Abstract
In this work, we report synthesis and characterization of the new La2SrCo2FeO9 triple perovskite material. The samples were produced by the solid-state reaction method. The analyses of the XRD patterns were made by Rietveld refinement through the GSAS code. The results reveal that the material crystallizes in an orthorhombic complex perovskite, space group Immm (#71) with lattice parameters a=5.4314(3) Ǻ, b=5.4583(3) Ǻ and c=7.7018(2) Ǻ. SEM micrographs evidence a strongly diffused granular morphology with mean grain size of 2 μm and the EDX spectra show that the chemical composition of samples are in good agreement with the nominal values of the stoichiometric formula. 57Fe Mössbauer spectroscopy recorded at 300 K reveals two sites in concordance with the X-ray diffraction measurements, and its valence state is +3, as determined from the isomer shift found. At 4.2 K magnetic ordering with canting of the Fe moments is found. Measurements of the magnetization as a function of temperature permitted us to determine the ferromagnetic characteristic of material with an effective magnetic moment of 9.7μ B.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The perovskite oxide materials have been extensively investigated in the last years, because small structural distortions, vacancies and compositional modifications can induce a great variety of physical and chemical properties [1]. When the ideal formula ABO3 is changed to introduce different types of A and B cations on the octahedral site of the primitive unit cell, the cationic ordering leads to complex perovskites [2]. One of these is known as triple perovskite, which is identified by the A2A′B2B′O9 formula. Then, it is possible to produce new materials by the introduction of an alkaline or rare-earth ion in the A, A′ sites and transition metal ions in the B, B′. Depending on the magnetic or electric characteristic of B and B′, it is relatively easy to create new perovskite systems with half-metallic properties [3, 4], magneto-electric response [5] or magnetic ordering [6], which offer promising perspectives in the recent spintronics technology [7].
In order to analyze the possibility to create new magnetic perovskite materials, in this work we report the synthesis, the structural characterization, magnetic studies from magnetization measurements and magnetic and structural properties from 57Fe Mössbauer spectroscopy, of the new ferromagnetic triple perovskite La2SrCo2FeO9, which was idealized as the introduction of a lanthanide ion in the A site, an alkaline earth in the A′ and a mixture of two magnetic transition metals in the B and B′ sites of A2A′B2B′O9 formula, to construct a triple complex perovskite.
2 Experimental
The samples were synthesized by the solid-state reaction recipe. The precursor powders La2O3, SrCO3, Co2O3 and Fe3O4 (Aldrich 99.9%) were mixed in stoichiometric proportions according to the chemical formula La2SrCo2FeO9. Mixture was ground to form a pellet and annealed at 1250 °C for 12 hours. The samples were then reground, repelletized and sintered at 1250 °C for 24 hours and 1350 °C for 24 hours. X-ray diffraction (XRD) experiment was performed by means a PW1710 diffractometer with λ CuKα =1.54064 Å. Rietveld refinement of the diffraction pattern was made by the GSAS code [8]. Scanning Electron Microscopy (SEM) images were obtained by using a FEI QUANTA 200 microscopy, which has a system for Energy Dispersive X-ray (EDX) analysis. Field cooling measurements of susceptibility as a function of temperature and magnetization as a function of applied field were carried out by using a MPMS Quantum Design SQUID. 57Fe Mössbauer spectra were taken at room temperature and at 4.2 K using a 50 mCi 57Co:Rh source in transmission configuration, kept at the same temperature of the sample, in sinusoidal movement. The isomer shift is relative to that of α-Fe.
3 Results and Discussion
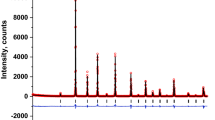
The analysis of XRD pattern showed in Fig. 1 reveals the presence of characteristic peaks of complex perovskite systems. In Fig. 1, crosses represent the experimental data and the line corresponds to simulated pattern by means of GSAS code. The base line is the difference between theoretical and experimental results.
Rietveld refinement permitted to establish that this material crystallizes in a orthorhombic triple perovskite with space group Immm (#71) and lattice constants are a=5.4314(3) Ǻ, b=5.4583(3) Ǻ and c=7.7018(2) Ǻ. These results are 99.78%, in agreement with the theoretical values obtained from the Structure Prediction Diagnostic Software SPuDS [9], which predicts that lattice constants a=5.458(8) Ǻ, b=5.427(1) Ǻ and c=7.688(9) Ǻ for the La2SrCo2FeO9 material.
