Abstract
Cancers are a great threat to humans. In cancer therapy, surgical removal of the tumor combined with radiotherapy and chemotherapy is the most routine treatment procedure and usually the most effective. However, radiotherapy and chemotherapy drugs that kill cancer cells efficiently also kill normal cells, thus exhibiting large side effects. Cancer-targeted drugs, which aim to specifically recognize proteins or signaling pathways associated with tumor proliferation and migration, have achieved marked progress in recent years. Azurin is a copper-containing redox protein secreted by Pseudomonas aeruginosa. Azurin and its derived peptide p28 preferentially enter a variety of cancer cells and induce apoptosis or cell cycle arrest. Mechanistic studies revealed that azurin and p28 target the p53 and receptor tyrosine kinase signaling pathways as well as other pathways. Two phase I trials of p28 have been carried out, with findings that p28 is safe and exhibits anticancer activity in both adult and pediatric patients. In this review paper, we provide an up-to-date summary of progress on the anticancer mechanisms and therapeutic strategies for azurin and p28.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Malignant tumors (cancers) are a great threat to humans, and comprise the second leading cause of death after cardiovascular disease. Cancers and their complications greatly reduce the quality of life of patients and their families and increase medical and healthcare spending. Therefore, it is of much value to explore efficient anticancer drugs. For cancer treatment, surgical resection of the tumor combined with radiotherapy and chemotherapy is currently the most common treatment strategy, and often the most effective. However, traditional radiotherapy and chemotherapy drugs kill both cancer cells and normal cells simultaneously, and often show strong toxicity and side effects. With the aim of specifically recognizing proteins or signal transduction pathways related to tumor proliferation and migration, cancer-targeted drugs, which are expected to achieve the dual goals of tumor treatment and reduced side effects, have undergone rapid development in recent years [1,2,3,4,5,6].
Azurin is a copper-containing redox protein secreted by Pseudomonas aeruginosa [7]. Based on their ability for selective entry into and induction of apoptosis or cell cycle arrest in many cancer cells, azurin and its derived peptide p28 have attracted much attention in the last two decades [8,9,10,11,12,13,14,15,16]. At present, two phase I clinical trials of p28 have been completed, with findings that p28 is safe and exhibits anticancer activity in both adult and pediatric patients [17, 18]. Because azurin and p28 target many different signaling pathways [19], such as the p53 and receptor tyrosine kinase pathways, azurin and p28 may not easily induce resistance and have the potential to become new anticancer drugs. In this review paper, we summarize recent progress on the anticancer mechanisms and therapeutic strategies for azurin and p28.
2 Properties of Azurin and p28
2.1 Amino Acid Sequences and Structures
Azurin (UniProt ID P00282) is a copper-containing redox protein secreted by P. aeruginosa [7] that contains 128 amino acids and a copper ion (Fig. 1). According to the SCOP classification, the three-dimensional structure of azurin belongs to the all-β folding class, which mainly comprises two groups of β-strands (4 strands per group) arranged in a sandwich structure (Fig. 1b) [20]. p28 is a fragment (Leu50-Asp77) of the azurin protein (Fig. 1a), encompassing a β-strand, an α-helix, a turn, and an irregular structure (Fig. 1b). It is worth noting that the structure of the p28 segment is separated from the sandwich structure of azurin. Although p28 is folded into a stable three-dimensional structure within azurin, it does not mean that the p28 fragment forms the same structure as an isolated peptide. In fact, molecular dynamics simulations showed that the α-helix of p28 was unstable after isolation [21, 22].
Amino acid sequences and three-dimensional structures of azurin and p28. a Amino acid sequence of azurin. The region corresponding to p28 is shown in orange. b Three-dimensional structure of azurin (PDB ID 2xv2). The region corresponding to p28 is shown in orange. The copper ion is shown as a purple sphere (Color figure online)
2.2 Preferential Entry into Cancer Cells
Azurin preferentially enters a variety of human cancer cells [23]. Truncation experiments revealed that the region of azurin mediating its penetration through the cell membrane is mainly composed of amino acids 50–77 (termed p28). Furthermore, fusion of p28 to cargo proteins, such as glutathione S-transferase and green fluorescent protein, enabled internalization of cargo proteins into macrophages, melanoma cells, or breast cancer cells [23]. Competition experiments and studies with inhibitors suggested that azurin may enter cells by a receptor-mediated endocytic process involving caveolin-1, the Golgi complex, and ganglioside GM-1 [23,24,25]. The membrane receptors that mediate azurin or p28 internalization often show higher expression levels in cancer cells compared with normal cells. Therefore, relative to normal cells, the contents of p28 in tumor cells are about 3–6-fold higher [24]. Although p28 is the main segment that mediates azurin cell penetration, hydrophobic residues adjacent to the p28 segment in space may also be involved. For example, alanine mutation of Phe114 significantly reduced the cell-penetrating activity of azurin [25].
