Abstract
This research reports the synthesis and characterization of two new donor–acceptor type conjugated polymers carrying indolocarbazole as donor, benzothiadiazole and diketopyrrolopyrrole as acceptor via stille coupling polymerization reactions. The structures of the polymer were established by spectroscopic techniques and polymer molecular weight by gel permeation chromatography. Further the linear optical and electrochemical properties of the polymers have been investigated. Polymers showed broad absorption and good fluorescence behaviour with good quantum yields. Optical bandgaps of the polymers were estimated using UV–visible absorption edge and found to be 2.34 and 2.12 eV respectively. Further, theoretical evaluation of the polymers was carried using density functional theory to investigate their geometrical, electrochemical and optical properties using DFT/B3LYP. The obtained results are in good agreement with the experimental results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conjugated polymers have become a key player in interdisciplinary research due to their applications across various fields of science and engineering (Li et al. 2013, 2018; Zeglio et al. 2019; Jin Cheon et al. 2020). Π-conjugated polymers show interesting structural, optical, and electronic properties that can be tuned through various molecular modifications and synthesizing processes (Chung et al. 2015; Park et al. 2019). This makes the conjugated polymers to be promising candidates for various optoelectronic applications such as organic solar cells (OSC) (Cheng et al. 2009; Zhan and Zhu 2010; Akkuratov et al. 2020), organic light-emitting diode (OLED) (Sekine et al. 2014; Wong 2017; Zhang et al. 2020), field-effect transistors (Kim et al. 2020) and chemosensors (Fan et al. 2009; Mahesh et al. 2019). Especially D–A type conjugated polymers offer enhanced flexibility in tuning their optoelectronic properties through intramolecular charge transfer (ICT). To alter the bandgap of these polymers, a remarkable number of donor–acceptor moieties and their combinations are studied. Among the other donor moieties, carbazole and its derivatives have been investigated extensively due to their enhanced hole transportation property and inherent electron-donating nature (Li and Grimsdale 2010; Janosik et al. 2018). Particularly, the indolocarbazole (ICZ) based donor moieties(Gokce and Icli Ozkut 2018) offer high thermal stability and charge carrier mobility which is supported by their rigid backbone and low HOMO level (Negru and Grigoras 2019). The fused carbazole ring in ICZ supports the extension of conjugation length and improves the planarity of the molecule thereby enhancing the π-π stacking(Suman et al. 2019). Additionally, ICZ derivatives can also act as acceptor when coupled with strong donors such as 3,4-propylenedioxyhtiophene(Gokce and Icli Ozkut 2018). Many acceptor moieties have been coupled with the ICZ donor to enhance the π-conjugation and optoelectronic properties of the resulting alternating copolymers (Tsai et al. 2009; Chen et al. 2013; Khetubol et al. 2015). For example, the incorporation of benzothiadiazole-thiophene core acceptor with the ICZ donor through the Suzuki cross-coupling method resulted in a lower bandgap copolymer exhibiting broad absorption in the range of 300–720 nm (Tong et al. 2015) that is suitable for organic solar cell application. The benzothiadiazole (BT) acceptor moieties have been used to obtain low bandgap D–A conjugated polymers for organic solar cell applications due to their broader solar light absorption supported from intramolecular charge transfer (Pei et al. 2011; Ekbote et al. 2018). Additionally, the diketopyrrolopyrrole (DPP) moiety also offers greater thermal stability and photophysical properties that are essential for organic optoelectronic devices. But, the major drawback of the DPP core was its insolubility in common organic solvent due to strong intermolecular hydrogen bonding. This is overcome by introducing substituents groups like alkyl and phenyl groups on DPP core (Grzybowski and Gryko 2015; Pop et al. 2019) which enhanced its solubility in organic solvents. Since this advancement, DPP has been widely used as acceptor moiety in various organic solar cells and organic field-effect transistors for its high electron-withdrawing effect and excellent charge carrier mobility facilitated by strong π-π interactions.
