Abstract
To study the vanadium addition effects on the BSCCO superconducting system, bulk samples with the general formula Bi1.7Pb0.3V x Sr2Ca2Cu3O10 + δ (x = 0.0, 0.1, 0.2, 0.3 0.4 and 0.5) were prepared by solid-state reaction method. Energy-dispersive X-ray spectroscopy (EDX) analysis was used to test the proportions and energies of the elements of the compound. The XRD analyses showed an orthorhombic structure with two phases: a high-2223 phase and a low-2212 phase in addition to which an impure phase was found. The highest T c at 118 K was obtained for the sample with x = 0.2. Scanning electron microscopy (SEM) was used to identify the morphology of the superconducting phase and to investigate the influence of vanadium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The discovery of the Bi-Sr-Ca-Cu-O (BSCCO) superconducting system by Maeda [1] was considered to be very important for practical applications since it is relatively stable in atmospheric pressure and has a large chemical resistance against moisture. For these reasons, many researchers study this system. Yaroslavsk et al. [2] studied the effect of various doping elements, such as V, Y, As, Sb, Pb, Ag, and Ge, in the range of 3–5 % atom on the phase stability of the Bi-Sr-Ca-Cu-O system. The Bi-2212 phase was found a predominant phase in all doped samples. Nováková et al. [3] focused on the effect of the stoichiometric replacement of Pb and Bi by V in Bi a Pb b V c Sr4Ca5Cu7O x composition. The concentration of Bi, Pb, and V was altered according to the formula a + b + c = 4 (a = 2.6–3.6, b = 0–0.8, c = 0–0.6). The influence of the dopant on the samples’ properties was detected by X-ray diffraction (XRD) analysis, electrical resistivity, critical current density, and magnetic susceptibility. The increasing rate of V on Pb led to a decrease of the 2223 phase and to the deterioration of the superconducting properties.
Watanabe and Kojima [4] studied the addition effect of V2O5 on the high-superconducting materials of the Bi-Pb-Sr-Ca-Cu-O system (2223 phase) for ambient samples by Ar-7.7 % atmospheres. The d.c. electrical resistivity, critical current density, and the a.c. magnetic susceptibility were measured by the standard four-point probe technique and the inductance method. They found that the addition of a small amount of V2O5 decreased the magnetic shielding effect and enhanced slightly both the critical transition temperature and the current density.
Trivijitkasem et al. [5] reported the effect of a doped vanadium-lead in the Bi-Sr-Ca-Cu-O superconducting system. A series of initial nominal composition Bi1.75Pb0.25−x V x Sr2Ca2Cu3O y with x = 0, 0.025, 0.050, 0.075, and 0.1 were fabricated. Their results showed that both critical temperature and the 2223 phase formations were affected by vanadium and lead concentration. Pure (Bi and Pb) samples showed nearly uniform homogenous microstructure. On the other side, V-doped samples revealed a more porous inhomogeneous microstructure.
Darsono et al. [6] prepared the Bi1.6Pb0.4Sr2Ca2Cu3O7 superconducting system by a solid-state reaction using high-energy milling for the starting materials. The effects of the sintering temperature and the time on the phase evolution, microstructure, and superconducting properties were examined by thermogravimetric analysis, X-ray diffraction, scanning electron microscopy, and four-point probe resistivity measurements. High-energy milling decreased the precursor particle size to approximately 30 nm with a relatively uniform distribution of multi-compositions. The samples showed good superconducting properties with T c∼ 100 K.
This research is aimed at investigating the effect of the vanadium addition on the superconducting properties of Bi1.7Pb0.3V x Sr2Ca2Cu3O10 + δ samples that were prepared by solid-state reaction method.
