Abstract
In this study a simple green route was used for producing worn-look denim garments with antibacterial property. Silver nanoparticles were successfully synthesized on denim fabrics by reducing silver nitrate using cellulosic chains of cotton, starch and/or glucose in alkali media. Glucose had the ability to reduce indigo dye and resulted in better control of particle size and stability. Transmission electron microscopy (TEM), dynamic light scattering (DLS) and UV–vis spectroscopy were employed to characterize the synthesized Ag nanoparticles. The treated denim fabrics were also characterized by scanning electron microscopy (SEM), Raman spectroscopy and colorimetric measurements. The results indicated the successful synthesis of nano silver particles with average particle size of 30–40 nm on the indigo dyed cellulosic fabric along with denim color reduction. Although the fabrics showed antibacterial activity against E. coli and S. aureus, a decrease in the antibacterial feature was observed in presence of glucose as a suitable nutrient for the growth of bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibacterial textile products have attracted great interest due to the ever-growing demand for healthy living [1, 2]. Many antimicrobial agents have been used including quaternary ammonium compounds, N-halamines, chitosan and nanoparticles of noble metals and metal oxides [3, 4]. Among different metals such as copper, zinc and cobalt, silver is known as one of the most promising antibacterial materials [5–7]. It can adhere to the bacterial membrane and kill microorganisms or inhibit their growth by cell wall damage, alteration of cell wall permeability and inhibition of enzyme actions [8]. Silver nanoparticles with different size, stability and morphology can be prepared using many synthesis methods including chemical and photochemical reductions such as polysaccharide, tollens reagent and irradiation methods [5, 9]. Various natural polymers such as starch and chitosan have been reported as reducing agents for the synthesis of silver nanoparticles [10]. Recent studies have shown that starch can play the dual role of reducing and stabilizing agent in nano silver preparation [10]. Panàček et al. used Four different saccharides namely glucose, galactose, maltose and lactose to reduce [Ag(NH3)2]+ complex, preparing Ag nanoparticles [11]. Raveendran et al. have fabricated Ag nanoparticles using starch as capping/reducing agent [12].
Various efforts have been made to impart antibacterial properties into cotton, owning to its proneness to the growth of bacteria [1, 13]. Cellulose fibers modified with silver nanoparticles were prepared using N-methyl morphine-N-oxide [14]. Cotton fabrics with excellent antimicrobial properties against common bacteria were treated by Ag/TiO2 nanocomposite along with citric acid as a crosslinking agent [15]. In situ formation of Ag nanoparticles in a grafted polymer network of cotton fabric has been reported as an effective method for the preparation of antibacterial fabrics [16]. In situ synthesis of silver nanoparticles on cotton fabric using cellulosic chains of cotton as reducing and stabilizing agent has been recently investigated [5, 17–19]. Glucose along with poly vinyl pyrrolidone (PVP) and sodium hydroxide was used to synthesize well-dispersed nano silver particles [20, 21]. Silver nanoparticles were also prepared by sodium salt of carboxymethyl cellulose (CMC) as both reducing and stabilizing agent [22].
Owning to the vast popularity of denim fabrics, researchers have been looking for new ways to process the fabric with more comfort and style, giving it a proper aged and worn look.
Stone washing, enzymatic process and laser treatment are among the current methods used to remove color from denim while adding contrast to the fabric [23–25].
In this study, the idea of imparting antibacterial property into cotton denim fabric together with discoloration effect was concerned, aiming to develop aged-looked denim with antibacterial activity. In this regard, silver nano particles were synthesized on denim fabric by a reduction method using glucose and cellulosic chains of cotton as reducing and stabilizing agents. Starch as a sizing agent in denim fabric also played a reducing/stabilizing role for the synthesis of silver nanoparticles. Therefore, the interplay of the glucose, starch and cellulosic chains of cotton with respect to the alkaline pH of the reaction medium was considered. Moreover, glucose as a green reducing agent for vat dyes undergoes a degradation sequence in alkaline solution, resulting in the indigo reduction process [26].
Experimental
Materials
A starch sized indigo dyed denim 100 % cellulosic fabric weighing 322 g/m2 with warp density of 26/cm and weft density of 20/cm was used. In order to evaluate the back-staining occurred during discoloration process, a piece of 100 % bleached woven cotton fabric weighing 166 g/m2 with weft and warp density of 26/cm was sewed on the denim fabric and labeled as white pocket. Silver nitrate, glucose and sodium hydroxide were all provided by Merck Co., Germany.
