Abstract
High performance silica/epoxy nanocomposites were prepared through mixing epoxy, tetraethyl orthosilicate (TEOS), γ-aminoproplytriethyoxy siliane(APTES), and triethyltrtramine (TETA) at 25 °C via sol-gel method on one-step. The effects of content of TEOS and coupling reagents on the mechanical and thermal properties of SiO2/EP composites were studied. Microcosmic morphology and properties of the hybrid materials were characterized by FT-IR, TEM, FESEM, and DSC. Results revealed that SiO2/EP composites achieve the optimal mechanical and thermal properties when the composites prepared with mass ratio of TEOS/APTES/epoxy for 3/2/100 without acetone. Compared with pristine epoxy, the tensile strength, elongation at break, impact strength and bend strength increased 67.6 %, 190 %, 82.1 % and 15.7 %, respectively. The further study was to investigate the content of TEOS and APTES effecting on mechanical properties and water sorption of fiber reinforced composites, which used the above compound as matrix resin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins which possess outstanding chemical resistance, thermal resistance and mechanical properties, is now being widely used in various applications, mainly in the paint, electron materials, wrapping materials and adhesives [1–7]. Silica has gained the reputation of one of the most suitable inorganic filler for epoxy resin, which can be dispersed in the polymer matrix as nanoparticles, or generated network by homogeneous mixing of the precursors in simultaneous polymerization [8–10]. Polymer nanocomposites reinforced with nanoparticles exhibit improved mechanical properties due to the formation of strong adhesive interfaces between highly dispersed nanoparticles and the polymer matrix [11–14]. The strong interface interaction between inorganic nanoparticle and organic polymer is obtained due to big interfacial area, and induce a nanocomposties with improved toughness, strength and heat resistance [15–19]. It is found that the homogenous dispersion is the crucial factor to prepare the nanocomposites with higher interface action, and the surface modifiers are often used to improve the dispersion of nanoparticles in polymer matrix. Silane coupling reagent, such as γ-aminopropyltriethoxysilane [H2N(CH2)3Si(OC2H5)3](APTES) is an essential coupling reagent for nano-silicas, which can form a layer of single molecule membrane on the surface of fillers and improve the wettability and dispersion of them [20, 21].
The use of the sol-gel process to prepare highly intermingled inorganic-organic hybrid polymer networks using coupling agents is of current scientific interest since it offers the possibility of tailoring the properties of the materials by variation of the relative composition of the inorganic and organic phases [22–25]. This network structure are formed together to achieve homogeneous phase morphologies when chemical bonds are developed between inorganic and organic components.
Various researchers have reported the feasibility of making glass fiber reinforced composites (FRC), which results in superior mechanical properties, high structural efficiency and cost effectiveness [3]. It is widely accepted that the mechanical behaviour of glass FRC is highly dependent on the interphase between fiber and matrix. An appropriately interphase can significantly improve tensile strength, bending strength, impact strength, stiffness and toughness of composites [26–28], as well as the environmental stability.
In this study, silica/epoxy nanocomposites with inorganic-organic hybrid network are prepared by one-step sol-gel method. The tetraethylorthosilicate (TEOS) and epoxy resin with alkoxy groups are used as silica precursor, and the triethyltrtramine (TETA) are used as curing agent and catalyst during sol-gel process. Compared with Nopphawan Phonthamachai [10], high performance silanized silica/epoxy nanocomposites were prepared through mixing epoxy, TEOS, 3-aminopropyl trimethoxysilane (APTMS) and ammonia solution at 50 °C. Epoxy resin with alkoxy groups are synthesis with the reaction between amino groups of APTES and epoxide groups of epoxy resin. Chemical bonds between silica and epoxy resin are induced by the hydrolysis and condensation of alkoxy groups from TEOS and epoxy resin modified with APTES, and more homogeneous nanocomposites are prepared. Moreover, the possibility of synthesis of inorganic-organic hybrid materials is expected by controlling the process of sol-gel. The morphology, microstructure, mechanical properties and thermal properties of the silica/epoxy hybrid materials are characterized, and the silica/epoxy/glass cloth composites are prepared and their tensile properties are tested.