2 Material and Method
A BSCCO sample with chemical composition Bi1.7Pb0.3 V x Sr2Ca2Cu3O10 + δ was prepared by solid-state reaction powders of Bi2O3, PbO, Sr(NO3)2, CaO, CuO, and V2O4 powders as the starting materials. The powders were mixed together using agate mortar. To get a homogeneous mixture, a sufficient quantity of 2-propanol was added to form a paste during the process of grinding for about 1 h. Later the mixture was calcined in air at 800 ∘C for 24 h. Then it was pressed into disk-shaped pellets 13 mm in diameter and 1–2 mm in thickness using a manual hydraulic press type (SPECAC) under pressure 0.7 GPa. The pellets were sintered in air at 835 ∘C for 140 h.
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) of type FEI-SEM model Inspect-S50 were used to provide compositional and surface morphology analysis of the samples. The structure of the prepared samples was obtained by using X-ray diffraction (XRD) (Philips) with CuK α source. The four-point probe method was used to measure the resistivity and to determine the critical temperature (T c).
3 Results and Discussion
Energy-dispersive X-ray spectroscopy (EDX) was used to analyze Bi1.7Pb0.3V x Sr2Ca2Cu3O10 + δ bulk samples that sintered at 835 ∘C for 140 h as shown in Fig. 1a–c. The spectrum illustrates the elemental distribution in the sample. The results in Fig. 1 demonstrate that the peaks are related to Bi, Pb, Sr, Ca, Cu, and V. Also, an extra peak of the carbon element is observed. This may be attributed to a thin carbon that evaporated on the sample during the synthesis process. A quantitative determination of the elements is not possible due to the strong peaks overlapping such as Bi and Pb which have the same energy values. This means that Pb atoms are successfully substituted into the Bi site.
In a comparison between samples a, b and c an addition of vanadium on the compounds was observed. The vanadium peaks appeared at 4.953 and 0.51 keV for the Bi1.7Pb0.3V0.4Sr2Ca2Cu3O10 + δ sample. Moreover, the peaks of other elements in these samples, such as Sr, Ca, and Cu, are still without any change. This is an excellent proof regarding the homogeneous distribution of elements in prepared samples.
Figure 2 represents the X-ray diffraction patterns of Bi1.7Pb0.3V x Sr2Ca2Cu3O10 + δ for x = 0, 0.1, 0.2, 0.3, 0.4, and 0.5. This figure demonstrates that the peak intensity decreases with the increasing of vanadium concentration for V-doped samples as comparable with the V-free samples. On the other side, the X-ray diffraction pattern for the V-doped samples shows an increasing in the reflection peak intensities when x changes from 0.1 to 0.2. However, most of these peaks are lowered with the increasing of vanadium concentration. The variation in the intensities could be related to the off stoichiometry of the lattice V 3+,5+ being substituted at Bi 3+ sites or may be held somewhere in the interstitial sites. Furthermore, off stoichiometry develops because of the difference of ionic radii of the substituted and the host atoms as indicated by Gul et al. [7]. The ionic radius of Bi 3+ is 0.96 Å while that of V 3+,5+ is 0.79 and 0.59 Å, respectively. The increasing of x leads to an increase in the resistance and the depression of the superconducting properties, in addition to the phase transformation from a high phase to a low phase which happens at x = 0.4. Another feature is observed that appears beyond H (0012) at 2 𝜃 = 28.8∘ for all samples which is necessary to prove the Bi-compound superconductivity.
Some reflection lines, such as H (0014), H (317) H (2210), and H (319), are lost for the composition with x = 0.4 and 0.5. For this reason, we suggested increasing the dopant concentration which allows the formation of the low phases such as Bi-2201 and 2212; the stability of Bi-2223 phase appears to be altered as indicated by Rodrigues et al. [8]. In addition to the above reflection lines, a new peak attributed to the Bi-2223 phase, such as H (115), appeared for the sample at x = 0.2 and 0.3. Table 1 shows a decreasing of the lattice parameters a and c for the V-addition samples as comparable with the V-free samples. Indeed, this behavior agreed with Novakova et al. [3] who found a decreasing in the lattice parameter c from 37.173? to 37.079 Å when the amount of V increased from 0 to 0.6 in Bi-based superconductors. It is well known that lattice parameter a is controlled by the length of the in-plane Cu-O bond [9]. The length may be expanded or contracted with the change of the electrons into the antibonding orbital. A non-systematic variation of the lattice parameters is observed with the increasing of the vanadium concentration. The deformation in the lattice constant as a result of the addition or deficiency of some atoms to adjust the amount of charge transfer from Bi layer to Cu layer will be a driving force for the pair generation of the superconductor. Indeed, the behavior of the lattice constant may be attributed to the incorporation of V ions into interstitial sites in the unit cell or on the Bi-sites as indicated in the previous item.