In this study, we report the design and synthesis of two new donor–acceptor copolymers ICBTD and ICDPP through stille coupling polymerization reactions. The polymers have indolocarbazole as donor moiety which is incorporated with benzothiadiazole and diketopyrrolopyrrole acceptor moieties having a strong electron-withdrawing effect. The obtained polymers had a fairly planar structure supported by planarity of the indolocarbazole unit and highly rigid phenyl backbone which enhanced the conjugation of polymers through π-π stacking. The optoelectronic properties of polymers such as HOMO–LUMO, excitation, and emission energies were characterized using cyclic voltammetry, UV–Vis, and fluorescence spectra analysis. The experimental calculations were supported by theoretical computations using density functional theory (DFT) and time-dependant DFT (TDDFT) studies. The structural and photophysical properties of the polymers were theoretically evaluated with a monomeric approximation to limit the computational cost. The TDDFT based calculations of HOMO–LUMO and excitation energies showed less sensitivity towards different basis sets that were used for the computation(Majidizadeh Fini et al. 2020).
Procedure
Materials and chemicals
All the solvents and chemicals are purchased from Aldrich and directly used without any further purifications. One of the monomer for preparation of polymer, 4,7-dibromo-2,1,3-benzothiadiazole was purchased from Aldrich.
Instrumentation
1H NMR spectra of compounds were recorded in Bruker 400 MHz NMR spectrometer. UV–Vis absorption and fluorescence emission spectra were recorded with Perkin Elmer Lambda 25 UV–Vis spectrometer using THF as a solvent. Autolab-30 was used to carry out electrochemical measurement of polymers where the platinum electrode was used as a counter electrode, silver–silver chloride electrode was used as reference electrode and glassy carbon electrode used as a working electrode. Molecular weight analysis of the polymer was determined by Waters to make gel permeation chromatography.
Synthesis and characterization
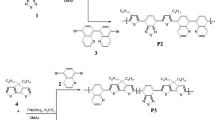
Indolocarbazole based monomer was synthesized from a series of reactions. Initially, indole was made to react with bromo benzaldehyde in presence of hydroiodic acid. Then aromatization of dihydroindolocarbazole was done using molecular iodine. Further alkyl chains were introduced to indolocarbazole to increase the hydrophobicity. In the final step, stannylation was done using tributyltinchloride. Synthesis of indolocarbazole based monomer 4 is depicted in Fig. 1. Further diketopyrrolopyrole based monomer was synthesized from the reaction of 2 methyl 2 butanol with bromobenzonitrile in presence of potassium tert butoxide. Obtained diketopyrrolopyrrole was alkylated with bromooctane to get soluble monomer 6. Then the obtained monomer 6 was made to react with monomer 4 to get polymer ICDPP. On similar lines, ICBTD polymer was synthesized from monomer 4 and dibromobenzothiadiazole.
12-bis(4-bromophenyl)-5,6,11,12-tetrahydroindolo[3,2-b]carbazole 1
5 g of indole(0.0426 mol) and 7.89 g of p-bromo benzaldehyde was dissolved in 100 ml of acetonitrile. HI (57%)(1.08 g, 0.00852 mol) was slowly added into the round bottom flask at room temperature. Then continued the stirring for about 1 h then reaction temperature was raised to 80 °C for about 20 h. Completion of the reaction was monitored by TLC(Thin layer chromatography). Then the reaction mass was cooled to room temperature and the obtained solid was filtered and washed with cold acetonitrile and dried under vacuum. The resulting solid was directly taken for next step without any further purification. During the reactions some portion of the compound was aromatized to next compound hence further analysis was not done.
12-bis(4-bromophenyl)-5,11-dihydroindolo[3,2-b]carbazole 2
The synthesized dihydroindolocarbazole 1 was dissolved in 60 ml of acetonitrile and 0.453 g of iodine was added then reaction mixture was heated at 80 °C for 20 h. Completion of the reaction was ensured by TLC. Then the solvent was removed under reduced pressure and the obtained precipitate was washed with cold acetonitrile and dried under vacuum. This crude product was purified by column chromatography using hexane:ethyl acetate (1:4) system. Yellow coloured solid was obtained as a product with a yield of 48%. The solid product did not melt upto 300 °C and above this temperature it started charring. 1H NMR (DMSOd6, 300 MHz) δ 6.98–6.99(m, 2H), 7.34–7.36(m, 6H), 7.66 (d, 4H), 7.82–7.87(m, 6H (including NH proton).