Experimental
Materials
Epoxy resin (industry grade), diglycidyl ether of bisphenol-A (DGEBA) with structure shown in Scheme 1, was purchased from DaLian QiHua Chemicals Co. Ltd.(Liaoning, China); γ-aminopropyltriethoxy silane (APTES) purchased from Nanjing Shuguang Chemicals Group Co. Ltd.(Jiangsu, China) and tetraethoxysilane (TEOS) was purchased from ChangShu ZhongJie Chemicals Co. Ltd.; triethylenetetramine (TETA) was purchased from Tianjin Chemical Products Factory and used as curing agent(Tianjin, China). Plain weaved glass cloth (1 mm thick, E glass) was purchased from Shannxi fiberglass factory general with APTE surface treatment agent.
Preparation of the SiO2/epoxy hybrid materials
Synthesis of silanized epoxy and SiO2/EP hybrid materials
A mixture of epoxy resin and APTES, with the weight ration of epoxy/APTES 100/2 or 100/5, was stirred vigorously at 80 °C for 2 h to get the silanized epoxy. In a typical synthesis of SiO2/EP hybrid materials, silanized epoxy (with the weight ration of epoxy/APTES 100/2), TEOS, distilled water, ethanol were mixed and stirred vigorously at room temperature for 30 min to obtain homogenous mixtures, with the molar ratio of –Si-OC2H5/H2O = 1/1 and the molar ratio of TEOS/ethanol = 2/1. The amount of TETA needed to achieve an overall -NH/epoxide molar ratio of 1/1 was then added to the mixtures at room temperature and mixed for another 5 min. the resulting mixtures were degassed under vacuum and poured into a steel mold with release agent in advance. The curing process was carried out under room temperature for 24 h followed additional 4 h at 80 °C to obtain the completely cured inorganic-organic hybrid materials.
Fabrication of SiO2/epoxy/galss cloth composite
The glass cloth was dried in oven under 120 °C for 2 h and used immediately after taking out to remove water absorbing during storage. Silanized epoxy (with the weight ration of epoxy/APTES 100/2), TEOS, distilled water, ethanol was mixed to get homogenous mixture, and TETA was added into the mixture to prepare the matrix for composites. The FRC was fabricated by fabric hand lay-up process with the weight ration of matrix/cloth 1/1 and cured under room temperature for 24 h followed additional 4 h at 80 °C. The resulting composites were 2 mm thick plats.
Materials characterization
Differential scanning calorimeter (DSC) analyses of the samples (approximately 5 mg) were carried out at a heating rate of 10 °C/min in the range of −50 ~ 250 °C under a nitrogen atmosphere by USA TAMDSC2910. The sample was placed on a KBr pellet and the FTIR spectrum was obtained using a Nicolet AVATAR 550 analyzer at room temperature. Scanning electron microscope (SEM) measurement was performed on Hitachi S-2700 to observe the fracture surface of hybrid materials after tensile tests, with accelerating voltage of 20KeV. The field emission scanning electron microscope (FE-SEM) observations were made using a Hitachi SU700 microscope. The fracture surface was coated with gold before SEM measurement. Transmission electron microscope (TEM) was conducted in high resolution mode using a Hitachi H-600 instrument and operated at 75 kV. The samples were cut using a Leica Ultracut UCT ultramicrotome and placed on 200 mesh copper grids.
The tensile strength and modulus of SiO2/epoxy were measured by electron omnipotence experiment machine (SANS-CMT5105, Shenzhen Xin-SanSi Corporation of China) according to GB/T 2567-2008. The dog-bone shaped specimens used in the tensile testing were 50 mm × 10 mm × 4 mm (L × W × D) in the narrow region. The tensile test for FRC was performed electron omnipotence experiment machine according to GB/T 1477-2005 using a loading rate 10 mm/min at ambient temperature. Samples were 250 mm × 20 mm × 2 mm. Bend strength was measured by ZMG1(Hebei Material experiment factory of China) according to GB/T 2567-2008. The dimension of specimens is 100 mm × 15 mm × 4 mm. The impact strength was measured by ZBC-4B impact testing machine (Shenzhen Xin-SanSi Coperation of China) according to GB/T2567-2008. The samples were 80 mm × 10 mm × 4 mm. All samples were treated at 80 °C for 2 h to eliminate the inner stress.