2.3 Inhibition of Cancer Cell Proliferation and Tumor Growth
Besides cell membrane penetration, azurin and p28 can inhibit proliferation or induce apoptosis in various cancer cell lines (Table 1). For example, only 29% of MCF-7 breast cancer cells survived after treatment with 53 μM azurin for 72 h [26] and the cell number of ZR-75-1 breast cancer cells was reduced by 44% after treatment with 100 μM p28 for 72 h [27]. Many azurin/p28-sensitive cancer cells express p53 protein and the levels of p53 protein are elevated after treatment with azurin or p28. On the contrary, azurin or p28 do not effectively induce apoptosis or cell cycle arrest in control p53-null cells [22, 26,27,28,29,30,31,32]. Therefore, azurin and p28 mainly inhibit cell proliferation or induce apoptosis through the p53 signaling pathway. The p53 protein is a transcription factor that plays key roles in mediation of DNA damage repair, apoptosis, and cell cycle progression through transcriptional regulation of downstream gene expression [33, 34]. It was demonstrated that azurin/p28-stabilized p53 enters the nucleus and induces expression of proapoptotic genes like Bax and Bcl-2 [26, 32] and cell cycle inhibitors like p21 and p27 [27]. Meanwhile, studies on mouse models, including MCF-7 breast tumor mice, 4T1 breast tumor mice, and Dalton’s lymphoma mice, showed that azurin and p28 can efficiently inhibit tumor growth [27, 35, 36].
3 Mechanism of the Anticancer Action of Azurin
3.1 Regulation of Redox Homeostasis
Reactive oxygen species (ROS), such as superoxide (O2−), hydroxyl radical (HO·), and hydrogen peroxide (H2O2), mediate redox signaling for numerous cellular functions [37]. High levels of ROS in cells usually cause cell death [38, 39]. Therefore, modulation of ROS levels may provide strategies for cancer treatment. Because azurin is a redox protein, treatment of macrophages with azurin generates a higher level of ROS. However, the cytotoxicity of azurin is not related to its redox activity because several redox-negative azurin mutants also generated ROS and induced macrophage apoptosis [40, 41]. Mechanistic analyses revealed that azurin and its mutants form complexes with p53 and increase its protein level, suggesting that azurin regulates redox homeostasis by a p53-mediated mechanism [40,41,42].
3.2 Stabilization of p53 Protein
Much evidence has indicated that the anticancer activity of azurin depends on the presence of p53 protein. For example, azurin readily induces apoptosis in cancer cells expressing functional p53, but to a much lesser extent in p53-null cells [26, 32]. The p53 protein levels in cancer cells are elevated, suggesting that azurin functions through the p53 pathway. MDM2 is a major E3 ubiquitin ligase that regulates p53 degradation by binding to its N-terminal transactivation domain (p53-TAD) [43]. Although azurin interacts with p53-TAD, the estimated dissociation constant of azurin/p53-TAD complex (Kd ~ 7 μM) is much larger than that of MDM2/p53-TAD complex (Kd ~ 34 nM) [44, 45]. Furthermore, azurin, MDM2, and p53 can form a ternary complex [46]. Thus, azurin is unable to inhibit MDM2 binding to p53-TAD. The interactions between azurin and the p53 DNA-binding domain (p53-DBD) were also characterized by molecular docking and Raman spectroscopy, revealing that azurin can bind to the flexible L1 and s7–s8 loops of p53-DBD and increase its structural stability [47,48,49]. The structural stability of p53-DBD may be related to the protein level and anticancer function of p53.
