Abstract
The morphology and affinity of a scaffold influence the attachment of cells to its surfaces. In this study, the morphology and hydrophilicity of chitosan/caffeic acid hybrid scaffolds were investigated. Grafting caffeic acid onto chitosan hybrid scaffolds by using high levels of potassium persulfate produced scaffolds with looser morphology and higher porosity, as indicated by scanning electron microscopy (SEM) and porosity analysis. SEM analysis showed that the prepared scaffolds had a macroporous morphology with interconnected pores. Differential scanning calorimetry (DSC) revealed that the scaffolds’ hydrophilicity decreased after caffeic acid grafting. The scaffolds were cultured with human osteosarcoma UMR-106 cells, but SEM analysis showed that cell attachment was poor. However, calcification of the scaffolds promoted the attachment of UMR-106 cells onto the scaffold. This study shows that calcified chitosan/caffeic acid hybrid scaffolds could be suitable for use in hard-tissue engineering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To be used as a scaffold, its matrix should meet the following requirements: (a) biocompatibility with the tissues, (b) biodegradability at the ideal rate corresponding to the rate of new tissue formation, (c) non-toxicity and non-immunogenicity, (d) optimal mechanical properties, and (e) adequate morphology and porosity for mass transfer both intra-scaffold and inter-scaffold and also with its local environment [1]. Many biodegradable polymers are either chemically or physically modified to meet these requirements, and much attention has been given to a natural biopolymer, chitosan (CS) [2–6]. Chitosan is a linear polysaccharide composed of randomly distributed β-(1–4)-linked d-glucosamine (deacetylated unit) and N-acetyl-d-glucosamine (acetylated unit). Chitosan is produced commercially by deacetylation of chitin, which is the structural element of the exoskeleton of crustaceans (crabs, shrimp, etc.) and cell walls of fungi.
Chitosan is widely employed as an excipient for drug delivery in the pharmaceutical industry and as a scaffold for tissue culture in tissue-engineering applications [4–8]. Chitosan is a renewable, biocompatible, biodegradable, antibacterial, and non-toxic biomaterial. It can be fabricated into the desired shapes of the scaffolds, and can be molded to porous structures. It also contains amino and hydroxyl groups which can be reacted and functionalized [9, 10].
Whereas increased porosity and pore size facilitate cell growth on scaffolds, the mechanical strength of the scaffolds is weakened. This could be the cause of infection, which could make scaffold implants fail after prolonged durations, increases the cost of medical care, and even leads to patient death [11]. The incidence of infection is typically around 1%, but has also been reported to be high as 16% [12]. There are two key ways to keep a patient away from infections: one is to take antibiotics before an operation, the other is to improve the antimicrobial properties of implants.
Many studies show polyphenols not only reduce oxidative stress but also possess anticancer ability [13–15]. In our earlier work, we found that grafting of caffeic acid onto a CS scaffold improves its compressive strength, antibacterial, antioxidant, and anticancer properties [16]. A number of studies indicate that both morphology and hydrophilicity influence the attachment of cells onto the surface of a scaffold [17–19]. However, as for CS/caffeic acid hybrids, the influence of morphology and hydrophilicity on growth and attachment of cells onto scaffolds has not been reported.
In this paper, the effect of grafting caffeic acid on the morphology and hydrophilicity of CS scaffolds was investigated. The calcification of CS/caffeic acid hybrid scaffolds and attachment of human osteosarcoma UMR-106 on it were studied.
Experimental
Materials
CS (medium molecular weight, deacetylation of 85%) and caffeic acid (purity ≥95%) were provided by Fluka. (3-Chloropropyl)trimethoxysilane (CPTMS, purity ≥98%), potassium persulfate (KPS, purity ≥99%), calcium chloride (purity ≥97.0%), and sodium phosphate (purity ≥99.0%) were obtained from Aldrich. NaCl particles (purity >99.5%) were purchased from Taiwan Salt Industry Corporation (Taiwan). All other chemicals are of reagent grade or higher grade and were used without further purification.
