiazole [40] (5) were prepared according to the literature procedures. Pivalic acid was purchased from Sigma Aldrich. Potassium carbonate (K2CO3) and dimethylacetamide (DMAc) were purchased from Spectrochem and used without further purification. All the reactions were carried out using dry solvents under a nitrogen atmosphere unless specified otherwise.
Instrumentation and methods
1H NMR spectra were recorded on a Bruker 300 MHz spectrometer in CDCl3 with TMS as an internal standard. Analytical Thin Layer Chromatography (TLC) was conducted using Merck silica gel precoated aluminium plates. The UV/Visible spectra for polymer solutions and films were obtained with a T90+ UV/Visible spectrophotometer and Perkin Elmer, Lambda 1050 UV/VIS/NIR Spectrometer, respectively. Cyclic Voltammetry (CV) measurements were carried out on an Autolab 30 at a scan rate of 50 mVs−1. Tetrabutylammoniumperchlorate (Bu4NClO4) in acetonitrile (0.1 M) was used as supporting electrolyte. A three electrode cell was used with Ag/AgCl electrode as the reference electrode and platinum (Pt) wires as both the working and counter electrodes. The films for electrochemical measurement were prepared on a Pt electrode by dipping in a viscous solution of the polymer in chloroform. Thermogravimetric traces for the polymers were recorded on a Q-50 TGA instrument from TA instruments. The experiment was carried out under nitrogen atmosphere at a heating rate of 20 °Cmin−1 with 5 ± 1 mg sample. DSC analysis was carried on a TA Instruments DSC Q200 instrument under nitrogen atmosphere at a heating/cooling rate of 10 °Cmin−1. Molecular weights were measured using a Gel permeation chromatograph (GPC) containing a PLgel column at a flow rate of 1 mL/min against polystyrene standards in chloroform.
OFET fabrication
Field effect transistors using the polymers were made on cleaned glass substrates, in bottom gate-top contact geometry (BG-TC). 40 nm Aluminium gate electrodes were deposited on the glass substrates by thermal evaporation of pure aluminium at 5 × 10−6 mbar pressure, at a rate of 1Ao/s. PMMA (poly(methyl methacrylate)) solution prepared in propylene carbonate solution (80 mg/mL) was spin coated on the gate electrode at 800 rpm and baked at 110 °C for 1 h in inert atmosphere. Polymer solute on (10 mg/mL concentration in chlorobenzene) was spin coated at 600 rpm and annealed at 100 °C in N2 filled glove box for 1 h. 40 nm thick gold top contacts were evaporated using thermal evaporation method at 5 × 10−6 mbar pressure, maintaining a deposition rate of 0.1 Ao/s.
Electronic characterization of hence prepared organic field effect transistors were carried out using a Keithley 4200-SCS system, while keeping the devices in vacuum (10−2 mbar). Mobility of transistors was calculated using standard transistor equation in saturation regime
Where I ds is the saturation current, μsat FET is the saturation mobility, C ox is the capacitance per unit area of dielectric, W is the width of channel, V G is the gate voltage, V t is the threshold voltage and L is the length of the channel.
Synthetic procedures
Synthesis of poly[(quinoline-5,8-diyl)-alt-(4,7-bis-(4-octylthiophene-2-yl)-2,1,3-benzothiadiazole-5ʹ,5ʹʹ-diyl)] P1
A mixture of Pd(OAc)2 (3 mg, 0.013 mmol), K2CO3 (96 mg, 0.69 mmol), pivalic acid (8.4 mg, 0.083 mmol), 1 (146 mg, 0.279 mmol) and 2 (80 mg, 0.279 mmol) was stirred in DMAc (5.5 mL) at 80 °C for 12 h under a nitrogen atmosphere. It was then cooled to room temperature, the polymer was precipitated in methanol, filtered, washed with EDTA and purified by soxhlet extraction with acetone and chloroform. The chloroform portion was concentrated and reprecipitated in methanol, filtered and dried. The product was obtained as a red solid (175 mg, 96 %).1H NMR (300 MHz, CDCl3): δ 8.87–8.73 (m, 1H), 8.56–8.33 (m, 2H), 8.08–7.58 (m, 6H), 2.79 (bm, 4H), 1.55–0.79 (m, 30H). Mn = 3.6 x 103 gmol−1, Mw = 7.56 x 103 gmol−1, Mw/Mn = 2.1.