3.3 Modulation of Cell Membrane Properties
Cell-surface receptors regulate the structural properties of the cell membrane by modulating cell attachment to the surrounding extracellular matrix, cell shape, cell migration, and membrane stiffness [50]. Therefore, the activity of anticancer drugs is related to the expression level of these cell-surface receptors. Bernardes et al. [51] found that azurin reduces the expression level of integrin β1 and disturbs its distribution on the cell membrane of A549 lung cancer cells. Recently, azurin was found to decrease the level of caveolin-1 and the order of the cell membrane in MCF-7 breast cancer cells and HeLa cervical cancer cells [25]. Direct physical interactions between caveolin-1 and azurin were confirmed by immunoprecipitation [25]. Changes to the surface structure of cancer cells by azurin treatment render the cells more vulnerable to anticancer drugs, such as epidermal growth factor receptor-specific inhibitors (e.g., gefitinib, erlotinib) and chemotherapeutic drugs (e.g., paclitaxel, doxorubicin) [25, 51]. Cadherins are crucial molecules that regulate cell–cell adhesion, and overexpression of P-cadherin is associated with increased cell invasion in many breast cancer cells [52]. Bernardes and colleagues showed that azurin decreases P-cadherin expression and inhibits P-cadherin-induced cell invasion [53, 54]. The modulation of cell membrane properties by azurin may also be associated with the intracellular signaling responses of non-receptor tyrosine kinases, because the phosphorylation levels of FAK, Src, Akt, and PI3K are usually attenuated [51, 53].
3.4 Interference with the Eph-Ephrin Pathway
Eph receptors comprise the largest family of receptor tyrosine kinases. The signaling pathways between Eph receptors and their ephrin ligands are known to be involved in cancer progression [55]. Therefore, Eph receptors are potential targets for cancer therapy [56,57,58,59]. Azurin shows remarkable structure similarity to ephrins and binds to EphB2 with an affinity (Kd = 6 nM) that is 5-fold higher than the affinity of ephrinB2 for EphB2 (Kd = 30 nM) [60]. Consequently, azurin efficiently competes with ephrinB2 for binding to EphB2 and interferes with tyrosine phosphorylation of EphB2. Truncation experiments identified a C-terminal segment (amino acids 88–113) of azurin that mediates the interactions between azurin and EphB2. Further experiments showed that azurin peptide (88–113) treatment leads to reduced cell viability in LN-229 glioblastoma cells and growth inhibition in MCF-7 breast cancer cells [60].
4 Mechanism of the Anticancer Action of p28
4.1 Stabilization of p53 Protein
The anticancer activity of p28 is also dependent on the p53 status in cancer cells [27, 28]. Similar to azurin, p28 does not compete with MDM2 for binding to p53 [27]. However, Yamada et al. [22] found that p28 interacts with p53-DBD. Utilizing a variety of biophysical characterization methods, the dissociation constant for p28/p53-DBD complex was reported to range from 7 μM to 0.7 nM [61,62,63]. p53-DBD is a hub domain that interacts with COP1, an E3 ubiquitin ligase that negatively regulates p53 and is overexpressed in many cancers [64,65,66,67]. GST pull-down experiments showed that p28 reduces COP1 binding to p53-DBD in a concentration-dependent manner, suggesting that p28 competes with COP1 for binding to p53-DBD [22]. Fluorescence resonance energy transfer-derived distance information was used to guide molecular docking and molecular dynamics simulations. In the simulated models, p28 binds to a pocket adjacent to Trp146 on p53-DBD [61], confirming that p28 competes with COP1 for binding to p53-DBD [22]. Recently, the dissociation constant for p53/COP1 complex measured by atomic force microscopy and surface plasmon resonance was determined as approximately 10 nM [68], and is thus in a comparable range to the Kd value of p28/p53-DBD complex. Therefore, one possible mechanism for the stabilization of p53 by p28 is through the COP1-mediated ubiquitination pathway, wherein p28 inhibits COP1 binding to p53-DBD. Besides wild-type p53, p28 also binds to p53 mutants and activates p53 mutants in a series of cancer cell lines [30, 63]. The affinity of p28 for p53 mutants was found to be positively correlated with the β-sheet content and negatively correlated with the random coil content of p53 [63]. Rational designs of p28 can be performed to achieve high affinity for various p53 mutants.
4.2 Inhibition of Angiogenesis
Vascular endothelial growth factor (VEGF) is a type of cytokine that promotes angiogenesis, and is often overexpressed in solid tumors or blood cancers [69]. The interaction between VEGFA and its receptor VEGFR-2 is a key regulator of angiogenesis in tumors [70]. Mehta et al. [71] found that p28 inhibits VEGF-induced migration, capillary tube formation, and neoangiogenesis in multiple xenograft models. Although p28 penetrates human umbilical vein endothelial cells and co-localizes with VEGFR-2, unlike other antiangiogenic agents that inhibit VEGFR-2 kinase activity, p28 decreases downstream phosphorylation of FAK and Akt, resulting in abnormal distribution of cell migration-related proteins, such as F-actin, paxillin, and PECAM-1 [71]. Therefore, p28 may inhibit angiogenesis in a different manner from other antiangiogenic agents. Further studies on the interactions between p28 and VEGFR-2 will be valuable to clarify the antiangiogenic effect of p28 on endothelial cells. Inhibition of angiogenesis suppresses tumor growth in Mel-6 (p53 null) melanoma cell xenografts in athymic mice [71], while p28 has little effect on proliferation of these melanoma cells [30].