The parameters of the refinement are: \(R_{\mathrm{F}}^{2}=2.67\%\); x 2=1.313; R WP=3.82% and R P=3.20%. The numeric results of atomic positions and occupancy obtained from the Rietveld analysis are shown in Table 1.
One important parameter in perovskite materials is the tolerance factor, which is related to the probability of formation of octahedral coordination of B and B′ cations with the oxygen anion. The tolerance factor calculated for the La2SrCo2FeO9 complex perovskite was 0.982, which is characteristic of perovskite-like lattices distorted from the ideal cubic structure of triple A2A′B2B′O9. From the Rietveld refinement the values for the bond distances and positions of cations relative to the neighbor ions were obtained (Table 2).
The surface morphology of La2SrCo2FeO9 samples was studied by SEM images as shown in Fig. 2. The analysis performed reveals the occurrence of granular topology with different grain sizes. As observed in the microphotography, grains are strongly diffused between them, but great empty spaces appear too. It is important to notice that the sample evidences a single type of grain. From the EDX semi-quantitative analysis, which considers the spectrum areas for each element showed in Fig. 3, we obtain the experimental composition of material. These values were compared with the theoretical composition, calculated from the La2SrCo2FeO9 stoichiometric formula. Results are presented in Table 3.
The results of Fig. 3 and Table 3 corroborate with 97% that there exists a single phase, which corresponds to the stoichiometry of La2SrCo2FeO9. From structural, morphologic and compositional characterizations we deduced that no other crystallographic phases or impurities are present in the samples.
The 57Fe Mössbauer spectra were fitted through the NORMOS software, using the Full Hamiltonian Site analysis. The spectrum taken at room temperature revealed only an absorption peak, which indicates that there are no impurities, as was shown through X-ray diffraction measurements. This absorption peak, however, is somewhat broad and asymmetric, indicating different environments for the Fe atom in the lattice. We fitted this absorption peak with two quadrupole doublets, keeping fixed the line width to Γ=0.251 mm/s, and we found the hyperfine parameters shown in Table 4.
The proportion in area for each site is roughly 50%, each site relative to each crystallographic site found through the Rietveld refinement; the difference in occupancy for Fe in each crystallographic site can be reflected in the quadrupole splitting for each site, minor differences in occupancy can represent a minor change in the electric field gradient that interacts with the Fe quadrupole moment in its nuclear excited state (I=3/2). The isomer shift is roughly the same for both sites, indicating same valence. These values reflect most likely a high spin Fe3+ valence, compatible with the fact that the Sr2+ doping on La3+ site will increase the volume of the lattice, being crystallographically more favorable a high spin Fe ion configuration. A mixed valence for Fe should have been expected, since the substitution in the perovskite A site would lead to charge imbalance [10], but the combination of Fe and Co atoms in the B site compensates it. This means that we must take +3.5 for the valence of Co atoms, which means Co3+ and a Co4+ ions.
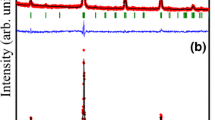
In Fig. 4 the Mössbauer spectrum of La2SrCo2FeO9 at 4.2 K reveals a well developed magnetic sextet, indicating magnetic ordering at lower temperatures, probably due to localization of moments and interaction via exchange mechanism.
This spectrum was fitted with two magnetic sites, equivalent to the sites found previously for RT spectrum. The values of the isomer shift and quadrupole splitting are different from the RT values due to thermal effects. No structural phase transition is evident, since there is not found a remarkably high variation in the quadrupole interaction eQVZZ/2, the energy term in the Hamiltonian that depends on lattice and electronic characteristics of the Fe environment. The line widths for these sites are somewhat broad (Γ=0.481 mm/s), probably related to small lattice inhomogeneities that could lead to inhomogeneous magnetic ordering or slightly inequivalent sites. The magnitude of the hyperfine fields confirms that Fe is in +3 valence state. The difference in magnetic hyperfine fields for the sites fitted is 3 T, as can be inferred from Table 4. We take the main component of the electric field gradient at the Fe sites, VZZ, to be in the c crystallographic direction. The angle that makes the magnetic hyperfine field with c axis within this model is close to 60 degrees for both sites, confirming an inhomogeneous alignment of the Fe moments. These results can be understood as follows: the Fe moments are canted with respect to c crystallographic axis, probably due to the interaction with Co moments. The approximate magnetic moment per Fe for each site is 3.40μ B and 3.22μ B, with an average magnetic moment of 3.31μ B.
