Abstract
Intravenous acetaminophen is a commonly used analgesic following surgery. The aims of this study were to determine the population pharmacokinetic profile of intravenous acetaminophen and its metabolites in adult surgical patients and to identify patient characteristics associated with acetaminophen metabolism in the postoperative period. 53 patients were included in the dataset; 28 were men, median age (range) 60 years (33–87), median weight (range) 74 kg (54–129). Patients received 1, 1.5 or 2 g of intravenous acetaminophen every 4–6 h. Plasma and urine samples were collected at various intervals for up to 6 days after surgery. Simultaneous modelling of parent acetaminophen and its metabolites was conducted in Phoenix® NLME™ to estimate pharmacokinetic parameters. The population mean estimate (CV%) for central (plasma) volume of distribution of parent acetaminophen (VC) was 13.9 (4.41) L, peripheral (tissue) volume of distribution (VT) was 50.9 (2.96) L, and intercompartmental clearance (Q) was 77.5 (9.29) L/h. The population mean (CV%) metabolic clearances for glucuronidation (CLPG) was 8.92 (3.25) L/h, sulfation (CLPS) was 0.903 (3.47) L/h, and oxidation (CLPO) was 0.533 (7.90) L/h. The population mean (CV%) urinary clearances of parent acetaminophen (CLRP) was 0.137 (5.46) L/h, acetaminophen glucuronide (CLRG) was 3.81 (6.71) L/h, acetaminophen sulfate (CLRS) was 3.13 (4.32) L/h, and acetaminophen cysteine + mercapturate (CLRO) was 3.51 (9.98) L/h. Age was found to be a significant covariate on the formation of acetaminophen glucuronide, and renal function (estimated as creatinine clearance) on the urinary excretion of acetaminophen glucuronide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acetaminophen has been used in postoperative pain management for decades, but its role was hindered by poor oral absorption in surgical patients. Its use has increased considerably since the licensing of an intravenous formulation and the concept of multimodal analgesia gained prominence [1]. Intravenous (IV) acetaminophen (PERFALGAN®, Bristol Myers Squibb Ltd, Auckland, New Zealand) was first available for use in 2001, and, to date, has been approved in approximately 80 countries. Doses of IV acetaminophen higher than recommended in the product information are often used in clinical care but there is little evidence of their safety in surgical patients [2, 3]. Currently, there are no population pharmacokinetic (PK) studies that have investigated the distribution and metabolism of IV acetaminophen during the postoperative period.
In healthy adult patients, the pharmacokinetics of acetaminophen are linear after single doses of up to 2 g and after repeated administration over 24 h [1, 4]. In this population, acetaminophen has a plasma half-life of 2.7 h and total body clearance of 18 L/h [5]. Acetaminophen is extensively metabolized in the liver by glucuronidation, sulfation and oxidation with less than 5 % excreted unchanged in the urine [6].
Following a single dose of acetaminophen, conjugation of acetaminophen with glucuronic acid is the major pathway for its deactivation and elimination in adult patients, accounting for approximately 55 % of urinary metabolites. Sulfation is catalysed by sulfotransferases in the liver and accounts for about 30 % of acetaminophen metabolism [6]. At therapeutic doses of acetaminophen, less than 10 % of its metabolism is accounted for by oxidation via the cytochrome P-450 system. An intermediary metabolite of acetaminophen oxidation is N-acetyl-p-benzoquinone imine (NAPQI), which is eliminated by glutathione conjugation forming cysteine and mercapturate metabolites [1, 5, 7, 8].
In certain circumstances such as acetaminophen overdose, malnourishment or inflammatory conditions, hepatic stores of glutathione may be depleted, leading to NAPQI-induced toxicity in the liver [9]. There are many reasons for altered pharmacokinetics after surgery, including poor hepatic perfusion and resulting hypoxia, as well as the influence of serum factors and cytokines on metabolic pathways [10]. Literature is limited with regard to changes to drug metabolism in the postoperative setting.