5 Therapeutic Strategies Based on Azurin and p28
Azurin and p28 preferentially enter cancer cells, and subsequently induce apoptosis or cell cycle arrest, or inhibit angiogenesis in tumors. Based on these anticancer activities, several therapeutic strategies have been designed.
5.1 Therapeutic Drugs
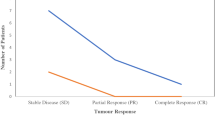
Azurin and p28 exhibit anticancer activity that has been verified in various cancer cells and mouse-based tumor models. Thus, azurin and p28 can be directly applied as anticancer drugs. Escherichia coli Nissle 1917 has been extensively used to treat acute diarrhea and possesses tumor-targeting activity [72]. Continuous expression of azurin in Nissle 1917 was shown to enable efficient inhibition of B16 melanoma and 4T1 breast tumor growth in mouse models [36]. Simultaneous expression of azurin with other anticancer agents has also been examined. For example, Ghasemi‑Dehkordi et al. [73] designed a vector to express azurin and Mammaglobin‑A and induce immune responses against breast cancer tumors, while Mehta et al. [74] designed a bacterial carrier that simultaneously expresses azurin and p53 under the control of a hypoxic promoter. Recently, two phase I clinical trials of p28 have been completed, with findings that confirm the anticancer activity and safety of p28 in human cancer patients. One clinical trial was carried out in 15 adult patients with metastatic solid tumors [17]. After p28 treatment, seven patients demonstrated stable disease for 7–61 weeks, three showed partial response, and one showed complete response. The other clinical trial was performed in children with brain tumors [18]. The results showed that p28 is safe and well tolerated, although its activity is not very high for central nervous system malignancies.
5.2 Cancer-Targeted Drug Carriers
The preferential entry of azurin and p28 into cancer cells enables them to function as cancer-targeted drug carriers. Azurin and p28 have been fused with several anticancer proteins/peptides to increase their activity. Granzyme B is released by the immune system and activates pro-apoptotic pathways. Paydarnia et al. [75] designed a granzyme B-azurin fusion protein and showed that the resulting protein induces significant apoptosis in several breast cancer cell lines. Upon fusion with p28, the NRC peptide and apoptin show higher anticancer activities toward breast cancer cells [76, 77]. Shahbazi et al. [78] fused HPV16 E7 protein with p28, and demonstrated that the resulting fusion protein efficiently targets cervical cancer cells and exerts immune activity. Furthermore, conjugation of the C-terminus of azurin to radiotherapy drugs enables ephrin receptor-targeted delivery, thereby improving the efficacy of radiation treatment for cancers overexpressing ephrin receptors [79]. p28 can also be conjugated to other cargos, such as liposomes and nanoparticles, enabling cancer-targeted drug delivery and release [5, 14].
5.3 Anticancer Drug Sensitizers
Besides directly inducing apoptosis and growth inhibition of cancer cells, azurin and p28 also disturb the membrane structure and inhibit cell migration, thus enhancing the sensitivity of cancer cells to anticancer drugs. For example, Bernardes and colleagues found that combined application with azurin enhances the sensitivity of A549 lung cancer cells to gefitinib and erlotinib [51], as well as the sensitivity of MCF-7 breast cancer cells, HeLa cervical cancer cells, and HT-29 colon cancer cells to paclitaxel and doxorubicin [25]. Yamada et al. [29] found that combined application with p28 improves the activity of DNA damage drugs and antimitotic drugs in a variety of cancer cells. Oral squamous carcinoma cells are resistant to many anticancer drugs. Choi et al. [31] showed that azurin treatment provides a way to enhance sensitivity to anticancer drugs, because the activities of 5-fluorouracil and etoposide in YD-9 oral squamous carcinoma cells are significantly increased after azurin treatment.