Heat distortion temperature (HDT) was measured by XRW-300 from Chengde shuiquan testing instrument CO.LTD according to GB/T 1634-2004, with heat rate 2 °C/min and load 1.81 N/mm2. HDT was determined by temperature when the distortion of samples is 0.33 mm.
Water sorption of the fiber reinforced epoxy resin composites was measured by using an electronic balance weights the specimen 3 times, and working out according to GB/T 1462-2005.
Results and discussions
The reaction of epoxy/APTES/TEOS/TETA mixture
During the course of reaction, amine functionalised silane coupling agents have been used as epoxy ring openers. Hydrolyzation and condensation of TEOS often concurred to form the polyhydric salt and an oxide by condensation reaction. In cured epoxy, the amine groups (TETA) of hardener are reacted with epoxy and silanized epoxy to form a polymer network. Since the amines are basic, they also catalyse the condensation of silanol groups to form chemical bonds and inorganic-organic hybrid network structure (Fig. 1). Some reactions are shown in Scheme 2.
Sol-gel process consists of hydrolyzation and condensation reactions of TEOS and APTES. However, different contents of catalyst caused different effects on the reaction process and morphology of the products. In order to verify the ability of APTES and TETA to react with epoxy, FT-IR measurements were used to test the sample of epoxy resin (EP), silanized uncured epoxy (EP-APTES) and cured silica/epoxy composites (EP-APTES-TETA).
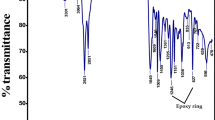
FT-IR spectra of uncured and cured SiO2/EP samples with different contents of TEOS are shown in Fig. 2. It is found that the hydroxyl-stretching band of epoxy resin appears a strong broad peak at 3,600 cm−1 ~ 3,100 cm−1, the N-H stretching and N-H bending peaks at 3,400 gradually broaden with introduction of amines. Moreover, 1,550 cm−1 indicate the amine group of APTES, the band appearing at 1,075 cm−1 assigned to Si-O-Si asymmetric stretching modes, which gradually shift toward the lower frequency because of the cross-linking reaction. Because of the breakage of epoxide groups to form bonds with amine during the curing process, the absorption peak appearing at 915 cm−1 almost disappears, implying the almost complete cured epoxy.
The viscosity curves of SiO2/EP hybrid materials are presented in Fig. 3. It is found that the viscosity of epoxy and silanized epoxy decreased greatly with temperature increased. Due to free volume fraction of the system improves, the movement of the chain segment enhances, leading to the intermolecular interaction force reduce, and the liquidity raised substantially.
However, at low temperature, the system modified by APTES has higher viscosity with higher APTES content. The results are attributed to chain extension arising from the reaction of amines of APTES and epoxide groups of epoxy resin. Then the movement among the chain segment is confined and the liquidity of the system reduced.
The exothermic reaction curves of hybrid materials are presented in Fig. 4. Due to the curing process was reacted on one-step, it was shown there is only one exothermic peak during cured exothermic reaction. Compared with the pristine epoxy (98 °C), introduction of ATPES and TEOS reduced the temperature of exothermic peak (93 °C). It is attributed that the heat release during the so-gel process accelerated the curing reaction of epoxy resin with TETA. However, no obvious difference was observed between pristine epoxy and hybrid materials.
Morphology and microstructure of SiO2/epoxy hybrid materials
The residue of stretching sample after high temperature treatment in air is shown in Fig. 5a. The organic ingredients have been completely decomposed and volatilized at high temperature. The rest white inorganic remains presented as a loose network structure, and still remain the original shape of sample. The XRD pattern of this residue shows a broad peak at 2θ = 22°, implying amorphous silica. We can conclude preliminary that the inorganic component distributes evenly in epoxy resin to form a hybrid network structure. In fact, the residual part of sample is the silica with network structure in the modified resin with crosslink network. The organic and inorganic hybrid networks are formed simultaneously to achieve homogenous structure. Compared with the composite prepared by mixing polymer with nanoparticles directly, the composites in our work with hybrid network have much smaller sized and homogenously dispersed inorganic phase.