Generally, studies of parent acetaminophen pharmacokinetics have found that its total clearance is not affected by surgery [11–13]. However, more recent studies have shown increases in the glucuronidation of acetaminophen after repeated supratherapeutic dosing, in surgical patients and in healthy volunteers [2, 4, 14]. In a previous study by Owens et al. [14], increased formation and excretion of acetaminophen glucuronide was reported following repeated therapeutic doses (4 g daily) of IV acetaminophen, and an increased overall clearance of acetaminophen in the postoperative period. However, this study was limited by the short duration of urine collection and only investigated a single dosing regimen (1 g every 6 h, with a maximum of 4 g as a total daily dose) for acetaminophen. Another study by Pickering et al. [15] found that acetaminophen metabolism shifted towards oxidative metabolism following major surgery and identified increased oxidative metabolism associated with advancing age. The aim of this study was to develop a population PK model for IV acetaminophen and its metabolites in the postoperative period and to identify potential patient characteristics related to acetaminophen metabolism, in particular to investigate covariates associated with the formation of oxidative metabolites (acetaminophen cysteine and mercapturate), as these represent the toxic metabolism of acetaminophen and could have potential implications on patient safety.
Methods
Patients
Patients were recruited from St John’s Hospital, Limerick, Ireland; Mercy University Hospital, Cork, Ireland; and Dunedin Hospital, Dunedin, New Zealand. The pharmacokinetics of IV acetaminophen and its metabolites has been previously described in the Dunedin patient group, and these patients were included in the population dataset [14]. The surgical procedures included in the study were major abdominal and breast surgeries, as described in Online Resource 1.
The exclusion criteria included: regularly taking acetaminophen and unable to stop prior to the study; hypersensitivity to acetaminophen; impaired liver function (determined by alanine aminotransferase (ALT)), renal dysfunction (<30 mL/min/1.73 m2) or failure (<15 mL/min/1.73 m2); type I diabetes; pregnancy; age <18 years; poor nutritional status (patient unable to take adequate oral feed, cachexia, very low body weight: low serum albumin level where available), eating disorder or body mass index (BMI) <16; chronic ethanol abuse; intolerance to oral medication; vomiting; porphyria; bleeding disorders; concomitant anticonvulsant medication (e.g. carbamazepine) and oral anticoagulants (e.g. warfarin).
Ethical approval
The studies conducted at St John’s and Mercy University Hospitals were granted ethical approval by the Clinical Research Ethics Committee of the Cork Teaching Hospitals, the Ethics Committee of St John’s Hospital, and the Drug and Therapeutics Committee of Mercy University Hospital. Those studies were approved by the Irish Medicines Board and registered with the European Medicines Agency (EudraCT 2009-010818-30). The Dunedin study had ethical approval from the Lower South Regional Ethics Committee and was registered with the Australia Clinical Trial Registry as an observational study (ANZCTR No: ACTRN12608000028303). The studies were conducted in accordance with the Declaration of Helsinki.
Study design
The study design was a combined multiple dose pharmacokinetic study with multiple study groups and sampling intervals (as described in Table 1), and analyzed as a population PK study.
Safety assessment
Vital signs (blood pressure, respiration, pulse and temperature) and blood tests (electrolytes, blood counts and liver function) were routinely monitored in the postoperative period. Liver function was determined by a daily ALT test. Patients were removed immediately from the study if they fulfilled any of the following criteria: AST or ALT level three times the upper limit of normal; acetaminophen toxicity or allergy (e.g. thrombocytopenia); loss of venous access. Any patient removed from the study continued to be monitored for the original proposed study period and their data were included in the final analysis.