12-bis(4-bromophenyl)-5,11-dioctyl-5,11-dihydroindolo[3,2-b]carbazole 3
Indolocarbaozle 2 (3.5 g, 6 mmol) and 1-bromooctane (4.7 g, 6 mmol) was slowly mixed in 100 ml of dry Dimethyl formamide (DMF) then powdered NaOH (1.5 g, 35 mmol) was added into it under vigorous stirring. Reaction temperature was raised to 100 °C and continued for 16 h in an inert atmosphere. The reaction mass was poured to crushed ice then extracted with chloroform and further dried to obtain the crude product. The crude product was purified by column chromatography using hexane: ethyl acetate system (1:2). Final product was yellowish solid with a yield of 79%. 1H NMR (CDCl3, 400 MHz, ppm): 7.79 (d, 4H (aromatic)), 7.6 (d, 4H, aromatic), 7.4 (t, 2H, aromatic), 7.30 (d, 2H), 6.90 (t, 2H), 6.63 (d, 2H), 3.82(t, 4H, N-CH2), 1.54 (t, 4H), 1.25 (m, 20H, alkyl protons), 0.91 (t, 6H terminal CH3).
11-dioctyl-6,12-bis(4-(tributylstannyl)phenyl)-5,11-dihydroindolo[3,2-b]carbazole 4
Alkylated indolocarbazole was dissolved in dry THF and then cooled to -78 °C using dry ice and acetone under argon atmosphere. Slowly n-butyl lithium (1.6 M) was added dropwise over a period of 30 min and the reaction was stirred for 1 h and then tributyltin was added at a stretch. Reaction was continued for about 3 h. After the completion of the reaction was quenched with crushed ice and then extracted with diethyl ether. Solvent was removed under reduced pressure and the product was washed repeatedly with cold methanol to remove excess tributyltin. The stannylated manomer was not characterized because of its instability in room temperature. Hence it is kept in a refrigerator and used in the reaction. Also it was observed that decomposition of the sample under air for long exposure. The resulting yellow syrupy product was taken to polymerization without further purification.
Synthesis of 3,6-bis(4-bromophenyl)-2,5-dihydropyrrolo[3,4-c]pyyol-1,4-dione (5)
Potassium tert-butoxide (4 g, 35.6 mmol) was completely dissolved in 2 methyl-2-butanol in inert atmosphere at 0 °C. 4-bromo benzonitrile (7.5 g, 41 mmol) was added slowly into the reaction mixture and the temperature was slowly raised to 98 °C for 40 min. Then reaction temperature was lowered to 60 °C and dimethyl succinate was added drop wisely into flak with constant stirring over a period of 2 h, and the temperature was again increased to 98 °C for about 4 h. Then the reaction was cooled to 60 °C and 50 ml of methanol was added and the resulting suspension was filtered while it was in hot condition. The filter cake was washed with methanol to remove impurities. Then the crude product was recrystallized using hot methanol and the product was dried under vacuum. The product observed was a red coloured solid with a low yield of 62%. FT-IR (KBr pellet, cm−1): 3145 (Ar‒H stretching), 1644 (C–O), 1608 (aromatic C–C), 1488, 1448, 1327, 1191, 1141, 816, 698 cm−1. 1H NMR (400 MHz, ppm): 7.72–7.75 (m, 8 aromatic protons).
Synthesis of 3,6-bis(4-bromophenyl)-2,5-dihexylpyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione 6.
Synthesized diketopyrrolopyrrole 3 (4 g, 0.08969 mol) was mixed with potassium tertbutoxide (2.2 g, 0.019 mol) in dry N-methyl pyrolidinone (30 ml). Reaction temperature was raised to 60 °C for 1 h followed by the addition of bromo octane drop by drop. Stittring was continued for about 24 h. After the completion of the reaction, it was poured to water and was extracted with ethyl acetate and dried with sodium sulphate. The solvent was removed under reduced pressure. The crude product was purified by column chromatography using hexane:Ethylacetate (1:5) system with a low yield of 35%. 1H NMR (300 MHz, CDCl3): δ is 7.6 (m, 8H, Aromatic), 3.68 (t, 4H, N-CH2), 1.22 (m, 16H, CH2), 0.9 (t, 6H CH3) (Fig. 2).