Synthesis of poly[(8ʹ,8-biquinolin-5,5ʹ-diyl)-alt-(4,7-bis-(4-octylthiophene-2-yl)-2,1,3-benzothiadiazole-5ʹ,5ʹʹ-diyl)] P2
P2 was synthesized according to the procedure followed for P1 using Pd(OAc)2 (3 mg, 0.013 mmol), K2CO3 (96 mg, 0.69 mmol), pivalic acid (8.4 mg, 0.083 mmol) 1 (146 mg, 0.279 mmol) and 3 (115 mg,0.279 mmol) in DMAc (5.5 mL) at 80 °C for 12 h. The product was obtained as a red solid (185 mg, 85 %).1H NMR (300 MHz, CDCl3): δ 8.86 (bs, 2H), 8.35–8.20 (m, 3H), 8.10–7.8 (m, 7H), 7.39 (bs, 2H), 2.68–2.55 (4H), 1.66–0.82 (m, 30H). Mn = 5.03 x 103 gmol−1, Mw = 7.94 x 103 gmol−1, Mw/Mn = 1.58.
Synthesis of poly{[2ʹ-(quinoline-5-yl)-4ʹ,4ʹ-dioctylcyclopenta[2,1-b:3,4-bʹʹ]dithiophene-8,6ʹ-diyl)]-co-[2ʹ-(2,1,3-benzothiadiazole-4-yl)-4ʹ,4ʹ-dioctylcyclopenta[2,1-b:3,4-bʹʹ]dithiophene-7,6ʹ-diyl]} P3
P3 was synthesized according to the procedure followed for P1 using Pd(OAc)2 (8.3 mg, 0.03 mmol), K2CO3 (257 mg, 1.864 mmol), pivalic acid (22 mg, 0.22 mmol), 4 (300 mg,0.746 mmol), 5 (109 mg, 0.373 mmol) and 2 (106 mg, 0.373 mmol) in DMAc (3.3 mL) at 80 °C for 24 h. The product was obtained as a dark solid (290 mg, 73 %).1H NMR (300 MHz, CDCl3): δ 9.10 (bs, ArH), 8.71 (bd, ArH), 8.10 (bs, ArH), 7.87 (bm, ArH), 7.55 (bs, ArH), 7.13 (m, ArH), 2.01 (bs, 8H α-CH2), 1.56–0.83 (m, 60H). Mn = 5.3 x 103 gmol−1, Mw = 9.43 x 103 gmol−1, Mw/Mn = 1.78.
2.3.4 synthesis of poly{[2ʹ-(8,8ʹʹ-biquinoline-5-yl)-4ʹ,4ʹ-dioctylcyclopenta[2,1-b:3,4-bʹʹʹ]dithiophene-5ʹʹ,6ʹ-diyl)]-co-[2ʹ-(2,1,3-benzothiadiazole-4-yl)-4ʹ,4ʹ-dioctylcyclopenta[2,1-b:3,4-bʹʹʹ]dithiophene-7,6ʹ-diyl]} P4
P4 was synthesized according to the procedure followed for P1 using Pd(OAc)2 (8.3 mg, 0.03 mmol), K2CO3 (257 mg, 1.864 mmol), pivalic acid (22 mg, 0.22 mmol), 1 (300 mg, 0.746 mmol), 5(109 mg, 0.373 mmol) and 3 (154 mg, 0.373 mmol) in DMAc (3.3 mL) at 80 °C for 24 h. The product was obtained as a dark solid (340 mg, 76.5 %).1H NMR (300 MHz, CDCl3): δ 8.88 (bs, ArH), 8.77 (bs, ArH), 8.10 (bd, ArH), 7.87 (bs, ArH), 7.44 (bs, ArH), 7.00 (bs, ArH), 2.03 (bd, 8H α-CH2), 1.21–0.84 (m, 60H). Mn = 7.6 x 103 gmol−1, Mw = 1.29 x 104 gmol−1, Mw/Mn = 1.71.