Drug administration and sample collection
Patients were given doses of either 1, 1.5 or 2 g of acetaminophen by intravenous (IV) infusion every 4–6 h. A combination of rich and sparse plasma and urine samples were collected over a 7-day period (day 0 being preoperative collection and days 1–6 being postoperative collection) for up to 12 h after each dosing. Dosing regimen details are described in Table 1. Plasma was isolated by centrifugation and stored at −20 °C until assayed for acetaminophen and its metabolites. The urine volumes were measured and sample aliquots (5 mL) were collected and were frozen at −20 °C until assayed for acetaminophen and its metabolites. Samples were stored for a maximum of 15 months prior to analysis. Freeze–thaw studies showed there was no significant loss of acetaminophen, acetaminophen glucuronide or sulfate on freezing and thawing. Quality control standards stored with clinical samples showed less than 10 % deviation from their nominal concentrations.
Bioanalytical methods
The HPLC–UV assay for acetaminophen and its metabolites, acetaminophen glucuronide, acetaminophen sulfate, acetaminophen cysteine and acetaminophen mercapturate, was conducted as previously described by Reith et al. [9]. Assay precision and accuracy were determined for concentrations within the linear range of each analyte. Validation details including coefficients of variation (COVs), accuracy and precision for intra-day and inter-day variability for both plasma and urine analysis, and lower limits of quantification have been previously reported [9].
Statistical analysis
Descriptive statistics of clinical data and patient demographics were conducted using STATA (version 11.2; College Station, TX, USA).
Population pharmacokinetic analysis
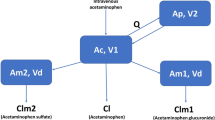
Parent acetaminophen, acetaminophen glucuronide, acetaminophen sulfate and acetaminophen cysteine + mercapturate plasma concentrations and urinary amounts were analyzed using nonlinear mixed effects modelling with Phoenix NLME (version 6.1; Pharsight, Mountain View, CA, USA). A parent-metabolite model (with a 2-compartment model describing parent plasma acetaminophen) including plasma and urine compartments, was used as previously described [14], with the addition of the oxidative pathway metabolites.
The parent-metabolite modelling process involved estimation of thirteen parameters using nine differential equations, as described in Fig. 1. The parameters included: central (plasma) volume of distribution of parent acetaminophen (VP), peripheral (tissue) volume of distribution of parent acetaminophen (VT), intercompartmental clearance (Q); metabolic clearance for glucuronidation (CLPG), sulfation (CLPS), and oxidation (CLPO) pathways; volumes of distribution for acetaminophen glucuronide (VG), acetaminophen sulfate (VS) and acetaminophen cysteine + mercapturate (VO); and urinary clearance of unchanged parent acetaminophen (CLRP), acetaminophen glucuronide (CLRG), acetaminophen sulfate (CLRS) and acetaminophen cysteine + acetaminophen mercapturate (CLRO). Urine data for parent acetaminophen, acetaminophen glucuronide, acetaminophen sulfate and acetaminophen cysteine + mercapturate were described in the model as cumulative amounts (μmol). All metabolic and urinary clearances were modelled as first-order processes.
The differential equations used in the model are given below (the initial conditions for all equations were 0):
where Eq. 1 describes the central (plasma) compartment of parent acetaminophen, Eq. 2 describes the peripheral (tissue) compartment of parent acetaminophen, Eq. 3 describes the plasma concentration of acetaminophen glucuronide, Eq. 4 describes the plasma concentration of acetaminophen sulfate, Eq. 5 describes the plasma concentration of acetaminophen cysteine + mercapturate, Eq. 6 describes the urinary amounts of parent acetaminophen, Eq. 7 describes the urinary amounts of acetaminophen glucuronide, Eq. 8 describes the urinary amounts of acetaminophen sulfate, and Eq. 9 describes the urinary amounts of acetaminophen cysteine + mercapturate.
The metabolites (acetaminophen glucuronide, acetaminophen sulfate, and combined acetaminophen cysteine + mercapturate) were modelled as each possessing a single plasma compartment and subsequent urinary compartment. All metabolic and urinary clearances were modelled as first order processes. The following two assumptions were made in the model: that all acetaminophen given as an IV dose is either eliminated unchanged or metabolized by glucuronidation, sulfation or oxidation, and all metabolites present in plasma are excreted into urine. Initial population parameter estimates for volumes of distribution and metabolic formation and urinary clearance were obtained from literature values [14]. Quasi-random parametric expectation maximisation (QRPEM) as an approximation method was applied to estimate the pharmacokinetic parameters [16, 17]. Interindividual variability of the pharmacokinetic parameters was modelled using an exponential random effects model. A proportional error model was used to describe the residual variability for both acetaminophen and its metabolites.