Synthesis of ICBTD polymer
Indolocarbazole monomer 4 was ( 2 g, 0.00177 mol) dissolved in dry toluene and completely purged with argon. Dibromobenzothiadiazole (0.521 g, 0.00177 mol)was dissolved in dry toluene and added dropwise to the above reaction and purging was continued for 30 min. Then a pinch of palladium tetrakis tryphenylphosphene was added and purging is continued for 30 min. The colour of the solution tuned in to dark orange red. Slowly reaction temperature was raised to 110 °C stirring was continued for 24 h. Completion of the reaction was ensured by Thin layer chromatography. The solvent was distilled off and the resulting crude gelly mass was reprecipitated in methanol. The obtained crude product was purified using Soxhlet extraction using hexane, and methanol. Finally, chloroform soluble fraction was taken and reprecipitated in methanol, filtered and dried under vacuum to obtain ICBTD polymer (Fig. 3). The obtained yield of the polymer was found to 55%. 1H NMR (300 MHz, CDCl3): δ δ, 7.42–6.78 (m, 18H (aromatic), 3.89 (m, 4H, (–NCH2–)), 1.95–1.27 (m, 24H (–(CH2)6–), 0.94–0.9 (t, 6H) (supplementary information). The gel permeation chromatography was done in THF solvent the weight average molecular weight was found to be, Mw = 12,800 Da with a polydispersity of 1.9.
Synthesis of ICDPP polymer
Monomer 4 (2 g, 0.00177 mol)and diketopyrrolopyrrole monomer 6 (1.09 g, 0.00177 mol) were dissolved in dry toluene and completely purged with argon atmosphere at room temperature. Then slowly palladium tetrakis triphenylphosphine palladium(0)(4 mol %)added into flak then purging is continued for another 30 min. The colour of the polymer was changed to dark red. Then reaction temperature was raised to 110 °C and continued for 24 h. After completion of polymerisation flask was cooled to room temperature solvent was stripped off and resulting solid was purified in soxhlet extraction with hexane, methanol solvent (each of 24 cycles) and finally chloroform fraction was taken and concentrated and resulting solid was precipitated in methanol and dried under vacuum (Fig. 4). Yield of the polymer was observed to be 48%. 1H NMR (300 MHz, CDCl3): δ 1H NMR (300 MHz, CDCl3): δ, 7.48–6.89 (m, 24H (aromatic)), 4.09, (m, 4H, (–NCH2–) diketopyrrolo pyrrole), -3.89 (m, 8H, (–NCH2–) indolocarbazole), 2.19–1.27 (m, 40H (–(CH2)6–), 0.94–0.90 (m, 12H)(supplementary information). The Gel permeation chromatography study to determine the molecular weight of the polymer in THF solutions, and it was found to be Mw = 12,800 Da with a polydispersity of 1.9.
Computational details
The structure and optical properties of the synthesized polymers were examined theoretically utilizing Density Functional Theory (DFT) in ORCA 4.0.1 program (Neese 2012). To limit the computational cost of the calculation, single repeating units of the polymers capped with hydrogen atoms were considered for all computations. The alkyl chains on the indolocarbazole and diketopyrrolopyrrole unit were replaced with methyl groups. The structure of the polymers ICBTD and ICDPP were established through geometry optimizations performed using Becke’s three-parameter hybrid functional (Becke 1993) with the Lee et al. (1988) correlation functional (B3LYP) with the Pople basis set, 6-31G(d) (Hehre et al. 1972) in gas phase considering default convergence criteria and gradient techniques. To confirm the minima of the obtained structures, analytical frequencies were calculated at the same level of theory. Further, the optical properties of the polymers like the HOMO–LUMO energy gap, electronic excitations, and oscillator strengths (f) were evaluated using Time-Dependent Density Functional Theory (TDDFT). The calculation of vertical singlet transitions and corresponding optical absorption simulations were considered at different basis sets such as 6-31G(d), cc-pVTZ and def2-TZVP in gas phase using the ground state optimized geometries.
Data, value and validation
Optical studies of the polymers
UV–visible spectra and fluorescence emission spectra of the polymers were recorded in thin films. Polymers were dissolved in teterahydrofuran (THF) and then spin coated on a quartz glass plate and dried under ambient conditions to prepare respective thin films of polymers. Absorption maxima of ICPBTD and ICPDPP polymers were found to be 498 nm and 427 nm respectively (Fig. 5). The ICDPP polymer showed broad absorption spectra when compared to that of ICBTD polymer along with 71 nm redshift in absorption maxima. This is mainly due to the presence of strong electron accepting nature of diketopyrrolopyrrole molecules. The red shift in the absorption maxima of polymer ICPDPP compared to other polymer is mainly attributed to the incorporation of highly electron accepting nature of diketopyrrolopyrrole moiety. These results suggest that diketopyrrolopyrrole molecule is strong electron acceptor when compared to benzothiadiazole moiety. Also the ICDPP polymer showed red-shifted absorption maxima with a broad absorption spectra indicating its suitability in polymer solar cells. Optical bandgaps were calculated using absorption band edges and were found to be 2.34 eV and 2.12 eV respectively for polymers ICBTD and ICDPP. Further, fluorescence emission spectra of a polymers ICBTD and ICDPP recorded in thin films were highly emissive with emission maxima of 565 nm and 486 nm respectively (Fig. 6). Also both the polymers showed a good stoke shift of 60 nm with good quantum yields which are further detailed in Table 1. This suggests that the polymers ICBTD and ICDPP may be a good candidate for light-emitting applications.