The TEM images of SiO2/EP hybrid materials are shown in Fig. 6. It was found that the lower TEOS content in the composites, the more excellent dispersion of silica in polymer matrix (Fig. 6a–c). Accordingly, the larger content of TEOS, the larger size of silica was, and more aggregated silica particles was observed. Figure 6e is the TEM image of hybrid materials with the mass ratio of epoxy/APTES/TEOS 100/0/3. The larger sized silica phase and clearer edge of silica particle were observed in comparison to hybrid materials with the mass ratio of epoxy/APTES/TEOS 100/2/3. It was suggested that APTES in sol-gel process benefit to formed chemical bonds and inorganic-organic hybrid network structure, and the interfacial interaction between the silica phase and matrix enhanced, finally agglomeration of silica disappeared. This nanometer sized silica particles homogeneously dispersed in epoxy resin could enhance the strength and toughness of epoxy resin.
Introduction of acetone induced that the silica phase became smaller gradually, and dispersed more evenly in the epoxy (Fig. 6e). The transparency of this sample enhanced obviously implying the possibility of hybrid network with molecular level dispersion. The dimension of the silica phase which dispersed homogeneously and isolated by the EP organic networks, can be effectively maintained under 100 nm. Moreover, nanometer SiO2 not only has a large specific surface area and high activity, but also has some uncondensed hydroxyls, which can act with epoxy to improve the interface interaction. Unfortunately, it is difficult to remove acetone from the system completely, and the mechanical and thermal properties are affected by the residual acetone strongly.
The SEM images and FESEM images of fracture surface of pristine epoxy and composites prepared with mass ratio of epoxy/APTES/TEOS 100/2/3 are presented in Fig. 7. Figure 7b showed that the fracture surfaces of hybrid materials exhibited unobvious inorganic particles, and formed ripple and squama in comparison to fracture surface of pristine epoxy (Fig. 7a). The much more micro-cracks and rough surface implied more energy consuming during the process of rupture, and lead to improved toughness and strength of composites. The FESEM image of composites (Fig. 7d) exhibited inorganic particles with radial caltrop shape dispersed homogenously in epoxy in comparison to pristine epoxy (Fig. 7c). It is attributed that the addition of TEOS leads to s ilica particles dispersed unevenly and appeared reunion gradually. However, particles are connected to form an inorganic network structure. So the composites have hybrid network structure composed with silica and cross-linked epoxy. Cooperated with the hydrogen bonds between hydroxyl of epoxy resin and silica particles, the compatibility is improved effectively. Consequently, the composites with hybrid network have better transparency than that prepare by mixing silica particles and epoxy directly.
Mechanical and thermal properties of the SiO2/epoxy hybrid materials
The effect of different content of TEOS on the mechanical properties of SiO2/EP hybrid materials are listed in Table 1. The tensile strength, elongation at break, impact strength and bend strength of SiO2/EP hybrid materials increased initially, but decreased gradually with the excess addition of TEOS, and the tensile strength, elongation at break, impact strength and bend strength of SiO2/EP materials achieve the optimum (62 MPa, 2.03 %, 17.3 kJ·m−2, 90.5 MPa, respectively) with mass ratio of epoxy/APTES/TEOS 100/2/3. Compared with pristine epoxy, the tensile strength, elongation at break, impact strength and bend strength of hybrid materials enhanced 67.6 %, 190 %, 82.1 %, 15.7 %, respectively. The results are attributed that the nanometer silica particles are dispersed homogeneously in epoxy resin, and it is easy to produce stress concentration under external force, which causing crazing in the surrounding matrix and absorbing more deformation energy. Moreover, the existence of nanometer rigid particles makes resin expansion blocked. It prevents the crack extending and finally stop cracking, which improves the mechanical properties of hybrid materials. In addition, the interfacial adhesion between the nanometer SiO2 particles and epoxy resin matrix could be enhanced through opening the epoxy rings and linked with the amine groups of APTES. So it has the ability to improve the strength and toughness.
