Abstract
Background and Objective
Acetaminophen (paracetamol) is a ubiquitously administered drug in critically ill patients. Considering the dearth of literature, we evaluated the population pharmacokinetics of intravenous acetaminophen and its principal metabolites (sulfate and glucuronide) in this population.
Methods
Critically ill adults receiving intravenous acetaminophen were included in the study. One to three blood samples were withdrawn per patient for the estimation of acetaminophen, and its metabolites (acetaminophen glucuronide and acetaminophen sulfate). High-performance liquid chromatography was used for measuring serum concentrations. We used nonlinear mixed-effect modeling for estimating the primary pharmacokinetic parameters of acetaminophen and its metabolites. The effect of covariates was evaluated followed by dose optimization using Monte Carlo simulation. Patient factors such as demographic information, liver and renal function tests were used as covariates in population pharmacokinetic analysis. The therapeutic range for serum acetaminophen concentration was considered to be 66–132 μM, while 990 μM was considered as the threshold for toxic concentration.
Results
Eighty-seven participants were recruited. A joint two-compartment acetaminophen pharmacokinetic model linked to glucuronide and sulfate metabolite compartments was used. The central and peripheral volume distributions were 7.87 and 8.87 L/70 kg, respectively. Estimated clearance (CL) was 0.58 L/h/70 kg, while intercompartmental clearance was 44.2 L/h/70 kg. The glucuronide and sulfate metabolite CL were 22 and 94.7 L/h/70 kg, respectively. Monte Carlo simulation showed that twice-daily administration of acetaminophen would result in a relatively higher proportion of patient population achieving and retaining serum concentrations in the therapeutic range, with reduced risk of concentrations remaining in the toxic range.
Conclusion
A joint pharmacokinetic model for intravenous acetaminophen and its principal metabolites in a critically ill patient population has been developed. Acetaminophen CL in this patient population is reduced. We propose a reduction in the frequency of administration to reduce the risk of supra-therapeutic concentrations in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Clearance of acetaminophen in critically ill patients is reduced. |
Dosing regimen modifications are suggested for intravenous acetaminophen in this patient population. |
1 Introduction
Critically ill patients are often have a need for multiple drug administration owing to the severity of their primary illness and the associated co-morbidities. A recent study estimated a median (range) of 11 (8–16) drugs administered in critically ill adults [1]. Factors altering the pharmacokinetics of drugs in critically ill patients include changes in the gastric emptying time, variations in the plasma protein binding and hepatic blood flow, and alterations in renal clearance [2]. Generally, an increased volume of distribution (Vd) is observed for hydrophilic drugs, necessitating loading doses [3]. Regarding changes in the renal clearance, a reduction is often observed owing to acute kidney injury [4]. However, recently augmented renal clearance (ARC) has been observed in critically ill patients (in particular with widespread systemic inflammation) to an extent of around 52.5% [5]. Together, these critical factors carry a greater potential to result in altered pharmacokinetics and consequently pharmacodynamics of drugs in this sub-population.
Acetaminophen is a hydrophilic drug, and the most used antipyretic and analgesic drug worldwide [6]. Several routes of administration of acetaminophen are used in clinical practice, namely, oral, rectal, and intravenous. In critically ill patients, oral bioavailability of paracetamol may be compromised due to reduced gastric motility as well as altered intestinal blood flow, and so an intravenous route with a better predictable pharmacokinetics (and accordingly pharmacodynamics) is preferred [7]. In adult healthy humans, clearance (CL) and volume of distribution (Vd) were 24.0 L/70 kg, while 43.5 L/70 kg was observed for acetaminophen [8]. Critically ill patients on ventilator support demonstrated an elimination half-life of 2.2 h, Vd of 1.03 L/kg, and CL of 0.33 L/kg/h [9]. A recent study in critically ill patients with traumatic brain injury observed a greater acetaminophen CL and a wide range of Vd of acetaminophen following intravenous administration, and recommended higher doses for the optimal control of pyrexia [10]. Considering a therapeutic reference range of 10–20 µg/ml for the antipyretic and analgesic effects, a significant number of critically ill patients (8 of 21) were observed with below-detectable limits of serum acetaminophen levels [11]. Despite a uniform recommendation that acetaminophen at 1 g every 6 h is ineffective and higher doses must be considered for the critically ill, none of these studies evaluated the impact of various dosing regimens on achieving therapeutic concentrations. Acetaminophen is primarily metabolized to its sulfate and glucuronide metabolites, while a small fraction did to an alkylating metabolite, N-acetyl-p-benzoquinone imine [12]. Nearly a fifth of critically ill patients have been observed with acquired liver injury and failure due to cholestatic/hypoxia/mixed etiology [13]. Critically ill patients often need administration of acetaminophen as a part of their pain or fever control; yet they are at increased risk of hepatotoxicity due to the severity of their illness and the toxic metabolite from acetaminophen. There is an absence of any published literature on the estimation of acetaminophen metabolites in critically ill adult patients. Hence, the present study was undertaken to evaluate the pharmacokinetics of acetaminophen and the principal metabolites following intravenous administration, using a population pharmacokinetic model approach, and to find out the optimized dosing regimen for obtaining therapeutic trough concentrations.