6 Conclusions and Future Perspectives
Cancer development is a complex process that involves many different factors. Consequently, it is difficult to achieve an ideal therapeutic effect using a single anticancer drug. Investigations on azurin and p28 during the last two decades have demonstrated that azurin and p28 are multi-target anticancer agents that can interfere with several different signaling pathways. Thus, azurin and p28 can induce apoptosis and cell cycle arrest by stabilizing p53 protein, inhibit downstream phosphorylation signaling pathways by binding with various receptor tyrosine kinases, and modulate the cell surface structure by interacting with lipid raft components. Therefore, azurin and p28 can be applied as anticancer agents alone or synergistically with other anticancer drugs. Furthermore, after conjugation to liposomes or nanoparticles, p28 has the potential to achieve cancer-targeted drug delivery. Although binding affinities have been measured for azurin/p28 and several of their binding partners, structure information on azurin/p28 in complex with these binding partners is extremely limited. It is urgently required to obtain the structures of p28/p53-DBD complex and azurin/p53-DBD complex to further understand the functions of azurin and p28 as well as perform rational designs for p28 to improve its activity. In addition, the discovery of azurin-like anticancer proteins will provide valuable information toward understanding the activities of azurin and p28 and clues toward improving these activities [80, 81].
References
Baudino TA (2015) Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol 12:3–20
Kumari P, Ghosh B, Biswas S (2016) Nanocarriers for cancer-targeted drug delivery. J Drug Target 24:179–191
Biswas S, Kumari P, Lakhani PM, Ghosh B (2016) Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci 83:184–202
Soudy R, Byeon N, Raghuwanshi Y, Ahmed S, Lavasanifar A, Kaur K (2017) Engineered peptides for applications in cancer-targeted drug delivery and tumor detection. Mini Rev Med Chem 17:1696–1712
Chatzisideri T, Leonidis G, Sarli V (2018) Cancer-targeted delivery systems based on peptides. Future Med Chem 10:2201–2226
Bernardes N, Fialho AM (2018) Perturbing the dynamics and organization of cell membrane components: a new paradigm for cancer-targeted therapies. Int J Mol Sci 19:E3871
van de Kamp M, Silvestrini MC, Brunori M, Van Beeumen J, Hali FC, Canters GW (1990) Involvement of the hydrophobic patch of azurin in the electron-transfer reactions with cytochrome C551 and nitrite reductase. Eur J Biochem 194:109–118
Fialho AM, Gupta TKD, Chakrabarty AM (2007) Designing promiscuous drugs? Look at what nature made! Lett Drug Des Discov 4:40–43
Mahfouz M, Hashimoto W, Das Gupta TK, Chakrabarty AM (2007) Bacterial proteins and CpG-rich extra chromosomal DNA in potential cancer therapy. Plasmid 57:4–17
Bernardes N, Seruca R, Chakrabarty AM, Fialho AM (2010) Microbial-based therapy of cancer: current progress and future prospects. Bioeng Bugs 1:178–190
Fialho AM, Bernardes N, Chakrabarty AM (2012) Recent patents on live bacteria and their products as potential anticancer agents. Recent Pat Anticancer Drug Discov 7:31–55
Chakrabarty AM, Bernardes N, Fialho AM (2014) Bacterial proteins and peptides in cancer therapy: today and tomorrow. Bioengineered 5:234–242
Van Dessel N, Swofford CA, Forbes NS (2015) Potent and tumor specific: arming bacteria with therapeutic proteins. Ther Deliv 6:385–399
Raucher D, Ryu JS (2015) Cell-penetrating peptides: strategies for anticancer treatment. Trends Mol Med 21:560–570
Karpinski TM, Adamczak A (2018) Anticancer activity of bacterial proteins and peptides. Pharmaceutics 10:54
Habault J, Poyet JL (2019) Recent advances in cell penetrating peptide-based anticancer therapies. Molecules 24:927
Warso MA, Richards JM, Mehta D, Christov K, Schaeffer C, Rae Bressler L, Yamada T, Majumdar D, Kennedy SA, Beattie CW, Das Gupta TK (2013) A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br J Cancer 108:1061–1070
Lulla RR, Goldman S, Yamada T, Beattie CW, Bressler L, Pacini M, Pollack IF, Fisher PG, Packer RJ, Dunkel IJ, Dhall G, Wu S, Onar A, Boyett JM, Fouladi M (2016) Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: a Pediatric Brain Tumor Consortium study. Neuro-oncology 18:1319–1325