Methods
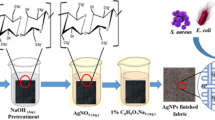
The sized denim samples were treated in an aqueous medium using distilled water with fabric-to-liquor ratio (L:R 1:40), according to the methods presented in Fig. 1. The concentration of silver nitrate, sodium hydroxide and glucose was 0.2 mg/L, 0.25 g/L and 0.5 mg/L, respectively. According to Fig. 1, two different methods were used, differing from the place at which denim fabric is introduced into the bath. In method (1), denim fabric was introduced into the bath at 60 °C, then the temperature was gradually raised up to boiling point within 60 min, and the treatment was continued for another 60 min. In method (2), the bath containing the chemicals was heated from 60 °C to boil within 60 min with no fabric, after which the denim sample was added and the process was continued for 60 min at boil. Both methods were carried out in absence (a) and presence of glucose (b), regarding its effects on the treatment.
Samples are labeled as S(1-a), S(1-b), S(2-a) and S(2-b), where (1) and (2) correspond to the applied method and (a) and (b) refer to absence and presence of glucose, respectively.
Characterization techniques
In order to study the formation of silver nanoparticles, UV–Vis absorption spectra of the treated denim fabrics solutions were obtained using a spectrophotometer, Camspec M-350. The samples for transmission electron microscopy (TEM) were prepared by drying a dispersion of the nano silver particles effluents on carbon-coated copper grids. The particles were imaged using TEM, model EM 208 Philips. Dynamic Light Scattering (DLS), Malvern, was used to analyze size distribution of the silver nanoparticles.
The morphology of the nanosilver particles distributed on denim cotton fabrics was examined by scanning electron microscope (SEM) model XL30, Philips Co., equipped with energy dispersive X-ray (Thermo Noran, EDX).
Raman spectra of the denim fabrics were obtained using an Almega Thermo Nicolet Dispersive Spectrometer instrument. Color coordinates of the denim fabrics (lightness (L*) and yellowness-blueness (b*)) were obtained using a Data color spectrophotometer. The CIELAB ∆E color difference values between the untreated and treated denim samples under illuminant D65 for 1964 standard observer were calculated based on Eq. 1.
Quantitative antibacterial assessment was conducted according to AATCC 100–2004 standard test method, using Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria. The number of bacteria colonies was counted according to colony forming unit (CFU) and decrease in microbial agents (C) was determined using Eq. 2.
Where M 1 is the number of colonies in control bacterial suspension and M 2 is the number of the colonies in the suspension after being co-located adjacent to the treated samples.
Tensile strength and elongation of the treated denim fabric were measured by tensile tester (SDL ATLAS) according to ASTM D5034–09. Five experiments in warp direction were carried out for each sample, and the mean value was reported.
The antibacterial kinetic of the treated denim was determined by considering the viable bacteria (S. aureus and E. coli bacteria) on inoculated fabric at different exposure time (5, 10, 20, 30, 45, 60, 90,120 and 150 min). The percent of bacterial reduction was calculated according to Eq. 3:
Where N and N 0 are the number of bacteria recovered from the samples at any defined time and zero contact time, respectively.
Results and discussion
Characterization of silver nanoparticles
UV–vis spectroscopy
According to Fig. 2a, the solutions of the treated denim S(1-a) exhibited a surface plasmon resonance absorption band with average maximum wavelength at around 406 nm indicating the synthesis of silver nano particles. Starch and cellulosic chains of cotton fabric played a reducing role, changing the silver ions into silver particles. Starch can also act as stabilizing agent and protect particles from growth and agglomeration, resulting in smaller silver particles formation. As shown in Fig. 2a, in presence of glucose (S(1-b)), the intensity of the peak at 400 nm was increased dramatically, indicating formation of larger number of nanoparticles by glucose. Silver ions with positive charge can be diffused and absorbed into the cotton denim fiber due to the electrostatic interaction (Fig. 3a) [27]. Therefore, the Ag+ ions were reduced by cellulose/starch/glucose and silver particles were generated (Fig. 3b). The possible reactions for nano silver synthesis are shown in Fig. 3.