2 Methods
2.1 Study Design and Ethics
This was a cross-sectional study carried out following Institutional Ethics Committee approval and written consent from patients or their legally acceptable representatives. The study was carried out between November 2021 and April 2022 in the Salmaniya Medical Complex, the largest intensive care unit in the Kingdom of Bahrain. We adhered to the latest Declaration of Helsinki guidelines.
2.2 Study Procedure
We included critically ill adults (aged 21 years) receiving intravenous paracetamol for any indications. Those admitted only for observatory purposes were excluded. The following details were captured: age, sex, body weight, APACHE II score (acute physiology and chronic health evaluation II score) within a day of admission, time-point of intravenous paracetamol administration, liver function tests [serum alkaline phosphatase (ALP), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), and total proteins], and serum creatinine. Creatinine clearance (CLCR) was estimated using the Cockcroft–Gault equation [14]. Those with CLCR exceeding 130 ml/min were categorized as having ARC [15]. The standard of care in our intensive care unit is to administer intravenous acetaminophen as infusion in 100 ml normal saline at 1 g over 30 min, irrespective of indications. One to three blood samples (5 ml) were conveniently withdrawn between 0.5 and 6 h following acetaminophen administration per study participant for the estimation of acetaminophen, and its metabolites (acetaminophen glucuronide and acetaminophen sulfate). The whole blood sample was kept at room temperature for 30 min and then centrifuged at 2000 rpm for 10 min. The obtained serum was stored at – 80 °C pending analysis. The therapeutic range for serum acetaminophen concentration was considered to be 66–132 μM (10–20 µg/ml), while 990 μM (150 µg/ml) was considered as the threshold for toxic concentrations [16].
2.3 Bioanalytical Method for Estimation of Serum Paracetamol and its Metabolites
High-performance liquid chromatography (HPLC) was used to measure serum acetaminophen, acetaminophen glucuronide, and acetaminophen sulfate, as described previously [17, 18]. A Waters USA UV/visible detector, consisting of an e2695 pump, and an autosampler,,was used to detect the compounds at the wavelength of 254 nm. All the reagents (acetaminophen, acetaminophen glucuronide, acetaminophen sulfate, potassium dihydrogen orthophosphate, and isopropanol) were obtained from Sigma-Aldrich. Tetrahydrofuran (THF) was purchased from Fluka. Methanol and water at the ratio of 3: 1 was used as the isocratic mobile phase that was passed on through the C18 analytical column with 1.2 ml/min flow rate for acetaminophen estimation. For the estimation of acetaminophen metabolites, the mobile phase consisted of potassium dihydrogen orthophosphate–isopropanol–THF combination that was passed on through C18 column at a flow rate of 1 ml/min. Serum acetaminophen was estimated with the limit of detection of 16 μM, and a range between 16 and 1324 μM was the linearity. Similarly, acetaminophen metabolites were estimated with the limit of detection of 0.1 μM with 0.1 and 10 μM as the linearity range. The percent coefficient of variations for all the analytes were less than 5%, thus establishing the accuracy and precision of the assays. Only one concentration exceeded the limit of quantification for serum acetaminophen and was included in the analysis, while none of the measured concentrations for the metabolites were out of the quantification limits.