However, excess addition of TEOS induces aggregation of silica, which leads to compromised mechanical properties. The mechanical properties of SiO2/EP hybrid materials prepared with acetone as solvent reduce because that it is difficult to remove solvent completely. So, the elongation at break enhanced significantly due to the plasticization of acetone. Thus, the introduction of acetone is beneficial for so-gel process, but it is harmful for final thermosetting of the material. Therefore, acetone dosage should be minimized in the preparation process.
The heat deflection temperature (HDT) and glass transition temperature (T g ) of SiO2/EP hybrid materials with different contents of TEOS are summarized in Table 2. The HDT and T g are improved significantly with the addition of TEOS. It is suggested that chemical bonds are formed between SiO2 and epoxy resin, which formed crosslinking sites to inhibit the movement of EP chain segment. The HDT of composites under dry state increase, it is possible that the inorganic nanometer particles formed from TEOS hydrolysis and polycondensation, dispersed in the EP matrix evenly. In addition, the interaction between inorganic nanometer particles with large specific surface area and epoxy resin is strong. The polymer chains around the nanometer particles are difficult to move due to the space steric hindrance. However, the HDT of composites under wet state decrease slightly, it indicates that the addition of TEOS has little effect on the water resisting property of the epoxy.
Mechanical properties and water absorbtion of the SiO2/epoxy/glass cloth composites
The effects of TEOS and APTES on the mechanical properties of FRC are presented in Table 3. Compared with pristine epoxy, integrative mechanical properties of FRC have been improved significantly with introduction of TEOS and APTES. The water adsorbtion of the FRC reduced with the content of ATPES increasing. Water can destroy the interaction between fiber and epoxy matrix and reduce the strength. In our work, the improvement of mechanical properties of FCR prepared with SiO2/epoxy hybrid materials is because that the improved interface between inorganic phase and polymer inhibit the harm of water.
Conclusions
The mechanical and physical properties of the inorganic-organic hybrid materials can be easily improved by the introduction of a low dosage of inorganic substances by the sol-gel process. It is indicated that SiO2/epoxy hybrid materials achieve the optimal mechanical and thermal properties when the mass ratio of epoxy/APTES/TEOS is 100/2/3. Compared with the pristine epoxy, the tensile strength, elongation at break, impact strength and bend strength of SiO2/epoxy materials increase 67.6 %, 190 %, 82.1 % and 15.7 %, respectively. The SiO2 phase dispersed homogeneously in epoxy resin. The T g of the composites enhanced over 30 °C, and the distortion temperatures are also improved. The SEM, TEM analysis showed that the dimension of nanometer silica particles was about 20 nm, and dispersed evenly in the curing system.
For the fiber reinforced epoxy resin composites, the mechanical properties increase with the content of TEOS and APTES increase. The addition of TEOS reduces the water adsorbtion of the FRC.
References
Guo H, Huang Y, Liu L et al (2010) Effect of epoxy coatings on carbon fibers during manufacture of carbon fiber reinforced resin matrix composites. Mater Des 31(3):1186–1190
Pihtili H (2009) An experim ental investigation of wear of glass fibre-epoxy resin and glass fibre-polyester composite materials. Eur Polym J 45(1):149–154
Nazir T, Afzal A, Siddiqi HM et al (2010) Thermally and mechanically superior hybrid epoxy silica polymer films via sol-gel method. Prog Org Coat 69(1):100–106
Johnsen BB, Kinloch AJ, Mohammed RD et al (2007) Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 48(2):530–541
Deng S, Ye L, Friedrich K (2007) Fracture behaviours of epoxy nanocomposites with nano-silica at low and elevated temperatures. J Mater Sci 42(4):2766–2774
Zhang K, Shen M, Wu K et al (2011) Comparative study on flame retardancy and thermal degradation of phosphorus- and silicon-containing epoxy resin composites. J Polym Res 18:2061–2070