Considering the preparation procedure used in method 2, it can be seen that although a broad absorption band was detected in sample S(2-a) (Fig. 2b), the UV–Vis absorption spectrum of sample S(2-b) depicted no distinctive peak around 400 nm. After introducing the cotton denim fabric (S(2-a)) into the bath, the Ag2O particles prepared by hydroxyl ions (Fig. 3c) were reduced to silver nanoparticles. Therefore, cellulosic chains of cotton together with starch acted as reducing agents. The results indicated that before addition of denim sample S(2-b), glucose was not able to reduce Ag2O particles and only acted as stabilizing agent, forming a layer around the Ag2O particles which could not be entirely reduced by cellulose/starch in the following step.
The formation of Ag nanoparticles can be also identified by the color change of the solution and denim treated fabrics. The solution was observed to change from colorless to yellow during the synthesis of silver nano particles. The color of the denim treated fabrics also changed into yellow confirming the adsorption of nano silver particles on the garment (Table 1).
Another obvious peak can be seen in the UV–vis spectra of the treated fabrics solutions around 660–670 nm (Fig. 2). This peak can be explained by the removal of indigo dye from denim fabric during the treatment which is more prominent in presence of glucose regarding its reducing ability in alkali media (Fig. 3d). Therefore, the discolored denim fabric with desirable worn-look appearance can be prepared under the applied conditions.
TEM images and DLS analysis
The TEM images along with respective particle size distribution of silver nano particles synthesized in solutions containing S(1-a), S(1-b), S(2-a) and S(2-b) denim fabrics are shown in Fig. 4a–d, respectively. In both methods the nano silver particles synthesized in presence of glucose dispersed individually and the diameter of the particles was in smaller range. While most of the nano particles were around 10–150 nm, very smaller particles were produced in presence of glucose. Moreover, no silver particles larger than 20 nm can be detected in presence of glucose using method 2. Therefore, stabilizing effect of glucose can be clearly demonstrated by forming a layer of membrane to hinder the inter-contact between the silver particles, protecting them from growth and agglomeration [20, 21, 28].
Characterization of the treated denim fabrics
SEM images
Figure 5 shows the SEM images of untreated, S(1-a) and S(1-b) denim fabrics. While the surface of untreated fabric is smooth with no particles (Fig. 5a), the synthesized silver nanoparticles were uniformly distributed on the surface of the treated denim samples (Fig. 5b–e). SEM images of S(1-a) treated fabric (Fig. 5b,c) compared to S(1-b) (Fig. 5d,e), demonstrated the ability of glucose in protecting the nano particles from agglomeration, producing smaller stabilized silver particles.
The existence of Ag element on the surface of the treated denim fabrics was further confirmed by EDX analysis (Figs. 5e and 6c,d). In addition to Ag, Au was the major element observed in EDX spectra, resulting from a gold layer covered the samples. Comparing the percentage of Ag, more silver nanoparticles were deposited on the surface of the fabric treated under method 1. The SEM pictures of the white pocket before and after the treatment are also shown in Fig. 6a, b. In comparison to the smooth surface of the fibers before the treatment (Fig. 6a), silver nano particles with average particle size of 55 nm were distributed on the surface of the treated fibers (Fig. 6b).
Raman spectra
As can be seen in Fig. 7a, untreated denim fabric has characteristic spectral bands corresponding to cellulose cotton. The bands assigned to CH, CH2 stretching around 3,000 cm−1, H-C-C, H-C-O, and H-O-C bending between ~1,300 and 1,400 cm−1, H-C-H and H-O-C bending around 1,500 cm−1, C-C and C-O stretching near 1,200 cm-1, skeletal C-O-C, C-C-C, O-C-C and O-C-O bending between ~350 and 510 cm−1 are the prominent bands in untreated denim fabric [29].
It is expected that cellulose chains of cotton denim can be oxidized through reduction of silver ions into silver nano particles (Fig. 2 (reaction (2)). According to Fig. 6b, Raman spectrum of the treated denim fabric has changed, due to the oxidation process. The main peaks belong to C–H groups of aldehyde compound in the region of 2,700–2,900 cm−1, the peak around 1,360–1,550 cm−1 corresponds to COO− compounds (carboxylic acids group) and the peak of C = O group resulting from oxidation appears at 1,550–1,650 cm−1 confirmed this expectation [17, 19, 30–32].
Color coordinates
Due to nano silver synthesis on denim fabrics and indigo dye removal through applied methods, colorimetric coordinates of the treated samples were changed and the obtained results are summarized in Table 1. In order to evaluate the back-staining occurred during discoloration process, the color coordinates were also measured on back of samples and white pocket. Lightness values of all the denim treated fabrics were increased due to the discoloration of indigo dye during the treatment. The result is consistent with UV–vis spectra indicating the presence of indigo dye in solutions (Fig. 2). Highest L* values belonged to samples S(1-b) and S(2-b) in which glucose acted as a reducing agent for indigo dye. Moreover, samples treated in method 1 were lighter than the identical specimens treated under method 2, due to the longer treatment time. ΔE values were also calculated and the maximum color difference was obtained in sample S(1-b) in presence of glucose.