2.4 Pharmacokinetic Modeling
We used a nonlinear mixed-effects approach for joint modeling of the pharmacokinetics of acetaminophen and its metabolites (acetaminophen glucuronide and acetaminophen sulfate). Estimation of pharmacokinetic parameters was achieved using the stochastic approximation expectation maximization algorithm implemented in Monolix 2021R2 software [19]. Since the data were very sparse, a Bayesian approach was implemented by considering prior pharmacokinetic parameters from the literature [8]. We used a two-compartment model to model the pharmacokinetics of acetaminophen. A one-compartment model with linear elimination was used to model acetaminophen glucuronide and the sulfate metabolites. Since the Vd of the two metabolites is not identifiable, it was set to the central volume of distribution (V1) of acetaminophen. The CL for the two metabolites was estimated from the data. The concentration–time profiles of acetaminophen, acetaminophen glucuronide, and acetaminophen sulfate were jointly modeled according to the series of differential equations (Eqs. 1–4) as illustrated in Fig. 1. The amount of acetaminophen in the central and peripheral compartments is denoted by Am and Ap, respectively. The acetaminophen central and peripheral volume of distributions and inter-compartmental clearance were represented by V1, V2, and Q, respectively. The rate constant associated with the formation of acetaminophen glucuronide is denoted at kpm1, while that of acetaminophen sulfate as kpm2. Am1 is the amount of acetaminophen glucuronide and Am2 the amount of acetaminophen sulfate.
Schematic of the parent-metabolite pharmacokinetic model of acetaminophen. Ac and Ap are the amounts of acetaminophen in the central and peripheral compartments, respectively, V1 is the central volume of distribution for acetaminophen which is assumed to be equal to acetaminophen sulfate and acetaminophen glucuronide volume of distribution, and Am1 and Am2 are the amounts of glucuronide and sulfate metabolite of acetaminophen, respectively
The residual unexplained variability was explored with proportional, combined (additive and proportional), and exponential models. The variability in the pharmacokinetic parameters was described by the interindividual variability model.
The effect of covariates on the pharmacokinetic parameters was investigated, to account for body size effect (weight), allometric scaling by fixing exponents of the volume of distribution and clearance to 1 and 0.75, respectively. The covariates tested were, age, sex, APACHE II score, serum total protein, creatinine clearance, alanine transaminase, alkaline phosphatase, and gamma-glutamyl transferase. All covariates were introduced in the model one at a time. If the introduction of the covariate resulted in a statistically significant change in interindividual variability and objective function value (OFV) of a minimum 3.84 points, it was retained in the model. This was followed by a backward deletion step of covariates in the full model. Deletion of the covariate was carried out if it led to the decrease in the OFV by at least 6.63 points and the Wald test was significant (p < 0.05).
2.5 Model Evaluation
The final pharmacokinetic model was assessed for possible misspecification using visual diagnostic plots such model-predicted concentration versus observed concentration, individual weighted residuals (IWRES) versus time/predicted concentration, and visual predicted checks (VPC). Numerical diagnoses were also performed to assess how well the observed data fit the final pharmacokinetic model. These included the decrease in the OFV defined as \(-2*loglikelihood\) precision in the estimated pharmacokinetic parameters described as the percentage relative standard error (%RSE). The robustness and stability of the final pharmacokinetic model were evaluated by performing a non-parametric bootstrap (500 bootstrap runs) resampling procedure. This was achieved using Rsmlx ® Speaks ‘Monolix’), which is a Monolix R package [20].
2.6 Assessment of Current Dose and Dosing Regimen Optimization
The minimum acetaminophen serum concentration needed for analgesia and antipyresis is 66 μM (10 μg/mL) [16] while a serum concentration of 990 μM (150 μg/mL) is considered the threshold for hepatotoxicity [21]. Therefore, the target for the pharmacokinetics simulation for the assessment of the present dose and dosing regimen was the acetaminophen serum concentration between ≥ 66 and < 990 μM in 24 h. Similarly, hepatoxicity was assessed by determining the time the acetaminophen plasma concentration was above 990 μM (T ≥ 990 μM) in 24 h. Monte Carlo simulations were performed in Simulx 2021 to determine the optimum dosing regimen.
3 Results
3.1 Demographic Characteristics
Eighty-seven participants were recruited, and Table 1 provides a summary of their characteristics. The following diagnoses were observed amongst the study participants: post-operative surgery (n = 31), sickle cell disease with vaso-occlusive crisis (n = 16), cerebro-vascular accidents (n = 12), sepsis (n = 11), road-traffic accident with head injury (n = 7), burns (n = 2), and one each with diabetic ketoacidosis, immune thrombocytopenic purpura, epilepsy, renal vein thrombosis, beta thalassemia, Hodgkin’s lymphoma, end-stage renal dysfunction, and acute pneumonia with cystic fibrosis. Following were the concomitant disorders observed: systemic hypertension (n = 24), diabetes mellitus (n = 19), and systemic lupus erythematosus (n = 2).