Results and discussion
The synthetic pathway for polymers P1-P4 is outlined in Scheme 1.All the polymers were synthesized using Pd catalyzed direct-arylation polycondensation reaction by heating corresponding monomers with Pd(OAc)2, Pivalic acid and DMAc at 80 °C under nitrogen atmosphere. All the monomers 1, 2, 3, 4 and 5 were synthesized based on literature procedures. [23, 37–40] Alternate polymers P1 and P2 were obtained by reacting 1 with 2 and 3, respectively. Random polymer P3 was obtained by reacting4with an equimolar mixture of the dibromides, 5and 2 whereas P4 was prepared using 3 instead of 2. Comparison of P1 and P3 with P2 and P4 provides an insight how the optical and electrochemical properties are affected by replacing quinoline unit with biquinoline unit. Also, comparing P1 and P2 with P3 and P4 show the effect of replacing thiophene unit by CPDT unit. This method gave the desired polymers in greater than 72 % yield. All the polymers showed good solubility in common organic solvents and gel permeation chromatography (GPC) measurements against polystyrene standards showed the number-average molecular weight to be 3.6 × 103 gmol−1, 5.03× 103 gmol−1, 5.3 × 103 gmol−1and 7.6 x 103 gmol−1for P1, P2, P3 and P4, respectively.
Optical properties
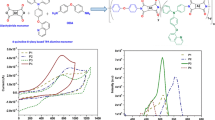
The UV-vis absorption spectra of the polymers P1-P4 in dilute chloroform solution and spin coated films are shown in Fig. 2. The absorption maxima and estimated optical band gaps are summarized in Table 1.The absorption spectra of all the polymers are characterized by two major absorption peaks, which is a common feature of alternating DA copolymers. The low wavelength absorption peak can be attributed to the higher energy π-π* transitions and long wavelength absorption peak can be attributed to the intermolecular charge-transfer transition (ICT) between the donor moiety and the acceptor unit. P1, P2, P3 and P4 showed their absorbance maxima at 494 nm, 481 nm, 664 nm and 649 nm, respectively. Absorbance maxima for polymers P1 and P3 containing quinoline unit in the backbone is red-shifted by 13 nm and 15 nm as compared to biquinoline based polymers P2 and P4, respectively. The higher value of absorbance maxima for P1 and P3 is most likely due to more efficient electron delocalization resulting from a more planar structure.[38, 37] P3 and P4 display a strongly red-shifted absorption band when compared to P1 and P2 indicating stronger ICT between the donor CPDT and acceptor unit due to more electron-donating nature of CPDT in comparison with thiophene.
Going from solution to solid state, a red-shifted and comparatively broader absorption bands for P1, P2, P3 and P4 were observed with absorbance maxima centered at 530 nm, 497 nm, 684 nm and 672 nm, respectively. A strong tailing effect in the solid state was observed making the determination of onset difficult. The optical band gap estimated from the thin film absorption onsets are 1.92 eV, 2.03 eV, 1.44 eV and 1.45 eV for P1, P2, P3 and P4, respectively. The optical band gap of polymers containing thiophene unit (P1 and P2) is higher than that of polymers having CPDT moiety (P3 and P4) due to more effective ICT in P3 and P4.
Electrochemical properties
The electrochemical properties of the polymers films were investigated using cyclic voltammetry. The positions of the HOMO energy levels and LUMO energy levels were estimated from the onset oxidation potential (Eox onset) and onset reduction potential (Ered onset) respectively, using the equations HOMO = − (Eox onset + 4.4) eV and LUMO = − (Ered onset + 4.4) eV. Cyclic voltammograms of the synthesized polymers are shown in Fig. 3 and the electrochemical properties estimated from cyclic voltammetry are summarized in Table 1.
The estimated HOMO energy levels for polymers P1-P4 lie in the range of −5.82 eV and −5.03 eV. The HOMO energy levels for P1 and P2 are −5.82 eV and −5.75 eV, respectively whereas the HOMO energy levels of P3 and P4 are positioned at −5.19 eV and −5.03 eV, respectively (Energy level diagram shown in Fig. 4). The HOMO energy levels of DA polymers are essentially dominated by the electron-donating unit and are affected by the electron-donating ability of the donor. Thus, the introduction of highly donating CPDT moiety in P3 and P4 by replacing thiophene unit in P1 and P2 results in higher HOMO energy levels. The deeper lying HOMO levels suggest that the incorporation of quinoline or biquinoline can help in designing conjugated materials with improved oxidative stability. The estimated LUMO energy levels for P1, P2, P3 and P4 are −3.84 eV, −3.87 eV, −3.79 eV and −3.83 eV, respectively. The LUMO energy levels of the polymers are essentially dominated by the acceptor unit (here BT and either quinoline or biquinoline unit) which explains the low and similar reduction potential and LUMO energy levels for all the four polymers. The reduction potential for polymers containing biquinoline unit in the backbone is slightly less than that of polymers containing quinoline unit. The ease in reduction of P2 and P4 compared to P1 and P3 shows that the introduction of biquinoline unit imparts better electron-accepting property to the polymer but the effect is small due to steric hindrance between the two quinoline units. Electrochemical band gap (Eg ec) for P1, P2, P3 and P4 estimated from difference between the HOMO energy level and LUMO energy level were found out to be 1.98 eV, 1.87 eV, 1.40 eV and 1.20 eV, respectively. Biquinoline based polymers, P2 and P4 show smaller electrochemical band gap as compared to quinoline containing polymers P1 and P3, respectively. A slight difference in the electrochemical band gap and optical band gap is observed for all the polymers since the determination of oxidation or reduction potential using cyclic voltammetry requires removal or addition of electrons, respectively, whereas optical band gap is the energy difference for intramolecular excitonic state. [41].