Preparation of various hydride scaffolds [16]
We used the heat cross-linking method to prepare a chitosan/CPTMS hybrid matrix (CSC), which is illustrated in Scheme 1. CS was firstly dissolved in a 5 wt% acetic acid solution to form a 4 wt% CS solution. Then 25 g of 4 wt% CS solution was mixed with 1 g of CPTMS and 25 g sieved NaCl particles (size 150–250 μm) by using a homo-mixer. The mixture was then cast in a 6-cm Teflon dish, and was heated in an oven at 70 °C for 36 h. The dried membrane was neutralized in 1 N NaOH solution. The wet membrane was dried at 70 °C to complete the nucleophilic aliphatic substitution, and then put into a water bath to remove both the NaCl particles and the remaining NaOH. The membrane was dried in a vacuum (15 Pa). CS membrane was prepared by the same procedure except that CPTMS was not added.
Potassium persulfate was used as an initiator for grafting of caffeic acid to the CS/CPTMS membrane (Scheme 2).
The grafted weight percent of these samples was calculated by using the following equation:
where \( {w_0} \) and \(w_{\text{g}}\) are the weight of CS/CPTMS hybrid membrane before and after grafting reaction.
Morphology and affinity
The morphology of the scaffolds was observed with a Jeol 5600 scanning electron microscope equipped with energy dispersive X-ray (EDX) analysis. Image analysis methods were used to measure the mean size of the pores. The porosity of the scaffolds was measured according to Archimedes’ principle, with absolute ethanol as the immersion medium.
Swelling behavior and differential scanning calorimetry (DSC) curves are employed to evaluate a scaffold’s affinity to water. To measure the swelling behavior of the membranes, dry samples were immersed in deionized water at 30 °C for 24 h to ensure they are saturated. Then the excess water was removed by suction. All experiments were repeated at least three times. The swelling percentage was calculated by:
where W w and W d are the weight of the swollen and dry membranes, respectively.
DSC was performed by using a Q series DuPont TA DSC instrument. A 10.5-mg sample was placed into an aluminum cup and sealed. A small hole was kept at the top of the cup, to allow the release of water. An empty cup was used as a reference. Samples were detected twice in the temperature range 30–200 °C, at scanning rate of 10 K/min.
Biological assessment
Cultivation of cells with scaffolds
Various scaffolds were first fixed on culture dishes with silicone gel. The scaffold was first placed in the dishes and two or three spots of silicone gel were then applied around the scaffold. A coverslip was placed on top of the scaffold and adjacent silicone gel spots. Silicone gel is used as adhesive for the coverslip and culture dish. Cells used in this study were human osteosarcoma UMR-106 cells. The cells were cultured in minimal essential medium (αMEM), supplemented with 10% FBS and 100 U/mL penicillin and 100 μg/mL streptomycin, at 37 °C in a humidified atmosphere of 5% CO2 in air. UMR-106 cells suspended in the culture medium (5 × 104 cells/mL) were then added to the dishes to allow the ingrowth of cells onto the scaffolds which were 5 mm in length, width, and height. Subsequently, the medium was changed every 2 days. After incubation for 7 days, cells on the scaffolds were harvested for cell attachment analysis.
Cell attachment analysis
The morphology of the cell attachment was observed by SEM. Scaffold with UMR-106 cells were fixed with a 2 wt% glutaraldehyde solution, and dehydrated in aqueous ethanol solutions. Drying with supercritical CO2 was performed to prevent deformation of the cells attached to the scaffolds. The samples were gold sputtered in a vacuum and then viewed with a Jeol 5600 scanning electron microscope.
Scaffold calcification
The scaffold was soaked in 20 mL of 1 M CaCl2/Tris HCl aqueous solution at 37 °C and pH 7.4 for 24 h, after which the aqueous phase was removed by suction. The sample was further soaked in 20 mL of 0.6 M (NH4)2HPO4 aqueous solution at 37 °C for another 24 h. Suction and drying were performed afterwards.
Results and discussion
We employed a differential dose ratio of CPTMS/CS to obtain the various hybrid scaffolds as CS, CSC0.2, CSC0.6, and CSC1.0, by the above preparation procedures. Thus, for CSC0.2, CSC0.6, and CSC1.0, 0.2, 0.6, and 1.0 g of CPTMS were added to 1 g of CS solution, respectively. Then, we observed the morphology of the resulting hybrid scaffolds by SEM. The SEM images show that the pore size decreased with the increasing dosage of CPTMS (Fig. 1).