Electrochemical study of the polymer
The electrochemical studies of the polymer were studied using cyclic voltammeric techniques using three-electrode systems. The conductive polymer films were coated on glassy carbon button electrodes by evaporating solution of polymer on top of the electrode by drop-casting method. The cyclic voltametric measurement was done using 0.1 M Tetrabytylammonium tetrafluoroborate solutions in acetonitrile solvent with a scan rate of 50 mV/s(Shivashankar et al. 2013). The argon was purged with acetonitrile prior to measurement to ensure the absence of moisture. The obtained electrochemical data are represented in Table 2. In oxidation cycle, polymers ICBTD and ICDPP showed oxidation peaks around 0.61 eV and 0.64 eV. This indicates that polymer can act as a good hole transport layer when it is used in polymer light-emitting material. However, there is no sharp peak during reduction cycle due to high rigidity of strong electron-accepting benzothiadiazole and diketopyrrolopyrrole as depicted by cyclic voltammetry curves in Fig. 7.
The onset oxidation and onset reduction potentials were used to calculate the highest occupied molecular orbital(HOMO) and lowest unoccupied molecular orbitals (LUMO). The calculation of HOMO and LUMO energy levels of the polymer was performed according to literature(de Leeuw et al. 1997) by comparing the results with ferrocine standard(Udayakumar and Adhikari 2006). HOMO of the polymers ICBTD and ICDPP were found to be − 4.88 eV and − 4.81 eV respectively. The close proximity in the HOMO values is mainly due to the presence of indolocarbazole moiety in the polymer backbone. This HOMO values indicate that the indolocarbazole monomer is a good donor molecule. Further, the LUMO values of the polymers ICBTD and ICDPP were found to be − 2.9 eV and − 3.2 eV due to the presence of benzothiadiazole and diketopyrrolopyrrole molecules. Thus, HOMO and LUMO values of the polymers indicate better electron injection ability when they are used in LED applications.
Molecular geometry
The minimum energy state of the polymer ICBTD and ICDPP were obtained through geometry optimization performed at the B3LYP/6-31G(d) level in the gas phase. The single monomer units were considered for all computations to reduce the computational cost. The respective optimized geometry structures are depicted in Figs. 8 and 9. The indolocarbazole moiety in both the optimized geometries showed high planarity illustrating the existence of effective π-π stacking. In optimized structure ICBTD, the orientation of indolocarbazole and benzothiadiazole moieties were found to be slightly nonplanar between the phenyl spacer, having a dihedral angle with the phenyl backbone as C1–C2–C3–C4 = − 73.4° and C5–C6–C7–C8 = 45.3° (see Fig. 8 for atom numbering). However, the optimized structure of ICDPP showed fair planarity between indolocarbazole and diketopyrrolopyrrole hetero moieties having a dihedral angle of C1–C2–C3–C4 = − 67.60 and C5–C6–C7–C8 = 38.7° with the phenyl spacer (see Fig. 9 for atom numbering) thereby enhancing the charge transport in the system. In polymer ICBTD structure the length of the C–C bond varied from 1.3709–1.4966 Å and the N–C bond length from 1.3392–1.4537 Å and in ICDPP structure the C–C bond length varied from 1.3818–1.4937 Å and the N–C bond length from 1.3863–1.4568 Å. The additional optimized geometry parameters are detailed in the supplementary material (Table S1-S2). Further, the calculation of analytical frequency showed no imaginary frequencies for both ICBTD and ICDPP, which indicated that the polymers were at their local minima. Hence these optimized geometries were used for further optoelectronic property calculations.