3.2 Pharmacokinetic Modeling
A two-compartment pharmacokinetic model has been built starting from the prior pharmacokinetic model [8]. The residual variability was best explained by the exponential error model. The interindividual variability and residual error values of the base model are displayed in Table 2. Inclusion of body weight through allometric scaling improved the model (ΔOFV, − 8.49), which explained 15.8% and 10% of the variation in clearance and Vd, respectively. Parameters were estimated with better precision when allometric exponents were fixed than when estimated. The clearance or Vd was not significantly affected by sex, age, serum total protein, alanine transaminase, alkaline phosphatase, and gamma-glutamyl transferase. The final pharmacokinetic model parameters are displayed in Table 3. Equations (5–10) represent the individual models for Vd and clearance:
where acetaminophen central and peripheral individual volumes of distribution models are denoted by V1,i and V2,i, respectively, individual clearance and intercompartmental clearance are denoted by CLi and Qi, respectively, CLm1i is the individual clearance of acetaminophen glucuronide, CLm2i denotes the individual clearance of acetaminophen sulfate, and the random effect in the individual parameter is denoted by ηi. APACHE and CLCR are covariates predictive of the pharmacokinetic parameters. All pharmacokinetic parameters have been normalized to the typical individual in the population weighing 70 kg.
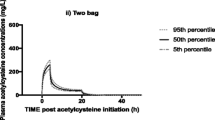
A good agreement was observed in the diagnostic plots between the predicted and observed concentration data points (Fig. 2). However, there was a slight underprediction of higher concentrations for both metabolites. The VPC (Fig. 3) for acetaminophen and the two metabolites showed that the observed data points were within the model-predicted range. The plots of the IWRES (Fig. 4) versus time or predicted concentration did not show any significant trends in the splines. The bootstrap parameters were comparable with the estimated parameters. There was high BSV in the CL, Q, and V2. Additionally, Q and kpm2 were not estimated with good precision as indicated by the high %RSE of 99 and 88.8%, respectively (Table 3).
3.3 Dosing Regimen Optimization
The original study design was a combination of 1000 mg BID (twice a day), TDS (three times a day) and QID (four times a day) dose regimens. Simulations of a BID dose regimen showed that 47% of the population attained the desired target concentration range within an average time of 15 h. Simulation of the TDS and QID dose regimes led to 40% of the population attaining the target concentration range within the time range of 13–14 h. The BID dose had the lowest average time in which plasma concentration was above 990 μM compared to the TDS and QID dose regimens. In terms of toxicity in the BID group, the acetaminophen serum concentration in 20% of the population was in the toxic range for 5 h. The plasma concentration in 34% of the population for both TDS and QID was in the toxic range for an average of 8 h.
4 Discussion
We evaluated the pharmacokinetic parameters of acetaminophen (parent drug and sulfate and glucuronide metabolites) following intravenous administration in 87 critically ill patients. The following were the primary pharmacokinetic parameters observed: Vd (7.87 L/70 kg) and CL (0.58 L/h/70 kg) of the parent drug. Dose optimization suggested twice-daily administration of acetaminophen at 1000 mg to ensure that a relatively higher proportion of the population retained their serum concentrations in the therapeutic range with reduced risk of their concentrations remaining in the toxic range.
Studies evaluating pharmacokinetics of intravenous acetaminophen in the critically ill are limited. In healthy humans, a mean (SD) CL following intravenous acetaminophen was 352 (40) ml/min [22], while in the present study (among the critically ill), it was 9.7 ml/min (0.58 L/h). Even the summary of product characteristics for intravenous acetaminophen recommends a mean CL of 18.9 L/h (0.27 L/h/kg) in general, which is nearly 32-fold higher than the critically ill [23]. A recent pooled analysis from seven studies and 2755 concentrations revealed a mean CL of 16.7 L/h/70 kg [24]. Morse et al., in 116 healthy volunteers, also observed a CL of 24 L/h/70 kg [8]. de Maat et al. evaluated the pharmacokinetics of intravenous acetaminophen among patients admitted in medium and intensive care units [25]. The authors used non-compartment analysis using formulae-based estimation of the pharmacokinetic parameters and observed a median CL of 23.65 L/h with the median body weight of 78 kg. They also observed a higher Vd (52 L) compared to the present study. Samson et al. [9] used population pharmacokinetic modeling in 19 critically ill patients following the first dose of intravenous acetaminophen (dosing regimen: 1 g every 6 h) and observed a clearance of 0.33 L/kg/h. The authors also observed that almost all patients had their serum concentrations below the therapeutic range. Acetaminophen is perceived as a relatively safer drug, ubiquitously compared to other non-steroidal anti-inflammatory drugs. However, we observed that a 1-g dose administered three or four times daily is associated with increased risk of toxicity (due to toxic drug concentrations). Mild to moderate hepatic dysfunction is commonly observed in the critically ill, attributed either to ischemic hepatitis manifesting as diffuse hepatic necrosis or to cholestatic liver disease [26]. This explains the reduced clearance of the parent drug and the simulation results pointing towards the administration of a twice-daily regimen which is associated with the least risk of toxicity in the present study.