A higher volume fraction of Bi-2223 was found for the Bi1.7Pb0.3Sr2V0.2Ca2Cu3O10 + δ composition. An enhancement of the 2223 phase indicates the catalytic action of vanadium, which is the same as to Pb. This could be explained as follows: The formation of the 2223 phase in the pure Bi samples is a very slow process. However, a rapid growth via the improved mobility of the ions can be catalyzed through the formation of a liquid phase, which is believed to be Ca2PbO δ , Sr2PbO δ , or an eutectic of 2201 and Ca2PbO δ . Partial melting of V-doped samples for low dopant concentration and the melting of these at higher dopant concentration suggests the addition of vanadium further extends the range of the liquid phase (as we show in the SEM item). Thus, increasing the possibility of occupying the substituent’s positions (interstitial or substitutional sites) in the crystal structure may get the predicator out if subjected to prolonged heat treatment. Now, if the addition is helpful in the formation of the CuO2 layer, it is expected that the mobility of ions will just favor the formation of 2223 as the dominant phase [10].
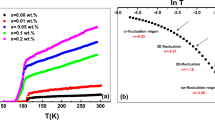
Electrical resistivity ρ (T) versus temperature for the pure and V-addition samples is shown in Fig. 3. It is clear from this figure that the resistivity for most samples decreases slowly with decreasing temperature and then drops to zero. The critical transition temperature (T c) improves with the initial increase in the vanadium content up to 0.2 and then decreases for x ≥ 0.3 as seen in Table 1, whereas the system goes from the optimally doped regime to an over-doped regime. These results are in agreement with those data obtained from the XRD spectra, which confirmed the increasing volume fraction of the Bi-2223 phase with an increasing amount of vanadium.
The reason of the vanadium addition to the Bi system is to fill the porosities, which is occurring due to the melting of some Bi atoms during synthesis. Bi melts at 818 ∘C and leaves the undesirable porosities in the lattice. In addition, the Bi-2223 crystal structure involves very weak coupling in the Ca-CuO2-Ca plane. During synthesis, the coordination between Cu and O atoms is destroyed and reveals an unstable (2223) phase. Substituting high-valence cations like V reveals the diffusing of oxygen into the lattice. In this way, the coupling between Ca and CuO2 will be stronger and generates a more stable (2223) phase [11].
The surface of the prepared samples is imaged by scanning electron microscopy (SEM) method. Figure 4a–e depicts the SEM pictures of the fractured surface of Bi1.7Pb0.3V x Sr2Ca2Cu3O10 + δ (0≤x≤0.5) samples that sintered at 835 ∘C for 140 h. All samples show the typical irregular structure with grain size from 5–15 μm for pure samples to 25–30 μm for samples with x = 0.2 that were accompanied by pores and voids. Moreover, the texturing needle-like grain clump of whisker and layered grain growth are found in most cases in Fig. 4b–e.
The grain morphology of the pure sample demonstrates the flaky grains with a uniform sharp edge and different size of stacks of plate-like grain which belongs to the high- T cphase. Each of these stacks is composed of thousands of layers; apparently, each grain (group of layers) grows in random direction. Some of the plates grow one through the other in different directions and create bigger grains. Figure 4b shows that the structure is composed of two types of particle features: irregular grains and spherical grains, and the latter may be attributed to the vanadium addition (the spherical grains here are more obvious). These different features reveal the fact of the formation of two phases (2212 and 2223).