Frontier molecular orbitals
The analysis of the highest occupied and lowest unoccupied molecular orbitals (HOMO, LUMO) favors the qualitative indication of the vertical excitations and the resulting optical properties of the π-conjugated polymer (Sun and Autschbach 2014). Also, the HOMO and LUMO values and corresponding energy gaps give information about the probable charge transport in the conjugated system. The frontier molecular orbitals of polymer ICBTD and ICDPP were obtained with three different basis set, 6-31G(d), cc-pVTZ and def2-TZVP using TDDFT. The isosurface plots of HOMO and LUMO of both the polymers at TD-B3LYP/6-31G(d) level are visualized in Fig. 10 (rest of the isosurfaces are depicted in the supplementary material, Figure S1–S2). For ICBTD the HOMO is localized on indolocarbazole donor moiety and the LUMO involves the benzothiadiazole acceptor moiety. Both molecular orbitals depict π type thus illustrating the probable intramolecular charge transfer from donor to acceptor upon excitation (Chung et al. 2020). Similarly, for ICDPP the HOMO is localized on indolocarbazole moiety and the LUMO located over diketopyrrolopyrrole moiety involving phenyl rings as shown in Fig. 10. In both polymers, the HOMO and LUMO orbitals were well separated over donor and acceptor moieties indicating efficient charge transport through the system. The isosurface plots obtained from other basis sets also depicted similar results. The HOMO and LUMO energy values of both the polymers obtained with three different basis sets are detailed in Table 3. From Table 3, the HOMO and LUMO energy values of ICBTD at 6-31G(d) level were found to be − 4.604 eV and − 2.326 eV having an optical energy gap of 2.278 eV which is close to the experimental value of 2.34 eV. Similarly, the HOMO and LUMO values of ICDPP were found to be − 4.647 eV and − 2.348 eV with an optical energy gap of 2.299 eV that was comparable with the experimental value of 2.12 eV.
Simulated absorption spectra
The ground state excitation energies of polymer ICBTD and ICDPP were calculated employing TDDFT with multiple basis sets. The theoretical absorption spectrum of the polymers is visualized in Figs. 11 and 12. Although the S0 → S1 excitations of both ICBTD and ICDPP were dominated by one electron transition from HOMO to LUMO, the absorption peaks resulted from the contribution of different orbital excitations having the highest oscillator strength. The absorption wavelengths along with oscillator strengths at different levels of theory are detailed in Tables 4 and 5.
The vertical excitation energies calculated using the def2-TZVP basis set were fairly close to experimental values. For ICBTD the absorption peak was located at 393.29 nm at def2-TZVP level, resulting from the transition of H → L + 1 (91%) having the highest oscillator strength of 0.1979. The absorption peak for ICDPP was found to be 448.87 nm due to the transition from H-1 → L (87%) with an oscillator strength of 1.1486. Compared to ICBTD the absorption peak value of ICDPP showed a red shift of 55.58 nm. This indicates the strong electron accepting capability of diketopyrrolopyrrole compared to benzothiadiazole acceptor moiety which makes the polymers suitable for organic solar cell application which correlates with the experimental findings.
Conclusions
In summary, two new polymers with a donor–acceptor structure having indolocarbazole as donor moiety, diketopyrrolopyrrole and benzothiadiazole as acceptor moieties were synthesized through stille coupling polymerization reactions. The synthesized polymers showed low bandgap energy values of 2.34 eV (ICBTD) and 2.12 eV (ICDPP) aided by the effective donor–acceptor nature indolocarbazole donor and of BTD and DPP moieties as acceptors. The linear optical studies indicated that the polymers show broad absorption and are highly emissive in the visible region with good quantum yields indicating their potential applications in light emitting devices. The electrochemical studies showed that the polymers had an efficient donor–acceptor system with their HOMO values closely located. This indicated the better electron injection capability of the polymers. Further, theoretical studies showed that the polymers had a fairly planar structure in their optimized state thus enhancing the conjugation and π–π stacking in the system. The frontier orbital studies showed notable separation between HOMO and LUMO orbital densities on donor and acceptor indicating the effective charge transfer in the system. Theoretically calculated HOMO of the polymers were very close to experimental values. There is no sharp peak in the reduction cycle of cyclic voltammetry was observed due to rigidity of acceptors hence experimental LUMO values are showing slight deviation to their simulated values. The computed absorption peaks showed good correlation with the experimental values and the broad absorption and high fluorescence emission of the polymers indicate that these polymers can find potential applications in polymer solar cells and light-emitting devices.