CSC1.0, which has the highest compressive strength among the CS/CPTMS hybrid scaffolds tested (shown in our earlier work [16]), was employed as the trunk matrix for grafting of caffeic acid. Its characteristics are mean pore size 125.3 ± 6.2 μm, porosity 78.5 ± 3.4%, diameter 4.95 ± 0.08 cm, and thickness 5.06 ± 0.04 mm.
The graft polymerization of caffeic acid onto CSC1.0 is illustrated in Scheme 2. Hsu et al. reported a free radical degradation of chitosan by potassium persulfate [20]. Mochalova et al. [21] found that at ammonium persulfate concentrations higher than 10−2 M, the rate of chitosan macrochain degradation is high; whereas, if the concentration of ammonium persulfate is around 10−4 M, the chitosan macrochain degrades insubstantially.
Therefore, to avoid serious chain breakage of CSC1.0 by free radical processes, low concentrations of potassium persulfate were used in this study: 2.5, 5.0, 7.5, and 10 mM of potassium persulfate aqueous solution were used as an initiator for the graft polymerization of 0.5 g caffeic acid (products were abbreviated as KPS2.5, KPS5.0, KPS7.5, and KPS10, respectively). And polymerization of 0.75 g, 1.0 g, and 1.25 g of caffeic acid was initiated by 2.5 mM potassium persulfate solution (products were abbreviated as CA0.75, CA1.0, and CA1.25, respectively), at 40 °C for 24 h. As shown in Fig. 2, the caffeic acid graft weight % increased with the increasing dosage of potassium persulfate and caffeic acid.
After caffeic acid was grafted, as shown in Fig. 3, the pore size of the scaffold was almost unchanged.
The EDX profiles (Fig. 4) show that the wt% values of Si were 0, 1.69, 3.30, and 5.29% in CS, CSC0.2, CSC0.6, and CSC1.0 respectively. However, the calculated Si wt%, referred to the formulation of CSC0.2, CSC0.6, and CSC1.0, should be 2.36, 5.30, and 7.07%, respectively. The EDX analysis indicated that some free CPTMS leaked out and did not incorporated into CS/CPTMS hybrid scaffolds.
As shown in Fig. 5 the porosity of CS is decreased after CPTMS is cross-linked. It was clear that the increase in CPTMS dosage caused the increasing in scaffold volume and decreased the efficiency of the NaCl porogen.
Moreover, the three-dimensional network increased as the dosage of CPTMS increased, making the CS/CPTMS scaffolds more burly for NaCl particles to form pores, so the porosity of the hybrid scaffold decreased. However, the porosity of the scaffolds increased with the increasing dosage of potassium persulfate. High potassium persulfate concentration may cause chain breakages that impart a looser morphology and a higher porosity.
The swelling (%) of CS/CPTMS hybrid scaffolds in potassium-buffered saline (PBS, pH 7.4) decreased as the CPTMS content increased. As shown in Fig. 6, the swelling (%) of the hybrid scaffolds further decreased when caffeic acid graft weight % increased to 16%. This result demonstrated that the incorporation of hydrophobic compounds (such as CPTMS with CS, and caffeic acid with CSC1.0) made the resultant scaffolds more hydrophobic.
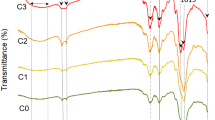
Figure 7 shows the DSC curves of CS, CSC1.0, KPS10, and CA1.25. All samples were stored in a dehumidifying cabinet before analysis. The samples were detected in the first and second heating scan run in the temperature range of 25–200 °C. The maximum temperature of 200 °C was selected in order to avoid possible CS degradation. In the first heating scan the peak was above 100 °C, presenting an apparently endothermic event for the evaporation of water. For samples in the second DSC run such a peak was not perceptible, which supports the view that the water evaporated during the first DSC run. A closer examination of Fig. 7 revealed differences in the peak area and position of the endotherm, indicating that CS, CSC1.0, CA1.25, and KPS10 differ in their water-holding capacity as well as the strength of the water–scaffold interaction. The endothermic peak area decreased indicating that the water content of the sample decreased. The water in scaffolds is held by hydroxyl groups and amino groups. Although the amount of hydroxyl groups increased with caffeic acid graft weight (%), caffeic acid is a hydrophobic compound and the amount of amino groups decreased after the grafting of caffeic acid. After preparation of the scaffolds, the amount of amino groups residue in the scaffolds was in the order of CS > CSC1.0 > CA1.25 > KPS10, so the water-holding capacity of the scaffolds was in the order of CS > CSC1.0 > CA1.25 > KPS10, too. This result was in agreement with the results of swelling analysis, as shown in Fig. 6. The peak position (102, 105, 106, and 107 °C for CS, CSC1.0, CA1.25, and KPS10, respectively) shifted to a higher temperature, indicating that hydrophilic interaction between water and the scaffold was strengthened.