Comparing b* values of samples’ back indicated higher back-staining (bluer) in samples treated along with glucose as a result of discoloration effect it had on indigo dye. Yellowness of all the treated denims is higher than the untreated fabric due to the synthesis of nano silver particles on treated samples. Therefore, in addition to the increasing effect of glucose on lightness, it resulted in higher back-staining.
Considering the white pocket of samples, an increase in yellowness value of all the treated samples was obtained due to synthesis and adsorption of nano silver. In this regard, samples treated with silver nitrate together with glucose were bluer as a result of discoloration effect of glucose. Due to the yellowing effect of silver synthesis on treated samples, whiteness index (WI) values of the pockets of samples S(1-a) and S(2-a) were negative. Discoloration ability of glucose led to higher whiteness in samples treated along with glucose.
Antimicrobial properties
Antibacterial activity of the untreated and denim treated samples against S.aureus and E.coli is shown in Table 1. Due to the presence of starch, as a source of nutrition for the growth of bacteria, in denim fabrics, antibacterial efficiency of the treated samples was not excellent. In comparison to sample S(2-a) with no antibacterial activity, sample S(1-a) had the highest bacterial reduction due to prolonged treatment time. Glucose as a suitable nutrient for the growth of bacteria led to decrease in antibacterial ability from 76 % to 34 %.
Therefore, in spite of the successful synthesis of nano silver particles on denim fabrics, residues of both glucose and starch on the garment surfaces had negative effect on the antimicrobial properties.
Antibacterial kinetic of treated denim
The antibacterial kinetic of samples S(1-a) and S(1-b) against S.aureus and E.coli was calculated based on Eq. 3 and the obtained results are shown in Fig. 8. The bacteria killing kinetic can be shown according to Chick-Watson, Eq. 4 and Eq. 5 (Kwok-Keung and LeChevallier 2004):
Where C is the concentration of synthesized nanosilver and K is the specific coefficient of lethality. The killing rate at specific concentrations can be shown as Eq. 5. Identical samples showed similar kinetic trends against Gram-positive and Gram-negative bacteria. Due to the role of glucose as nutrient for the bacteria, killing rate of the nano silver particles in sample S(1-a) is higher than S(1-b).
Tensile strength
Mean value of tensile strength and elongation for treated denim fabrics are shown in Table 2. The results indicated that nano silver synthesis under the applied methods (alkaline condition) led to a decrease in tensile strength and an increase in elongation of the treated denim fabrics. Furthermore, an increase in treatment time in samples S(1-a) and S(1-b) caused more reduction in tensile strength.
Conclusion
In this research old-look denim garment with a desirable yellow appearance, antibacterial property and acceptable tensile strength was successfully produced. Silver nano particles were prepared on denim cotton fabric by reducing silver nitrate with cellulose, starch and/or glucose. Furthermore, discoloration of indigo dyed denim garment was simultaneously carried out due to the reducing effect of glucose in alkali media. Although glucose was effective in preventing the silver nanoparticles from further growth and agglomeration, it had a negative influence on antibacterial activity.