References
Akkuratov AV, Nikitenko SL, Kozlov AS et al (2020) Design of novel thiazolothiazole-containing conjugated polymers for organic solar cells and modules. Sol Energy 198:605–611. https://doi.org/10.1016/j.solener.2020.01.087
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Chen Q, Zhang L, Ebrahim S et al (2013) Synthesis and structure study of copolymers from thiadiazole fused indolocarbazole and dithienosilole. Polymer 54:223–229. https://doi.org/10.1016/j.polymer.2012.11.006
Cheng Y-J, Yang S-H, Hsu C-S (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109:5868–5923. https://doi.org/10.1021/cr900182s
Chung K, McAllister A, Bilby D et al (2015) Designing interchain and intrachain properties of conjugated polymers for latent optical information encoding. Chem Sci 6:6980–6985. https://doi.org/10.1039/C5SC02403J
Chung HY, Oh J, Park J-H et al (2020) Spectroscopic studies on intramolecular charge-transfer characteristics in small-molecule organic solar cell donors: a case study on ADA and DAD triad donors. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.0c06741
de Leeuw DM, Simenon MMJ, Brown AR, Einerhand REF (1997) Stability of n-type doped conducting polymers and consequences for polymeric microelectronic devices. Synth Met 87:53–59. https://doi.org/10.1016/S0379-6779(97)80097-5
Ekbote A, Mobin SM, Misra R (2018) Structure–property relationship in multi-stimuli responsive D-A–A′ benzothiazole functionalized isomers. J Mater Chem C 6:10888–10901. https://doi.org/10.1039/C8TC04310H
Fan L-J, Zhang Y, Murphy CB et al (2009) Fluorescent conjugated polymer molecular wire chemosensors for transition metal ion recognition and signaling. Coord Chem Rev 253:410–422. https://doi.org/10.1016/j.ccr.2008.03.008
Gokce G, Icli Ozkut M (2018) An indolocarbazole based yellow-to-cyan soluble electrochromic polymer. Org Electron 52:317–322. https://doi.org/10.1016/j.orgel.2017.11.017
Grzybowski M, Gryko DT (2015) Diketopyrrolopyrroles: synthesis, reactivity, and optical properties. Adv Opt Mater 3:280–320. https://doi.org/10.1002/adom.201400559
Hehre WJ, Ditchfield R, Pople JA (1972) Self—consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261. https://doi.org/10.1063/1.1677527
Janosik T, Rannug A, Rannug U et al (2018) Chemistry and properties of indolocarbazoles. Chem Rev 118:9058–9128. https://doi.org/10.1021/acs.chemrev.8b00186
Jin Cheon H, Li X, Jin Jeong Y et al (2020) A novel design of donor–acceptor polymer semiconductors for printed electronics: application to transistors and gas sensors. J Mater Chem C 8:8410–8419. https://doi.org/10.1039/D0TC01341B
Khetubol A, Van Snick S, Clark ML et al (2015) Improved spectral coverage and fluorescence quenching in donor-acceptor systems involving indolo[3-2-b]carbazole and boron-dipyrromethene or diketopyrrolopyrrole. Photochem Photobiol 91:637–653. https://doi.org/10.1111/php.12437
Kim M, Ryu SU, Park SA et al (2020) Donor–acceptor-conjugated polymer for high-performance organic field-effect transistors: a progress report. Adv Funct Mater 30:1904545. https://doi.org/10.1002/adfm.201904545
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Li J, Grimsdale AC (2010) Carbazole-based polymers for organic photovoltaic devices. Chem Soc Rev 39:2399–2410. https://doi.org/10.1039/B915995A
Li J, Liu J, Wei C-W et al (2013) Emerging applications of conjugated polymers in molecular imaging. Phys Chem Chem Phys 15:17006–17015. https://doi.org/10.1039/C3CP51763B
Li W, Guo Y, Wang Y et al (2018) A “chain–lock” strategy to construct a conjugated copolymer network for supercapacitor applications. J Mater Chem A 7:116–123. https://doi.org/10.1039/C8TA08766K
Mahesh K, Karpagam S, Pandian K (2019) How to design donor-acceptor based heterocyclic conjugated polymers for applications from organic electronics to sensors. Top Curr Chem (Z) 377:12. https://doi.org/10.1007/s41061-019-0237-4