Gao M, Zhou J, Su Z, Huang Y (2017) Bacterial cupredoxin azurin hijacks cellular signaling networks: protein–protein interactions and cancer therapy. Protein Sci 26:2334–2341
Nar H, Messerschmidt A, Huber R, van de Kamp M, Canters GW (1991) Crystal structure analysis of oxidized Pseudomonas aeruginosa azurin at pH 5.5 and pH 9.0. A pH-induced conformational transition involves a peptide bond flip. J Mol Biol 221:765–772
Santini S, Bizzarri AR, Cannistraro S (2011) Modelling the interaction between the p53 DNA-binding domain and the p28 peptide fragment of Azurin. J Mol Recognit 24:1043–1055
Yamada T, Christov K, Shilkaitis A, Bratescu L, Green A, Santini S, Bizzarri AR, Cannistraro S, Gupta TK, Beattie CW (2013) p28, a first in class peptide inhibitor of cop1 binding to p53. Br J Cancer 108:2495–2504
Yamada T, Fialho AM, Punj V, Bratescu L, Gupta TK, Chakrabarty AM (2005) Internalization of bacterial redox protein azurin in mammalian cells: entry domain and specificity. Cell Microbiol 7:1418–1431
Taylor BN, Mehta RR, Yamada T, Lekmine F, Christov K, Chakrabarty AM, Green A, Bratescu L, Shilkaitis A, Beattie CW, Das Gupta TK (2009) Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res 69:537–546
Bernardes N, Garizo AR, Pinto SN, Canico B, Perdigao C, Fernandes F, Fialho AM (2018) Azurin interaction with the lipid raft components ganglioside GM-1 and caveolin-1 increases membrane fluidity and sensitivity to anti-cancer drugs. Cell Cycle 17:1649–1666
Punj V, Bhattacharyya S, Saint-Dic D, Vasu C, Cunningham EA, Graves J, Yamada T, Constantinou AI, Christov K, White B, Li G, Majumdar D, Chakrabarty AM, Das Gupta TK (2004) Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene 23:2367–2378
Yamada T, Mehta RR, Lekmine F, Christov K, King ML, Majumdar D, Shilkaitis A, Green A, Bratescu L, Beattie CW, Das Gupta TK (2009) A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol Cancer Ther 8:2947–2958
Abuei H, Behzad-Behbahani A, Faghihi F, Farhadi A, Rafiei Dehbidi GR, Pirouzfar M, Zare F (2019) The effect of bacterial peptide p28 on viability and apoptosis status of p53-null HeLa cells. Adv Pharm Bull 9:668–673
Yamada T, Das Gupta TK, Beattie CW (2016) p28-mediated activation of p53 in G2-M phase of the cell cycle enhances the efficacy of DNA damaging and antimitotic chemotherapy. Cancer Res 76:2354–2365
Yamada T, Das Gupta TK, Beattie CW (2013) p28, an anionic cell-penetrating peptide, increases the activity of wild type and mutated p53 without altering its conformation. Mol Pharm 10:3375–3383
Cho JH, Lee MH, Cho YJ, Park BS, Kim S, Kim GC (2011) The bacterial protein azurin enhances sensitivity of oral squamous carcinoma cells to anticancer drugs. Yonsei Med J 52:773–778
Yamada T, Goto M, Punj V, Zaborina O, Chen ML, Kimbara K, Majumdar D, Cunningham E, Das Gupta TK, Chakrabarty AM (2002) Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc Natl Acad Sci USA 99:14098–14103
Valente JFA, Queiroz JA, Sousa F (2018) p53 as the focus of gene therapy: past, present and future. Curr Drug Targets 19:1801–1817
Niazi S, Purohit M, Niazi JH (2018) Role of p53 circuitry in tumorigenesis: a brief review. Eur J Med Chem 158:7–24
Ramachandran S, Mandal M (2011) Induction of apoptosis of azurin synthesized from P. aeruginosa MTCC 2453 against Dalton’s lymphoma ascites model. Biomed Pharmacother 65:461–466
Zhang Y, Zhang Y, Xia L, Zhang X, Ding X, Yan F, Wu F (2012) Escherichia coli Nissle 1917 targets and restrains mouse B16 melanoma and 4T1 breast tumors through expression of azurin protein. Appl Environ Microbiol 78:7603–7610
Holmstrom KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15:411–421
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A (2020) ROS in cancer therapy: the bright side of the moon. Exp Mol Med. https://doi.org/10.1038/s12276-020-0384-2
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374
Yamada T, Goto M, Punj V, Zaborina O, Kimbara K, Das Gupta TK, Chakrabarty AM (2002) The bacterial redox protein azurin induces apoptosis in J774 macrophages through complex formation and stabilization of the tumor suppressor protein p53. Infect Immun 70:7054–7062