References
Yadav V, Prasad A, Kathe A, Raj S (2006) Functional finishing in cotton fabrics using zinc oxide nanoparticles. Mater Sci B 29:641–645
Wang HH, Lin MS (1998) Biocidal polyurethane and its antibacterial properties. J Polym Res 5:177–186
Simoncic B, Tomsic B (2010) Structures of novel antimicrobial agents for textiles: a review. Text Res J 80:1721–1737
Fc C, Lai SM, Hsie IC, Don TM, Huang CY (2012) Preparation and properties of chitosan/clay (nano) composites: a silanol quaternary ammonium intercalated clay. J Polym Res 19:9781–9790
Sharma VK, Yngard RA, Lin Y (2009) Silver Nanoparticles: green synthesis and their antimicrobial activity. Adv Colloid Interfac 145:83–96
Montazer M, Malekzadeh SB (2012) Electrospun antibacterial nylon nanofibers through in situ synthesis of nanosilver: preparation and characteristics. J Polym Res 19:9980–9985
Chalal S, Haddadine N, Bouslah N, Benaboura A (2012) Preparation of Poly(acrylic acid)/silver nanocomposite by simultaneous polymerization–reduction approach for antimicrobial application. J Polym Res 19:24–31
Akhavan O (2009) Lasting antibacterial activities of Ag–TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J Colloid Interf Sci 336:117–124
Mishra S, Shimpi NG, Sen T (2012) The effect of PEG encapsulated silver nanoparticles on the thermal and electrical property of sonochemically synthesized polyaniline/silver nanocomposite. J Polym Res 20:49–59
Singh M, Sinha I, Mandal RK (2009) Role of pH in the green synthesis of silver nanoparticles. Mater Lett 63:425–427
Panacek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N, SharmaVK NT, Zboril R (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110:16248–16253
Raveendran P, Fu J, Wallen SL (2006) A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem 8:34–38
Kaur I, Bhalla TC, Sharma B (2011) Functionalization of cotton fabric orienting towards antibacterial activity. J Polym Res 18:347–358
Smiechowicz E, Kulpinski P, Niekraszewicz B, Bacciarelli A (2011) Cellulose fibers modified with silver nanoparticles. Cellulose 18:975–985
Montazer M, Behzadnia A, Pakdel E, Rahimi MK, Moghadam MB (2011) Photo induced silver on nano titanium dioxide as an enhanced antimicrobial agent for wool. J Photoch Photobio B103:207–214
Gupta P, Bajpai M, Bajpai SK (2008) Investigation of antibacterial properties of silver nanoparticle-loaded poly (acrylamide-co-itaconic acid)-grafted cotton fabric. J Cotton Sci 12:280–286
El-Shishtawy RM, Asiri AM, Abdelwahed NAM, Al-Otaibi MM (2011) In situ production of silver nano particle on cotton fabric and its antimicrobial evaluation. Cellulose 18:75–82
Marambio-Jones C, Hoek EMV (2010) A review of the antibacterial effects of silver Nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551
Montazer M, Alimohammadi F, Shamei A, Rahimi MK (2012) In situ synthesis of nano silver on cotton using Tollens’ reagent. Carbohydr Polym 87:1706–1712
Wang H, Qiao X, Chen J, Ding S (2005) Preparation of silver nanoparticles by chemical reduction method. Colloid Surface A 256:111–115
Wang H, Qiao X, Chen J, Wang X, Ding S (2005) Mechanisms of PVP in the preparation of silver nanoparticles. Mater Chem Phys 94:449–453
Chen J, Wang J, Zhang X, Jin Y (2008) Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater Chem Phys 108:421–424
Stepankova M, Wiener J, Rusinova K (2011) Decolourization of vat dyes on cotton fabric with infrared laser light. Cellulose 18:469–478
Montazer M, Sadeghian Maryan A (2009) Influences of different enzymatic treatment on denim garment. Appl Biochem Biotech 160:2114–2128
Montazer M, Sadeghian Maryan A (2008) Application of laccase with cellulase on denim for clean effluent and repeatable biowashing. J Appl Polym Sci 110:3121–3129
Vuorema A, John P, Keskitalo M, Marken F (2011) Electrochemical determination of plant-derived leuco-indigo after chemical reduction by glucose. J Appl Electrochem 38:1683–1690
Barani H, Montazer M, Samadi N, Toliyat T (2012) In situ synthesis of nano silver/lecithin on wool: enhancing nanoparticles diffusion. Colloid Surface B 92:9–15
Gulrajani ML, Gupta D, Periyasamy S, Muthu SG (2008) Preparation and application of silver nanoparticles on silk for imparting antimicrobial properties. J Appl Polym Sci 108:614–623
Cho LL (2007) Identification of textile fiber by Raman microspectroscopy. Forensic Sci J 6:55–62
Tipson RT (1968) Infrared spectroscopy of carbohydrates. National Bureau of Standards Monograph
Jenkins SL, Almond MJ, Atkinson SDM, Hollinsm P, Knowles JP (2005) Gas–solid reactions of single crystals: a study of reactions of NH3 and NO2 with single crystalline organic substances by infrared microspectroscopy. Spectrochim Acta A 62:1131–1139
Yuen SN, Choi SM, Philips DL, Ma CY (2009) Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem 114:1091–1098
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maryan, A.S., Montazer, M. & Harifi, T. One step synthesis of silver nanoparticles and discoloration of blue cotton denim garment in alkali media. J Polym Res 20, 189 (2013). https://doi.org/10.1007/s10965-013-0189-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0189-2