Majidizadeh Fini A, Kalantari Fotooh F, Nateghi MR, Shahi S (2020) Combined experimental and theoretical investigation of optical and structural properties of poly aniline derivatives. Chem Pap. https://doi.org/10.1007/s11696-020-01273-4
Neese F (2012) The ORCA program system. WIREs Comput Mol Sci 2:73–78. https://doi.org/10.1002/wcms.81
Negru OI, Grigoras M (2019) Synthesis and properties of copolyarylenes containing indolo[3,2-b]carbazole moieties in the backbone. J Polym Res 26:30. https://doi.org/10.1007/s10965-018-1693-1
Park KS, Kwok JJ, Dilmurat R et al (2019) Tuning conformation, assembly, and charge transport properties of conjugated polymers by printing flow. Sci Adv 5:eaaw757. https://doi.org/10.1126/sciadv.aaw7757
Pei J, Wen S, Zhou Y et al (2011) A low band gap donor–acceptor copolymer containing fluorene and benzothiadiazole units: synthesis and photovoltaic properties. New J Chem 35:385–393. https://doi.org/10.1039/C0NJ00378F
Pop F, Humphreys J, Schwarz J et al (2019) Towards more sustainable synthesis of diketopyrrolopyrroles. New J Chem 43:5783–5790. https://doi.org/10.1039/C9NJ01074B
Sekine C, Tsubata Y, Yamada T et al (2014) Recent progress of high performance polymer OLED and OPV materials for organic printed electronics. Sci Technol Adv Mater 15:034203. https://doi.org/10.1088/1468-6996/15/3/034203
Shivashankar SM, Anantapadmanabha VK, Adhikari AV (2013) Optical limiting materials: synthesis, electrochemical and optical studies of new thiophene based conjugated polymers carrying 1,3,4-oxadiazole units. Polym Eng Sci 53:1347–1356. https://doi.org/10.1002/pen.23362
Suman SA, Keshtov ML et al (2019) New indolo carbazole-based non-fullerene n-type semiconductors for organic solar cell applications. J Mater Chem C 7:543–552. https://doi.org/10.1039/C8TC05318A
Sun H, Autschbach J (2014) Electronic energy gaps for π-conjugated oligomers and polymers calculated with density functional theory. J Chem Theory Comput 10:1035–1047. https://doi.org/10.1021/ct4009975
Tong J, Guo P, Zhang H et al (2015) Synthesis of modified benzothiadiazole-thiophene-cored acceptor and carbazole/indolocarbazole alternating conjugated polymers and their photovoltaic applications. Polym Bull. https://doi.org/10.1007/s00289-014-1292-1
Tsai J-H, Chueh C-C, Mei-Hsiu L et al (2009) Synthesis of new indolocarbazole-acceptor alternating conjugated copolymers and their applications to thin film transistors and photovoltaic cells. Macromolecules. https://doi.org/10.1021/ma802720n
Udayakumar D, Adhikari AV (2006) Synthesis and characterization of new light-emitting copolymers containing 3,4-dialkoxythiophenes. Synth Met 156:1168–1173. https://doi.org/10.1016/j.synthmet.2006.07.007
Wong MY (2017) Recent advances in polymer organic light-emitting diodes (PLED) using non-conjugated polymers as the emitting layer and contrasting them with conjugated counterparts. J Electr Mater 46:6246–6281. https://doi.org/10.1007/s11664-017-5702-7
Zeglio E, Rutz AL, Winkler TE et al (2019) Conjugated polymers for assessing and controlling biological functions. Adv Mater 31:1806712. https://doi.org/10.1002/adma.201806712
Zhan X, Zhu D (2010) Conjugated polymers for high-efficiency organic photovoltaics. Polym Chem 1:409–419. https://doi.org/10.1039/B9PY00325H
Zhang D-W, Li M, Chen C-F (2020) Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes. Chem Soc Rev 49:1331–1343. https://doi.org/10.1039/C9CS00680J
Acknowledgements
Authors are grateful to RV College of Engineering for providing characterization facility to carry out this research work .
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I certify that there is no actual or potential conflict of interest in relation to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vishnumurthy, K.A., Girish, K.H. Synthesis, photophysical, electrochemical and computational study of indolocarbazole based donor acceptor type conjugated polymers. Chem. Pap. 75, 1969–1980 (2021). https://doi.org/10.1007/s11696-020-01445-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01445-2