Population covariate analysis
The effects of the covariates such as age (years), body weight (kg), gender and creatinine clearance (mL/min) were evaluated for the final model. Creatinine clearance was calculated from preoperative serum creatinine levels using the Cockcroft–Gault equation [18]. Creatinine clearance was also used as simplified covariate to investigate the effect of age, weight and gender on renal elimination. These patient data are incorporated into the calculation of renal function using the Cockcroft–Gault equation. Continuous covariates were centered at the median values and were included in the model using linear relationships. Categorical covariates were incorporated using indicator variables. Patient characteristics were first examined visually as having potential covariate effects on the pharmacokinetic parameters of interest. For the final model, stepwise forward addition followed by backward deletion was used. A covariate was considered significant when the addition of this covariate resulted in a decrease in the Akaike Information Criterion (AIC) of >6.635 (P < 0.01) and elimination of this covariate resulted in an increase in the AIC of >10.828 (P < 0.001) [19, 20]. It was decided a priori that if >10 % of study patients were missing any covariate data, the covariate was excluded from the covariate analysis.
Model evaluation
The improvement of fit of the model was evaluated using the AIC. Visual model evaluation was done by inspection of scatter plots of plasma concentrations and urinary amounts (of parent acetaminophen and all metabolites) versus individual and population predicted values. The relative standard error (RSE) of the mean was graphically described by conditional weighted residuals (CWRES) plotted against population predicted (PRED) plasma concentrations and urinary amounts and time (h). To further evaluate the model, simulations were conducted in Phoenix NLME using the study dataset. Simulated percentiles (5th, 50th, and 95th) were calculated and visual predictive checks (VPCs) were created in OriginPro (version 8.5; Northhampton, MA, USA) showing the observed plasma or urinary data over the simulated predictions based on the model.
Results
Patients and surgical procedures
A total of 53 patients were included in the dataset, as described in Table 2. Of these patients, 28 (53 %) were men; the median age (range) was 60 years (33–87), median weight (range) was 74 kg (54–129), and median height (range) was 168 cm (154–185). Of the patients included in the study dataset, 13 had a BMI greater than 30 kg/m2, and five were greater than 80 years of age. Surgical procedures and anesthesia are described in Online Resource 1. None of the patients in the study had elevated AST or ALT during the study period. The effect of nutritional status was not investigated because all patients were fed during the study period.
Population pharmacokinetic analysis
The population mean estimate (95 % CI) for central (plasma) volume of distribution of parent acetaminophen (VP) was 13.9 (12.7–15.1) L, peripheral (tissue) volume of distribution (VT) was 50.9 (47.9–53.8) L, and apparent intercompartmental clearance (Q) was 77.5 (63.4–91.6) L/h. Mean (95 % CI) metabolic clearance for glucuronidation (CLPG) was 8.92 (8.35–9.49) L/h, metabolic clearance for sulfation (CLPS) was 0.903 (0.841–0.964) L/h, and metabolic clearance for oxidation (CLPO) was 0.533 (0.451–0.616) L/h. Mean (95 % CI) urinary clearance (L/h) of parent acetaminophen (CLRP) was 0.137 (0.123–0.152) L/h, urinary clearance of acetaminophen glucuronide (CLRG) was 3.81 (3.31–4.31) L/h, urinary clearance of acetaminophen sulfate (CLRS) was 3.13 (2.86–3.39) L/h, and mean urinary clearance of acetaminophen cysteine + mercapturate (CLRO) was 3.51 (2.83–4.20) L/h. The pharmacokinetic model parameters (fixed effects) are shown in Table 3.