Yang P, Wang XF, Gu Y (2012) Copolymers of phenolphthalein-aniline-based benzoxazine and biphenyl epoxy curing behavior and thermal and mechanical properties. J Polym Res. doi:10.1007/s10965-012-9901-x
Jian J, Xin S, Pinnavaia TJ (2008) Reinforcement of a rubbery epoxy polymer by mesostructured silica and organo-silica with wormhole framework structures. Adv Funct Mater 18(7):1067–1074
Wan J, Zhi-Yang B, Cun-Jin X (2011) Preparation, curing kinetics, and properties of a novel low-volatile starlike aliphatic-polyamine curing agent for epoxy resins. Chem Eng J 171(15):357–367
Phonthamachai N, Chia H, Li X et al (2010) Solvent-Free One-Pot Synthesis of high performance silica/epoxy nanocomposites. Polymer 51(23):5377–5384
Costil S, Lukat S, Langlade C et al (2008) Surface modification of ceramic matrix composites induced by laser treatment. Appl Surf Sci 255(5):2425–2432
Mir I, Kumar D (2008) Recent advances in isotropic conductive adhesives for electronics packaging applications. Int J Adhes Adhes 28(7):362–371
Chen C, Justice RS, Schaefer DW et al (2008) Highly dispersed nanosilica-epoxy resin with enhanced mechanical properties. Polymer 49(17):3805–3815
Al-Hartomy OA Al-Ghamdi AA, Al-Solamy F et al (2012) Influence of matrices chemical nature on the dynamic mechanical and dielectric properties of rubber composites comprising conductive carbon black. J Polym Res. doi:10.1007/s10965-012-0016-1
Barabanova AI, Philippova OE, Askadskii A et al (2012) Transparent epoxy/silica nanocomposites with increased glass transition temperatures. Procedia Chem 4:352–359
Dittanet P, Pearson RA (2012) Effect of silica nanoparticle size on toughening mechanisms of filled epoxy. Polymer 53(9):1890–1905
Lekakou C, Kontodimopoulos I, Murugesh AK et al (2008) Processability studies of silica-thermoset polymer matrix nanocomposites. Polym Eng Sci 48(2):216–222
Battistella M, Cascione M, Fiedler B et al (2008) Fracture behaviour of fumed silica/epoxy nanocomposites. Compos A: Appl Sci Manuf 39(12):1851–1858
Chen S, You B, Zhou S et al (2009) Preparation and characterization of scratch and mar resistant waterborne epoxy/silica nanocomposite clearcoat. J Appl Polym Sci 112(6):3634–3639
Friedrich K, Schlarb AK (2008) Tribology of polymeric nanocomposites. Tribol Interf Eng 1–7
Zhang FH, Wang RG, He XD et al (2009) Fabrication and characterization of electrospun titania nanofibers. J Mater Sci 44(5):1198–1205
Hsieh TH, Kinloch AJ, Masania K et al (2010) The mechanisms and mechanics of the toughening of epoxy polymers modified with silica nanoparticles. Polymer 51(26):6284–6294
Gu J, Zhang Q, Dang J et al (2008) Preparation and properties research of SiC LLDPE thermal conductivity composites. Mod Chem Ind 28(9):42–45
Khoee S, Hassani N (2010) Adhesion strength improvement of epoxy resin reinforced with nanoelastomeric copolymer. Mater Sci Eng 527(24):6562–6567
Jiao J, Sun X, Pinnavaia TJ (2009) Mesostructured silica for the reinforcement and toughening of rubbery and glassy epoxy polymers. Polymer 50(4):983–989
Dong Y, Chaudhary D, Ploumis C et al (2011) Correlation of mechanical performance and morphological structures of epoxy micro/nanoparticulate composites. Appl Sci Manuf 42(10):1483–1492
Gao X, Jensen RE, McKnight SH et al (2011) Effect of colloidal silica on the strength and energy absorption of glass fiber/epoxy interphases. Appl Sci Manuf 42(11):1738–1747
Chung-Hwei S, Yi-Pang C, Chih-Chun T et al (2010) Preparation, characterization and thermal properties of organic–inorganic composites involving epoxy and polyhedral oligomeric silsesquioxane(POSS). J Polym Res 17(5):673–681
Acknowledgments
This study was supported by graduate starting seed fund of Northwestern Polytechnical University (NO. Z2013149).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiao, J., Liu, P., Wang, L. et al. One-step synthesis of improved silica/epoxy nanocomposites with inorganic-organic hybrid network. J Polym Res 20, 202 (2013). https://doi.org/10.1007/s10965-013-0202-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0202-9