Hydrogen bonding was enhanced in the hydrophilic interaction with the hydroxyl groups of the chitosan/caffeic acid hybrid scaffolds, resulting in the higher temperature needed to remove such water molecules as reflected by the DSC peak position.
The cell attachment in the scaffold can be improved by calcification [15]. Scaffold CA1.25 was calcified to increase its ability for cell attachment. The SEM image of UMR-106 cells grown on calcified CA1.25 (Fig. 8b) showed that the cell attachment was better than on CA1.25 (cf. Fig. 8a). This result indicated that calcification of caffeic acid grafted scaffolds was beneficial for UMR-106 cell attachment.
Conclusion
CA1.25, obtained by grafting caffeic acid onto CSC1.0, is less hydrophilic than CSC1.0 and showed poor cell attachment to UMR-106 cells. The cell attachment in the scaffold can be improved by calcification of the scaffold. The calcified chitosan/caffeic acid hybrid scaffold could be suitable for use in hard-tissue engineering.
References
Yoon K, Kim K, Wang X, Fang D, Hsiao BS, Chu B (2006) Polymer 47:2434–2441
Yang MC, Lin WC (2002) J Polym Res 9:135–140
Deemak P, Wanichwecharungruang S, Nonthabenjawan R, Jornjangjun C (2010) J Polym Res. 18:419–424. doi:10.1007/s10965-010-9432-2
Kim J, Cai Z, Lee HS, Choi GS, Lee DH, Jo C (2010) J Polym Res. doi:10.1007/s10965-010-9470-9
Elkholy SS, Khalil KD, Elsabee MZ (2010) J Polym Res. 18:459–467. doi:10.1007/s10965-010-9437-x
Wang Y, Yang JF (2010) J Polym Res 17:221–232
Enescu D, Hamciuc V, Pricop L, Hamaide T, Harabagiu V, Simionescu BC (2009) J Polym Res 16:73–80
Wu Y, Liu C, Zhao X, Xiang J (2008) J Polym Res 15:181
Mi FL, Shyu SS, Chen CT, Lai JY (2002) Polymer 43:757–765
Rutnakornpituk M, Ngamdee P, Phinyocheep P (2005) Polymer 46:9742–9752
Campoccia D, Montanaro L, Arciola CR (2006) Biomaterials 27:2331–2339
Engelsman AF, Van der Mei HC, Ploeg RJ, Busscher HJ (2007) Biomaterials 28:2314–2327
Park HK, Han DW, Park YH, Park JC (2005) Curr Appl Phys 5:449–452
Fujii H, Yokozawa T, Kim YA, Tohda C, Nonaka GI (2006) Biosci Biotech Bioch 70:2104–2111
Feng R, Ni HM, Wang SY, Tourkova IL, Shurin MR, Harada H, Yin XM (2007) J Biol Chem 282:13468–13476
Shiu JC, Ho MH, Yu SH, Chao AC, Su YR, Chen WJ, Chiang ZC, Yang WP (2010) Carbohydr Polym 79: 724–30
Idota N, Tsukahara T, Sato K, Okano T, Kitamori T (2009) Biomaterials 30:2095–2101
Wang W, Fan M, Li Z, Liu SH, Sun L, Wang, CY (2009) Biomed Mater 4(2):025011
Wang LY, Wang YJ, Cao DR (2009) J Macromol Sci 46:765–773
Hsu SC, Don TM, Chiu WY (2002) Polym Degrad Stab 75:73–83
Mochalova AE, Zaborshchikova NV, Knyazev AA, Smirnova LA, Izvozchikova VA, Medvedeva VV, Semchikov YuD (2006) Polym Sci Ser 48:918–923
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiang, ZC., Li, HY., Chao, AC. et al. Characterization of the morphology and hydrophilicity of chitosan/caffeic acid hybrid scaffolds. J Polym Res 18, 2205–2212 (2011). https://doi.org/10.1007/s10965-011-9631-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-011-9631-5