Inter-subject variability (random effects) was estimated for the following parameters in the model: VP, VG, VS, VO, CLPG, CLPS, CLPO, CLRG, CLRS, CLRO, and CLRP. The population parameter estimates for the base model (the population pharmacokinetic model without covariate effects), with coefficients of variation (CV%) are given in Table 3.
Population covariate analysis
The population parameter estimates for the base (no covariate effects) and final (significant covariate effects) models, with coefficients of variation (CV%) are given in Table 3. In the current study, two patients were missing preoperative serum creatinine measurements, comprising <4 % of the total dataset, therefore no covariate data were imputed. The covariates found to be statistically significant were age on the formation of acetaminophen glucuronide (shown in Fig. 2), and renal function (estimated as creatinine clearance) on the urinary excretion of acetaminophen glucuronide (shown in Fig. 3).
Model evaluation
Individual fits of acetaminophen and its metabolites in plasma and in urine are shown for four individuals in Figs. 4 and 5. Visual fit of plots and AIC were used to discriminate between models. There was little to no apparent visual improvement in model fit with the addition of covariates, hence the significant reduction in AIC was used as the final diagnostic. Scatter plots of plasma concentrations and urinary amounts (of parent acetaminophen and all metabolites) versus individual predicted values are shown in Figs. 6 and 7, indicating goodness of fit. In both the base and final models, the data points are evenly distributed along the line of identity. The RSE of the population prediction was graphically described by conditional weighted residuals (CWRES) plotted against population predicted (PRED) plasma concentrations and urinary amounts, and also plotted against time (h) for parent acetaminophen and its metabolites, as shown in Online Resources 3–6. Both parent acetaminophen and all metabolites had CWRES within ±2 across the study timeline and the range of predicted concentrations, indicating good model fit. Percentiles (5th, 50th and 95th) of predicted values were calculated by simulation (using the original dataset) in Phoenix NLME. The simulated percentiles of predicted values are overlaid with observed plasma and urinary data as VPCs in Online Resource 7.
Observed (circle acetaminophen, square acetaminophen glucuronide, triangle acetaminophen sulfate, diamond acetaminophen cysteine + mercapturate) and individual predicted (solid lines acetaminophen, dashed lines acetaminophen glucuronide, dotted lines acetaminophen sulfate, dash-dotted lines, acetaminophen cysteine + mercapturate) plasma concentrations (µmol/L) versus time for four representative patients
Observed (circle, acetaminophen, square acetaminophen glucuronide, triangle acetaminophen sulfate; diamond acetaminophen cysteine + mercapturate) and individual predicted (solid lines acetaminophen, dashed lines acetaminophen glucuronide, dotted lines acetaminophen sulfate, dash-dotted lines acetaminophen cysteine + mercapturate) cumulative urinary amounts (µmol) versus time for four representative patients
Discussion
This study has described the population pharmacokinetics of therapeutic and supratherapeutic IV acetaminophen and its metabolites in adult patients undergoing major abdominal surgery. Acetaminophen has increasingly been a part of multimodal analgesic regimens throughout the postoperative period. Despite toxicity in overdose, acetaminophen doses exceeding those recommended have been advocated and supratherapeutic use of acetaminophen has been reported in children and adults without adverse effects [2, 21–25]. The pharmacokinetics of IV acetaminophen have been reported in both postoperative patients and healthy adult subjects, providing historical controls [13, 14, 26–29]. Our research group has previously reported altered postoperative metabolism of acetaminophen, particularly apparent increases in the metabolic conversion to acetaminophen glucuronide and its urinary clearance, suggesting potential induction of acetaminophen glucuronidation after major abdominal surgery [14].
The point of difference for the current study was to attempt to identify potential patient characteristics (covariates) associated with previously observed pharmacokinetic changes. Age was found to influence the intersubject variability in the formation of the acetaminophen glucuronide metabolite (CLPG), and creatinine clearance was associated with the urinary clearance of acetaminophen glucuronide (CLRG), as shown in Fig. 2. There were no covariates associated with the population parameter estimates for the formation of oxidative metabolites (acetaminophen cysteine and mercapturate) after major surgery.