Field effect transistors
FET characteristics of these semiconductors were studied. The transfer characteristics and the output characteristics for transistor based on P3 and P4 are shown in Figs. 5 and 6, respectively. It is to be noted that apart from the interfacial mobility, the varying HOMO levels of the different polymers also play a decisive factor and the presence of sizable electrode-semiconductor barrier can dominate the I-V characteristics. It was observed that in all the devices tested, CPDT based random copolymers P3 and P4 exhibit hole-type transport with average mobility values (10 sample average) 11 × 10−3 cm2/Vs and 6 × 10−3 cm2/Vs respectively. On the other hand, the polymers TBTT based P1 and P2 did not exhibit typical FET characteristics. This can be attributed to the existence of a dominant contact resistance and/or to transport parameters arising from reduced planarity and double bond character prevailing in P1 and P2 systems.
Thermal properties
The 5 % weight loss temperatures for the polymers P1-P4 were found to be between 350 ̊C and 405 ̊C, with no weight loss up to approximately 300 ̊C, indicating that all the four polymers are thermally stable. P2, P3 and P4 show clear glass transitions at 90 ̊C, 81 ̊C and 85 ̊C, respectively whereas P1 does not shown any clear transition. The insertion of biquinoline unit in the backbone increased the thermal stability and the glass transition temperature of the polymer.
Conclusions
A series of DA polymers based on quinoline and biquinoline unit in the backbone have been designed and synthesized by palladium catalyzed direct-arylation polycondensation reaction. Two alternating polymers containing TBTT segment and either quinoline (P1) or biquinoline (P2) unit and two random polymers incorporating donor CPDT moiety and randomly distributed 2,1,3-benzothiadiazole and either quinoline (P3) or biquinoline (P4) unit were synthesized and their thermal, optical and electrochemical properties of the synthesized polymers were investigated. Quinoline based polymers P1 and P3 show absorbance maxima centered at longer wavelength as compared to biquinoline based polymers P2 and P4 due to non-planar conformation of two quinoline rings in P2 and P4.The HOMO energy levels of polymers containing thiophene as donor (P1 = −5.82 eV, P2 = −5.75 eV) are deeper lying as compared to CPDT based polymers (P3 = −5.19 eV, P4 = −5.03 eV). Also, the absorbance maxima for polymers P3 and P4 are red-shifted as compared to P1 and P2 due to the more electron-donating nature of CPDT compared to thiophene leading to stronger ICT. The estimated electrochemical band gap for P1, P2, P3 and P4 are 1.98 eV, 1.87 eV, 1.4 eV and 1.2 eV, respectively. The deeper lying HOMO levels suggest high oxidative stability for this class of polymers and all of them showed thermal stability greater than 300 ̊C. Field effect transistors made using P3 and P4 polymers exhibited hole type charge transport, where as those made using P1 and P2 does not showed any device characteristics.
References
Lu F, Nakanishi T (2015) Alkyl-π engineering in state control toward versatile optoelectronic soft materials. Sci Technol Adv Mater 16(1):14805
Jou JH, Kumar S, Agrawal A, Li TH, Sahoo S (2015) Approaches for fabricating high efficiency organic light emitting diodes. J Mater Chem C 3(13):2974–3002
Kola S, Sinha J, Katz HE (2012) Organic transistors in the new decade: Toward n-channel, printed, and stabilized devices. J Polym Sci B Polym Phys 50(15):1090–1120
Ragoussi M-E, Torres T (2015) New generation solar cells: concepts, trends and perspectives. Chem Commun 51(19):3957–3972
Zhou H, Yang L, Stoneking S, You W (2010) A weak donor-strong acceptor strategy to design ideal polymers for organic solar cells. ACS Appl Mater Interfaces 2:1377–1383
Ajayaghosh A (2003) Donor-acceptor type low band gap polymers: polysquaraines and related systems. Chem Soc Rev 32:181–191
Roncali J (1997) Synthetic principles for bandgap control in linear π-conjugated systems. Chem Rev 97:173–205
Bundgaard E, Krebs FC (2007) Low band gap polymers for organic Photovoltaics. Sol Energy Mater Sol Cells 91:954–985.