Goto M, Yamada T, Kimbara K, Horner J, Newcomb M, Gupta TK, Chakrabarty AM (2003) Induction of apoptosis in macrophages by Pseudomonas aeruginosa azurin: tumour-suppressor protein p53 and reactive oxygen species, but not redox activity, as critical elements in cytotoxicity. Mol Microbiol 47:549–559
Punj V, Das Gupta TK, Chakrabarty AM (2003) Bacterial cupredoxin azurin and its interactions with the tumor suppressor protein p53. Biochem Biophys Res Commun 312:109–114
Moll UM, Petrenko O (2003) The MDM2-p53 interaction. Mol Cancer Res 1:1001–1008
Aberg E, Karlsson OA, Andersson E, Jemth P (2018) Binding kinetics of the intrinsically disordered p53 family transactivation domains and MDM2. J Phys Chem B 122:6899–6905
Gabellieri E, Bucciantini M, Stefani M, Cioni P (2011) Does azurin bind to the transactivation domain of p53? A Trp phosphorescence study. Biophys Chem 159:287–293
Domenici F, Frasconi M, Mazzei F, D’Orazi G, Bizzarri AR, Cannistraro S (2011) Azurin modulates the association of Mdm2 with p53: SPR evidence from interaction of the full-length proteins. J Mol Recognit 24:707–714
De Grandis V, Bizzarri AR, Cannistraro S (2007) Docking study and free energy simulation of the complex between p53 DNA-binding domain and azurin. J Mol Recognit 20:215–226
Signorelli S, Cannistraro S, Bizzarri AR (2019) Raman evidence of p53-DBD disorder decrease upon interaction with the anticancer protein azurin. Int J Mol Sci 20:3078
Xu C, Yin JJ, Zhao BL (2010) Structural characteristics of the hydrophobic patch of azurin and its interaction with p53: a site-directed spin labeling study. Sci China Life Sci 53:1181–1188
Pontier SM, Muller WJ (2009) Integrins in mammary-stem-cell biology and breast-cancer progression—a role in cancer stem cells? J Cell Sci 122:207–214
Bernardes N, Abreu S, Carvalho FA, Fernandes F, Santos NC, Fialho AM (2016) Modulation of membrane properties of lung cancer cells by azurin enhances the sensitivity to EGFR-targeted therapy and decreased beta1 integrin-mediated adhesion. Cell Cycle 15:1415–1424
Ribeiro AS, Albergaria A, Sousa B, Correia AL, Bracke M, Seruca R, Schmitt FC, Paredes J (2010) Extracellular cleavage and shedding of P-cadherin: a mechanism underlying the invasive behaviour of breast cancer cells. Oncogene 29:392–402
Bernardes N, Ribeiro AS, Abreu S, Mota B, Matos RG, Arraiano CM, Seruca R, Paredes J, Fialho AM (2013) The bacterial protein azurin impairs invasion and FAK/Src signaling in P-cadherin-overexpressing breast cancer cell models. PLoS ONE 8:e69023
Bernardes N, Ribeiro AS, Abreu S, Vieira AF, Carreto L, Santos M, Seruca R, Paredes J, Fialho AM (2014) High-throughput molecular profiling of a P-cadherin overexpressing breast cancer model reveals new targets for the anti-cancer bacterial protein azurin. Int J Biochem Cell Biol 50:1–9
Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133:38–52
Saha N, Robev D, Mason EO, Himanen JP, Nikolov DB (2018) Therapeutic potential of targeting the Eph/ephrin signaling complex. Int J Biochem Cell Biol 105:123–133
Barquilla A, Pasquale EB (2015) Eph receptors and ephrins: therapeutic opportunities. Annu Rev Pharmacol Toxicol 55:465–487
Xi HQ, Wu XS, Wei B, Chen L (2012) Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med 16:2894–2909
Lodola A, Giorgio C, Incerti M, Zanotti I, Tognolini M (2017) Targeting Eph/ephrin system in cancer therapy. Eur J Med Chem 142:152–162
Chaudhari A, Mahfouz M, Fialho AM, Yamada T, Granja AT, Zhu Y, Hashimoto W, Schlarb-Ridley B, Cho W, Das Gupta TK, Chakrabarty AM (2007) Cupredoxin–cancer interrelationship: azurin binding with EphB2, interference in EphB2 tyrosine phosphorylation, and inhibition of cancer growth. Biochemistry 46:1799–1810
Bizzarri AR, Moscetti I, Cannistraro S (2019) Interaction of the anticancer p28 peptide with p53-DBD as studied by fluorescence, FRET, docking and MD simulations. Biochim Biophys Acta Gen Subj 1863:342–350
Bizzarri AR, Santini S, Coppari E, Bucciantini M, Di Agostino S, Yamada T, Beattie CW, Cannistraro S (2011) Interaction of an anticancer peptide fragment of azurin with p53 and its isolated domains studied by atomic force spectroscopy. Int J Nanomed 6:3011–3019