Overall, the population estimates (reported as population mean estimates with 95 % CI) for pharmacokinetic parameters were within the expected ranges from previous studies. The estimates of central volume of distribution (VC), intercompartmental clearance (Q), and peripheral volume of distribution (VT) for parent acetaminophen were similar to those reported previously by our research group [14].
The mean (95 % CI) metabolic formation clearance of acetaminophen glucuronide (CLPG) was 8.92 (8.35–9.49) L/h, and volume of distribution (VG) was 102 (89.1–114) L, both greater than previously reported. The mean (95 % CI) urinary clearance of acetaminophen glucuronide was 3.81 (3.31–4.31) L/h; which is less than previously reported 10.5 ± 2.6 L/h [14]. The mean (95 % CI) volume of distribution of acetaminophen sulfate (VS) of 5.72 (5.07–6.38) L and urinary clearance (CLRS) of 3.13 (2.86–4.20) L/h, were lower than previously reported. However, with consideration of similar results found by our research group (with ranges for VS of 10.5–72.8 L/70 kg, and CLRS of 2.8–18.2 L/h/70 kg), these estimates were expected [14]. Clements et al. [30] and Morris and Levy [31] have reported that acetaminophen sulfate urinary clearance has an inverse relation to its plasma concentrations.
The pharmacokinetic parameters of acetaminophen oxidation, including metabolic formation (CLPO), volume of distribution (VO) and urinary clearance (CLRO), have not been previously described in literature when described as first-order processes. Previous pharmacokinetic analysis of the oxidative metabolites of acetaminophen have described Michaelis–Menten parameters (95 % CI) Vmax 0.25 (0.14–0.36) mmol/h and Km 0.3 (0.13–0.48) mmol/L instead of first-order clearances (Reith). The estimates for Vmax and Km by Reith et al. were based on individual model fits and had very high intra-subject variability (CV > 100 %). For the present study, a first-order parameterisation was chosen to improve model parsimony and avoid over parameterisation.
Liukas et al. [32] compared the pharmacokinetics of IV acetaminophen in elderly patients with younger adult patients. Despite differences in pharmacokinetics with increasing age, dose adjustment was not advised due to minimal clinical significance during short-term infusions [32]. Whereas increasing age is associated with reduced hepatic drug clearance by impaired CYP–mediated phase I reactions such as oxidation, conjugation mediated phase II reactions are relatively unaffected in the elderly [33]. Pickering et al. [34] reported an increase in the amounts of oxidative metabolites present in urine in the days following surgery, an observation that was more prominent with increasing age. However, the urinary recovery of metabolites was not related to dose or plasma concentration, thus pharmacokinetic parameters quantifying the formation of metabolites and urinary clearance were not calculated. These results were in contrast to those observed in this study, where age was found to be associated with the formation of acetaminophen glucuronide, and renal function (measured as creatinine clearance) with its urinary elimiantion.
Martin et al. [35] investigated the effect of renal function on acetaminophen elimination. Patients with renal failure consistently had significantly higher plasma concentrations of acetaminophen, acetaminophen glucuronide, and acetaminophen sulfate throughout the study period. While no patients in the present study had renal failure, renal function was investigated for two reasons. Firstly the urinary clearance of parent acetaminophen may be affected by renal function, and secondly because the calculation of creatinine clearance incorporates patient details of age, weight and gender.
A limitation of the study was that mass balance of acetaminophen was unable to be determined due to short urine collection intervals. The greater residual variability of the urinary metabolite data could potentially be due to error in timing of sample collections. Due to the multimodal nature of postoperative analgesia and the potential influence of stronger opioid analgesics on patient pain scores, it was not possible to assess clinical efficacy of IV acetaminophen.