Bundgaard E, Krebs FC (2006) Low-band-gap conjugated polymers based on thiophene, benzothiadiazole, and benzobis(thiadiazole). Macromolecules 39(8):2823–2831
Chu TY, Lu J, Beaupre S, Zhang Y, Pouliot J-R, Wakim S, Zhou J, Leclerc M, Li Z, Ding J, Tao Y (2011) Bulk heterojunction solar cells using thieno[3,4-c]pyrrole-4,6-dione and dithieno[3,2-b:2',3'-d]silole copolymer with a power conversion efficiency of 7.3 %. J Am Chem Soc 133:4250–4253
Amb CM, Chen S, Graham KR, Subbiah J, Small CE, So F, Reynolds JR (2011) Dithienogermole as a fused electron donor in bulk heterojunction solar cells. J Am Chem Soc 133:10062–10065
Zhou H, Yang L, Stuart AC, Price SC, Liu S, You W (2011) Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7 % efficiency. Angew Chem Int Ed Engl 50:2995–2998
Park SH, Roy A, Beaupre S, Cho S, Coates N, Moon JS, Moses D, Leclerc M, Lee K, Heeger AJ (2009) Bulk heterojunction solar cells with internal Quantum efficiency approaching 100 %. Nat Photonics 3:297–303
Price SC, Stuart AC, Yang L, Zhou H, You W (2011) Fluorine substituted conjugated polymer of medium band gap yields 7 % efficiency in polymer-fullerene solar cells. J Am Chem Soc 133:4625–4631
Li Y, Xue L, Li H, Li Z, Xu B, Wen S, Tian W (2009) Energy level and molecular structure engineering of conjugated donor − acceptor copolymers for photovoltaic applications. Macromolecules 42(13):4491–4499
Chen GY, Cheng YH, Chou YJ, Su MS, Chen CM, Wei KH (2011) Crystalline conjugated polymer containing fused 2,5-di(thiophen-2-yl)thieno[2,3-b]thiophene and thieno[3,4-c]pyrrole-4,6-dione units for bulk heterojunction solar cells. Chem Commun 47(17):5064–5066
Jiang JM, Yang PA, Hsieh TH, Wei KH (2011) Crystalline low-band gap polymers comprising thiophene and 2,1,3-benzooxadiazole units for bulk heterojunction solar cells. Macromolecules 44(23):9155–9163
Peet J, Kim JY, Coates NE, Ma WL, Moses D, Heeger AJ, Bazan GC (2007) Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat Mater 6:497–500
Biniek L, Chochos CL, Leclerc N, Hadziioannou G, Kallitsis JK, Bechara R, Leveque P, Heiser T (2009) A [3,2-b]thienothiophene-alt-benzothiadiazole copolymer for photovoltaic applications: design, synthesis, material characterization and device performances. J Mater Chem 19(28):4946–4951
Ko S, Mondal R, Risko C, Lee JK, Hong S, McGehee MD, Brédas J-L, Bao Z (2010) Tuning the optoelectronic properties of vinylene-linked donor − acceptor copolymers for organic photovoltaics. Macromolecules 43(16):6685–6698
Liang F, Lu J, Ding J, Movileanu R, Tao Y (2009) Design and synthesis of alternating regioregular oligothiophenes/benzothiadiazole copolymers for organic solar cells. Macromolecules 42:6107–6114
Wu WC, Liu CL, Chen WC (2006) Synthesis and characterization of new fluorene-acceptor alternating and random copolymers for light-emitting applications. Polymer 47(2):527–538
El Shehawy AA, Abdo NI, El Barbary AA, Lee JS (2011) Alternating copolymers based on 2,1,3-benzothiadiazole and hexylthiophene: positioning effect of hexyl chains on the photophysical and electrochemical properties. Eur J Org Chem 25:4841–4852
Botiz I, Schaller RD, Verduzco R, Darling SB (2011) Optoelectronic properties and charge transfer in donor–acceptor all-conjugated diblock copolymers. J Phys Chem C 115(18):9260–9266
Chen MH, Hou J, Hong Z, Yang G, Sista S, Chen LM, Yang Y (2009) Efficient polymer solar cells with thin active layers based on alternating polyfluorene copolymer/fullerene bulk heterojunctions. Adv Mater 21:4238–4242