The magnetic properties of La2SrCo2FeO9 have been investigated by measuring the DC susceptibility in the temperature range from 2 to 300 K and at an applied magnetic field of 50 Oe. Figure 5 shows the dependence of the susceptibility on temperature for La2SrCo2FeO9 when it is measured using the Zero Field Cooling and Field Cooling recipes.
The fitting susceptibility as a function of temperature was made by the Curie–Weiss equation,
which had permitted us to establish the ferromagnetic character of material with a Curie temperature T C=123.3 K, a Curie constant C=11.7438 emu⋅K/mol and a susceptibility independent of temperature of χ 0=2.56×10−2 emu/mol. From the Curie constant we obtain the effective magnetic moment of material μ eff=9.7μ B. Theoretical calculations by the Hund rule, with \(P_{\mathrm{eff}} = g\sqrt{J(J + 1)}\), predict that magnetic moments of the isolated ions Co4+ and Fe2+ must be \(\mu_{\mathrm{Co}}^{4+}=3.87\mu_{\mathrm{B}}\) and \(\mu_{\mathrm{Fe}}^{2+}=5.5\mu_{\mathrm{B}}\), respectively [10]. The effective magnetic moment of La2SrCo2FeO9 is obtained to be μ eff=9.33μ B, where we have used \(\mu_{\mathrm{eff}} = \sqrt{\mu_{\mathrm{Co}^{4 +}} ^{2} +\mu _{\mathrm{Fe}^{2 +}} ^{2}}\), by considering two Co4+ cations in the crystallographic unit cell. This result corresponds to 96% agreement between experimental and theoretical values.
In order to verify the ferromagnetic behavior of the La2SrCo2FeO9 triple perovskite, in Fig. 6 we show the curve of 1/χ as a function of temperature. In the picture, the value of T C is corroborated by the extrapolation of the high temperature linear-like behavior on the temperature axis.
4 Conclusions
The synthesis and structural characterization of the new La2SrCo2FeO9 perovskite-like material was performed. The Rietveld analyses reveal that this material crystallizes in an orthorhombic complex perovskite which corresponds to Immm (#71) space group. 57Fe Mössbauer spectroscopy revealed two sites for iron, both with valence +3 for Fe, and magnetic ordering at 4.2 K. Measurements of the magnetic susceptibility showed a ferromagnetic ordering transition for T C=123.3 K, which was fitted by the Curie–Weiss model. From the fitting we obtain the magnetic moment of the unit cell to be μ=9.7μ B.
References
Hazen, R.M.: Sci. Am. 258, 54 (1988)
Ortiz Diaz, O., Jayro Arbey Rodriguez, M., Fajardo, F., Landínez Téllez, D.A., Roa-Rojas, J.: Physica B 398, 248 (2007)
Philips, J.B., Majewski, P., Alff, L., Gross, R., Graf, T., Branst, M.S., Simon, J., Walther, T., Mader, W., Topwal, D., Sarma, D.D.: Phys. Rev. B 68, 144431 (2003)
Palkar, V.R., Malik, S.K.: Solid State Commun. 134, 783 (2005)
Lofland, S.E., Scabarozi, T., Moritomo, Y., Xu, Sh.: J. Magn. Magn. Mater. 260, 181 (2003)
Di Trolio, A., Larciprete, R., Testa, A.M., Fiorani, D., Imperatori, P., Turchini, S., Zema, N.: J. Appl. Phys. 100, 13907 (2006)
Larson, A.C., Von Dreele, R.B.: General Structure Analysis System (GSAS). Los Alamos National Laboratory Report LAUR, pp. 86–748 (2000)
Lufaso, M.W., Woodward, P.M.: Prediction of the crystal structures of perovskites using the software program spuds. Acta Crystallogr., B Struct. Crystallogr. Cryst. Chem. 57, 725 (2001)
Neov, L., Dabrowski Hofmann, M., Bouwmeester, H.J.M.: Appl. Phys. A 74, S664 (2002)
Kittel, C.: Introduction to Solid State Physics, 8th edn. University of California, Berkeley (2005)
Acknowledgements
This work was partially supported by Division of Investigations (National University of Colombia, Bogotá DC) and Brazilian agency CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albornoz, J.C., Landínez Téllez, D.A., Roa-Rojas, J. et al. Structural and Magnetic Properties of the New La2SrCo2FeO9 Triple Perovskite. J Supercond Nov Magn 26, 2313–2317 (2013). https://doi.org/10.1007/s10948-012-1425-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1425-4