Conclusions
The present study developed a population pharmacokinetics model of IV acetaminophen and its metabolites in the postoperative period. Of the covariates investigated in the population pharmacokinetic model, age was found to be significant on the formation of acetaminophen glucuronide (CLPG), and renal function (incorporating patient information on age, weight and gender) was found to be a significant on urinary clearance of acetaminophen glucuronide (CLRG). These covariate effects on the glucuronidation pathway of acetaminophen metabolism were minor and are not likely to be of clinical significance in the postoperative period, at the doses investigated. There were no significant covariates associated with the formation of oxidative metabolites (acetaminophen cysteine and mercapturate) suggesting no relation between patient characteristics and oxidative metabolism of acetaminophen after major surgery. The postoperative period is a time of marked changes to patient physiology and consequently pharmacokinetics. Acetaminophen is being increasingly utilized in multimodal analgesia regimens, therefore it is becoming increasingly relevant to consider the specific patient population and how clinical characteristics can influence drug metabolism after surgery.
References
Duggan ST, Scott LJ (2009) Intravenous paracetamol (acetaminophen). Drugs 69(1):101–113
Murphy PGM (2012) Paracetamol metabolism in postoperative patients. National University of Ireland, Cork, Ireland
Perfalgan Medicine Data Sheet: Medsafe Data Sheet: Perfalgan [Online] (2012) http://www.medsafe.govt.nz/profs/datasheet/p/Perfalganinf.pdf. Accessed 05 Apr 2012
Gelotte CK, Auiler JF, Lynch JM, Temple AR, Slattery JT (2007) Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Clin Pharmacol Ther 81(6):840–848
Ward B, Alexander-Williams JM (1999) Paracetamol revisited: a review of the pharmacokinetics and pharmacodynamics. Acute Pain 2(3):139–149
Forrest JAH, Clements JA, Prescott LF (1982) Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet 7:93–107
Manyike PTP, Kharasch EDMDP, Kalhorn TFBS, Slattery JTP (2000) Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther 67(3):275–282
Slattery JT, Wilson JM, Kalhorn BS, Nelson SD (1987) Dose-dependent pharmacokinetics of acetaminophen: evidence of glutathione depletion on humans. Clin Pharmacol Ther 41:413–418
Reith DM, Medlicott NJ, Kumara De Silva R, Yang L, Hickling J, Zacharias M (2009) Simultaneous modelling of the Michaelis–Menten kinetics of paracetamol sulphation and glucuronidation. Clin Exp Pharmacol Physiol 36(1):35–42
Kennedy JM, van Rij AM (1998) Effects of surgery on the pharmacokinetic parameters of drugs. Clin Pharmacokinet 35(4):293–312
Lewis RP, Dunphy JA, Reilly CS (1991) Paracetamol metabolism after general anaesthesia. Eur J Anaesthesiol 8(6):445–450
Schuitmaker M, Anderson BJ, Holford NHG, Woollard GA (1999) Pharmacokinetics of paracetamol in adults after cardiac surgery. Anaesth Intensive Care 27(6):615–622
Würthwein G, Koling S, Reich A, Hempel G, Schulze-Westhoff P, Pinheiro PV, Boos J (2005) Pharmacokinetics of intravenous paracetamol in children and adolescents under major surgery. Eur J Clin Pharmacol 60(12):883–888
Owens KH, Medlicott NJ, Zacharias M, Curran N, Chary S, Thompson-Fawcett M, Reith D (2012) The pharmacokinetic profile of intravenous paracetamol in adult patients undergoing major abdominal surgery. Ther Drug Monit 34(6):713–721
Pickering G, Loriot MA, Libert F, Eschalier A, Beaune P, Dubray C (2006) Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther 79(4):371–378
Leary R, Dunlavey M (eds) (2012) QRPEM, a quasi-random parametric EM method. PAGE, Venice