Shi F, Fang G, Liang F, Wang L, Mu Z, Zhang X, Xie Z, Su Z (2010) Broad absorbing low-bandgap polythiophene derivatives incorporating separate and content-tunable benzothiadiazole and carbazole moieties for polymer solar cells. Eur Polym J 46:1770–1777
Tamilavan V, Song M, Jin S-H, Hyun MH (2011) Synthesis of conjugated polymers with broad absorption bands and photovoltaic properties as bulk heterojuction solar cells. Polymer 52(11):2384–2390
Biniek L, Chochos CL, Hadziioannou G, Leclerc N, Lévêque P, Heiser T (2010) Electronic properties and photovoltaic performances of a series of oligothiophene copolymers incorporating both thieno[3,2-b]thiophene and 2,1,3-benzothiadiazole moieties. Macromol Rapid Commun 31(7):651–656
Padhy H, Huang JH, Sahu D, Patra D, Kekuda D, Chu CW, Lin HC (2010) Synthesis and applications of low-bandgap conjugated polymers containing phenothiazine donor and various benzodiazole acceptors for polymer solar cells. J Polym Sci, Part A: Polym Chem 48:4823–4834
Kanbara T, Saito N, Yamamoto T, Kubota K (1991) Preparation and properties of poly(quinolinediyl)s and poly(isoquinoline-1,4-diyl) with new pi-conjugation systems. Macromolecules 24(21):5883–5885
Saito N, Kanbara T, Nakamura Y, Yamamoto T, Kubota K (1994) Electrochemical and chemical preparation of linear pi-conjugated poly(quinoline-2,6-diyl) using nickel complexes and electrochemical properties of the polymer. Macromolecules 27(3):756–761
Saito N, Yamamoto T (1995) Preparation of new n-type conducting poly(arylene)s by organometallic process and their electrical and optical properties. Synth Met 69(1–3):539–540
Zhang X, Shetty AS, Jenekhe SA (1999) Electroluminescence and photophysical properties of polyquinolines. Macromolecules 32(22):7422–7429
Agrawal AK, Jenekhe SA (1996) Electrochemical properties and electronic structures of conjugated polyquinolines and polyanthrazolines. Chem Mater 8(2):579–589
Zhang X, Shetty AS, Jenekhe SA (1998) Efficient electroluminescence from a new n-type conjugated polyquinoline. Acta Polym 49(1):52–55
Kim JL, Kim JK, Cho HN, Kim DY, Kim CY, Hong SI (2000) New polyquinoline copolymers: synthesis, optical, luminescent, and hole-blocking/electron-transporting properties. Macromolecules 33(16):5880–5885
Tomar M, Lucas NT, Kim H, Laquai F, Müllen K, Jacob J (2012) Facile synthesis of 5,8-linked quinoline-based copolymers. Polym Int 61(8):1318–1325
Tomar M, Lucas NT, Gardiner MG, Muellen K, Jacob J (2012) Facile synthesis and coupling of functionalized isomeric biquinolines. Tetrahedron Lett 53(3):285–288
Drozdov FV, Myshkovskaya EN, Susarova DK, Troshin PA, Fominykh OD, Balakina MY, Bakirov AV, Shcherbina MA, Choi J, Tondelier D (2013) Novel cyclopentadithiophene based D–A copolymers for organic photovoltaic cell applications. Macromol Chem Phys 214(19):2144–2156
Pilgram K, Zupan M, Skiles R (1970) Bromination of 2,1,3-benzothiadiazoles. J Heterocycl Chem 7(3):629–633
Kim J, Yun MH, Anant P, Cho S, Jacob J, Kim JY, Yang C (2011) Copolymers comprising 2,7-carbazole and bis-benzothiadiazole units for bulk-heterojunction solar cells. Chem Eur J 17(51):14681–14688
Acknowledgments
The authors acknowledge the Department of Science and Technology, India (SB/S1/OC-12/2013) and Max Planck Society, Germany for generous financial support. M.T. acknowledges a research fellowship from the Indian Institute of Technology Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomar, M., Ashar, A.Z., Narayan, K.S. et al. Tuning the HOMO energy levels in quinoline and biquinoline based donor-acceptor polymers. J Polym Res 23, 50 (2016). https://doi.org/10.1007/s10965-016-0945-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-0945-1