The best structure regarding the porosity and homogeneity of the material is that for Bi1.7Pb0.3V0.2Sr2Ca2Cu3O10 + δ samples. The reaction that takes place during the sintering of this ratio favors the growth of the 2223 phase with improvement of the density of the material. Also, we note good connectivity between grains, less porosity, and high growth (continuous microstructure). Good connectivity forms bigger grains. The T c for this sample was higher than that of the other samples. It is obvious that the samples with x = 0.3 and x = 0.4 have a glassy phase (liquid phase) as a dominant phase besides the solid phase. The liquid phase covers the original structure. Also, a microcrack is observed in these samples. This may relate to the increasing of the sintering temperature close to the melting point.
The liquid phase effectively increases the contact area between grains (the plate-like structure is eliminated). In our opinion, the occurrence of partial melting on the surface causes the microstructure to coalescence, thus leading to a decrease of the pores and an increase in density.
From the above results, we can conclude that vanadium addition may promote the formation of oxide liquid during the sintering partly due to the melting temperature of the vanadium of about 690 ∘C [12]. Consequently, vanadium addition will reduce the sintering temperature since the liquid phase is found in the doped samples.
4 Conclusion
XRD analyses showed the orthorhombic structure of all the samples had at least two superconducting phases. The maximum transition temperature was 115 K with a higher volume fraction 70 % of Bi-2223 which was found for the composition at x = 0.2. Also, SEM micrographs confirmed that vanadium addition for the Bi1.7Pb0.3Sr2V x Ca2Cu3O10 + δ compounds promoted the formation of oxide liquid during the sintering process.
References
Maeda, H., Tanaka, Y., Fukutomi, M., Asano, T.: Jpn. J. Appl. Phys. 27(2), L 209 (1988)
Yaroslavsky, Y., Schieber, M., Beilin, V., Litvin, S., Burtman, V., Cinodman, V., Shaltiel, D.: J. Physica C 209(1–3), 179–182 (1993)
Nováková, K., Smrcková, O., Sýkorová, D., Vasek, P.: J. Supercond. Sci. Technol. 9(9) (1996)
Watanabe, K., Kojima, M.: Supercond. Sci. Technol. 11(4) (1998)
Trivijitkasem, S., Kluenrat, C., Pumchuchit, V.: Kasetsart J. (Nat. Sci.) 40, 28–34 (2006)
Darsono, N., Imaduddin, A., Raju, K., Yoon, D.-H.: J. Supercond. Nov. Magn. 28(8), 2259–2266 (2015)
Gul, I.H., Rrehman, M.A., Maqsood, M.A.: Phys C: Superconductivity 432, 71–80 (2005)
Rodrigues1, V.D., de Souza, G.A., de Lima, R.G., Carvalho, C.L., Zadorosny, R.: Univ Estadual Paulista - UNESP, Department of Physics and Chemistry, 15385-000 IlhaSolteira-SP, Brazil
Sarun, P.M., Vinu, S., Shabna, R., Biju, A., Syamaprasad, U.: Mater. Res. Bull. 44, 1017 (2009)
Mishra, D.R., Sharma, S.V., Sharma, R.G.: PRAMANA J. Phys. 54(2), 317–330 (2000)
Yazici, N., Erdem, M., Ozcelik, B.: J. Supercond. Nov. Magn. 25, 725–729 (2012)
Kim, B., Bohandy, J., Phillips, T., Green, W., Agostinelli, E., Adrian, F.: Appl. Phys. Lett. 53(4), 321–323 (1988)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermiz, G.Y., Jassim, A.K. & Alwan, E.K. Vanadium Addition Effect on the Superconducting Properties of BPSCCO System. J Supercond Nov Magn 29, 2003–2009 (2016). https://doi.org/10.1007/s10948-016-3496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3496-0