Leary R, Dunlavey M, Chittenden J, Matzuka B, Guzy S (2012) QRPEM—a new standard of accuracy, precision, and efficiency in NLME population PK/PD methods. Pharsight® A Certara™ Company © 2011 Tripos, L.P. All Rights Reserved
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Ludden T, Beal S, Sheiner L (1994) Comparison of the Akaike information criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Pharmacodyn 22(5):431–445
Yamaoka K, Nakagawa T, Uno T (1978) Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 6(2):165–175
Gregoire N, Hovsepian L, Gualano V, Evene E, Dufour G, Gendron A (2007) Safety and pharmacokinetics of paracetamol following intravenous administration of 5 g during the first 24 h with a 2-g starting dose. Clin Pharmacol Ther 81(3):401–405
Zacharias M, De Silva RK, Hickling J, Medlicott NJ, Reith DM (2007) Comparative safety and efficacy of two high dose regimens of oral paracetamol in healthy adults undergoing third molar surgery under local anaesthesia. Anaesth Intensive Care 35(4):544–549
Prescott L (1996) Paracetamol (acetaminophen): a critical bibliographic review. Taylor & Francis Ltd, London
Rumack BH (2002) Acetaminophen hepatotoxicity: the first 35 years. Clin Toxicol 40(1):3–20
Anderson B, Woollard G, Holford N (2001) Acetaminophen analgesia in children: placebo effect and pain resolution after tonsillectomy. Eur J Clin Pharmacol 57(8):559–569
Fouad EA, Ali MS, Kotb HMI, Emara S, Fracs MS, El Minshawy A (2009) Effect of cardiopulmonary bypass on the pharmacokinetics of intravenous paracetamol. Saudi Pharm J 17(2):130–136
Hahn TW, Mogensen T, Lund C, Jacobsen LS, Hjortsoe N-C, Rasmussen SN, Rasmussen M (2003) Analgesic effect of i.v. paracetamol: possible ceiling effect of paracetamol in postoperative pain. Acta Anaesthesiol Scand 47(2):138–145
Allegaert K, Van der Marel CD, Debeer A, Pluim MAL, Van Lingen RA, Vanhole C, Tibboel D, Devlieger H (2004) Pharmacokinetics of single dose intravenous propacetamol in neonates: effect of gestational age. Arch Dis Child Fetal Neonatal Ed 89(1):F25–F28
Rawlins MD, Henderson DB, Hijab AR (1977) Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol 11(4):283–286
Clements JA, Critchley JAJH, Prescott LF (1984) The role of sulphate conjugation in the metabolism and disposition of oral and intravenous paracetamol in man. Br J Clin Pharmacol 18(4):481–485
Morris ME, Levy G (1984) Renal clearance and serum protein binding of acetaminophen and its major conjugates in humans. J Pharm Sci 73(8):1038–1041
Liukas A, Kuusniemi K, Aantaa R, Virolainen P, Niemi M, Neuvonen PJ, Olkkola KT, Liukas A, Kuusniemi K, Aantaa R, Virolainen P, Niemi M, Neuvonen PJ, Olkkola KT (2011) Pharmacokinetics of intravenous paracetamol in elderly patients. Clin Pharmacokinet 50(2):121–129
Klotz U (2009) Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 41(2):67–76
Pickering G, Schneider E, Papet I, Pujos-Guillot E, Pereira B, Simen E, Dubray C, Schoeffler P (2011) Acetaminophen metabolism after major surgery: a greater challenge with increasing age. Clin Pharmacol Ther 90(5):707–711
Martin U, Temple RM, Winney RJ, Prescott LF (1991) The disposition of paracetamol and the accumulation of its glucuronide and sulphate conjugates during multiple dosing in patients with chronic renal failure. Eur J Clin Pharmacol 41(1):43–46
Acknowledgments
The authors would like to thank the School of Pharmacy, University of Otago for Katie Owens’ PhD Scholarship and Pharsight (Mountain View, CA, USA) for the provision of Phoenix® NLME™.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Owens, K.H., Murphy, P.G.M., Medlicott, N.J. et al. Population pharmacokinetics of intravenous acetaminophen and its metabolites in major surgical patients. J Pharmacokinet Pharmacodyn 41, 211–221 (2014). https://doi.org/10.1007/s10928-014-9358-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-014-9358-0