Signorelli S, Santini S, Yamada T, Bizzarri AR, Beattie CW, Cannistraro S (2017) Binding of amphipathic cell penetrating peptide p28 to wild type and mutated p53 as studied by Raman, atomic force and surface plasmon resonance spectroscopies. Biochim Biophys Acta 1861:910–921
Ka WH, Cho SK, Chun BN, Byun SY, Ahn JC (2018) The ubiquitin ligase COP1 regulates cell cycle and apoptosis by affecting p53 function in human breast cancer cell lines. Breast Cancer 25:529–538
Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, O’Rourke K, Koeppen H, Dixit VM (2004) The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 429:86–92
Dornan D, Bheddah S, Newton K, Ince W, Frantz GD, Dowd P, Koeppen H, Dixit VM, French DM (2004) COP1, the negative regulator of p53, is overexpressed in breast and ovarian adenocarcinomas. Cancer Res 64:7226–7230
Zou S, Zhu Y, Wang B, Qian F, Zhang X, Wang L, Fu C, Bao H, Xie M, Gao S, Yu R, Shi H (2017) The ubiquitin ligase COP1 promotes glioma cell proliferation by preferentially downregulating tumor suppressor p53. Mol Neurobiol 54:5008–5016
Moscetti I, Bizzarri AR, Cannistraro S (2018) Imaging and kinetics of the bimolecular complex formed by the tumor suppressor p53 with ubiquitin ligase COP1 as studied by atomic force microscopy and surface plasmon resonance. Int J Nanomed 13:251–259
Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6:273–286
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23:1011–1027
Mehta RR, Yamada T, Taylor BN, Christov K, King ML, Majumdar D, Lekmine F, Tiruppathi C, Shilkaitis A, Bratescu L, Green A, Beattie CW, Das Gupta TK (2011) A cell penetrating peptide derived from azurin inhibits angiogenesis and tumor growth by inhibiting phosphorylation of VEGFR-2, FAK and Akt. Angiogenesis 14:355–369
Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, Szalay AA (2007) Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol 297:151–162
Ghasemi-Dehkordi P, Doosti A, Jami MS (2019) The concurrent effects of azurin and Mammaglobin-A genes in inhibition of breast cancer progression and immune system stimulation in cancerous BALB/c mice. 3 Biotech 9:271
Mehta N, Lyon JG, Patil K, Mokarram N, Kim C, Bellamkonda RV (2017) Bacterial carriers for glioblastoma therapy. Mol Ther Oncolytics 4:1–17
Paydarnia N, Khoshtinat Nikkhoi S, Fakhravar A, Mehdiabdol M, Heydarzadeh H, Ranjbar S (2019) Synergistic effect of granzyme B-azurin fusion protein on breast cancer cells. Mol Biol Rep 46:3129–3140
Soleimani M, Mirmohammmad Sadeghi H, Jahanian-Najafabadi A (2019) A bi-functional targeted p28-NRC chimeric protein with enhanced cytotoxic effects on breast cancer cell lines. Iran J Pharm Res 18:735–744
Noei A, Nili-Ahmadabadi A, Soleimani M (2019) The enhanced cytotoxic effects of the p28-apoptin chimeric protein as a novel anti-cancer agent on breast cancer cell lines. Drug Res (Stuttg) 69:144–150
Shahbazi S, Bolhassani A (2018) Comparison of six cell penetrating peptides with different properties for in vitro and in vivo delivery of HPV16 E7 antigen in therapeutic vaccines. Int Immunopharmacol 62:170–180
Micewicz ED, Jung CL, Schaue D, Luong H, McBride WH, Ruchala P (2011) Small azurin derived peptide targets ephrin receptors for radiotherapy. Int J Pept Res Ther 17:247–257
Nguyen C, Nguyen VD (2016) Discovery of azurin-like anticancer bacteriocins from human gut microbiome through homology modeling and molecular docking against the tumor suppressor p53. Biomed Res Int 2016:8490482
Nguyen VD, Nguyen TT, Pham TT, Packianather M, Le CH (2019) Molecular screening and genetic diversity analysis of anticancer Azurin-encoding and Azurin-like genes in human gut microbiome deduced through cultivation-dependent and cultivation-independent studies. Int Microbiol 22:437–449
Acknowledgements
This work was supported by the Natural Science Foundation of Hubei Province (Grant Number 2019CFB713) and funding from Hubei University of Technology. The authors thank Alison Sherwin, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, F., Shu, Q., Qin, Z. et al. Anticancer Actions of Azurin and Its Derived Peptide p28. Protein J 39, 182–189 (2020). https://doi.org/10.1007/s10930-020-09891-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09891-3