The present study is the first to evaluate the pharmacokinetics of intravenous acetaminophen and its principal metabolites in critically ill patients through a population pharmacokinetic approach. However, we did not evaluate the hypotensive effect of intravenous acetaminophen and its relationship to the dose and pharmacokinetic parameters. Due to feasibility concerns, we did not collect blood samples for analysis in steady state as a steady state was not achieved based on the required doses. Concomitant drugs with potential interaction with paracetamol were not considered as there are hardly any well-established pharmacokinetic interactions with the drug and a small sample size. Pharmacodynamic studies evaluating the therapeutic response to various dosing regimens in addition to PK parameters would help in a better understanding of the therapeutic implications of various dosing regimens in this vulnerable population.
5 Conclusion
Primary pharmacokinetic parameters for intravenous acetaminophen in critically ill populations have been established along with their principal metabolites. A reduced clearance of the parent drug was observed, and a reduction in the frequency of administration should be considered to reduce the risk of supra-therapeutic drug concentrations in this population.
References
Sridharan K, Hasan H, Al Jufairi M, Al Daylami A, Abdul Azeez Pasha S, Al Ansari E. Drug utilisation in adult, paediatric and neonatal intensive care units, with an emphasis on systemic antimicrobials. Anaesthesiol Intensive Ther. 2021; 53(1): 18-24.
Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. Introduction to drug pharmacokinetics in the critically iII patient. Chest. 2012;141:1327–36.
Tansley G, Hall R. Pharmacokinetic considerations for drugs administered in the critically ill. Br J Hosp Med. 2015;76:89–94.
Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11.
Sridharan K, Pasha SAA, Qader AM, Hasan HMSN, El Seirafi MM. Drug Utilization in critically Ill adults with augmented renal clearance compared to normal renal clearance: implications for use of antimicrobials with predominant renal excretion. Curr Rev Clin Exp Pharmacol. 2021;16(2):174–81.
Freo U, Ruocco C, Valerio A, Scagnol I, Nisoli E. Paracetamol: a review of guideline recommendations. J Clin Med. 2021;10:3420.
White PF. Cost-effective multimodal analgesia in the perioperative period: Use of intravenous vs. oral acetaminophen. J Clin Anesth. 2020; 61: 109625.
Morse JD, Stanescu I, Atkinson HC, Anderson BJ. Population pharmacokinetic modelling of acetaminophen and ibuprofen: the influence of body composition, formulation and feeding in healthy adult volunteers. Eur J Drug Metab Pharmacokinet. 2022. https://doi.org/10.1007/s13318-022-00766-9.
Samson AD, Hunfeld NG, Touw DJ, Melief PH. Efficacy and pharmacokinetics of intravenous paracetamol in the critically ill patient. Crit Care. 2009;13(Suppl 1):P407.
Parker SL, Saxena M, Gowardman J, Lipman J, Myburgh J, Roberts JA. Population pharmacokinetics of intravenous paracetamol in critically ill patients with traumatic brain injury. J Crit Care. 2018;47:15–20.
Geronimo-Pardo M, Fuster-Lluch O, Peyro-Garcia R, Lizan-Garcia M. Paracetamol pharmacokinetics in critically ill trauma patients. Eur J Anaesthesiol. 2010;27:153.
Athersuch TJ, Antoine DJ, Boobis AR, Coen M, Daly AK, Possamai L, Nicholson JK, Wilson ID. Paracetamol metabolism, hepatotoxicity, biomarkers and therapeutic interventions: a perspective. Toxicol Res (Camb). 2018;7(3):347–57.
Horvatits T, Drolz A, Trauner M, Fuhrmann V. Liver injury and failure in critical illness. Hepatology. 2019;70(6):2204–15.
Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–9.
Chen IH, Nicolau DP. Augmented renal clearance and how to augment antibiotic dosing. Antibiotics (Basel). 2020;9(7):393.
Oscier CD, Milner QJ. Peri-operative use of paracetamol. Anaesthesia. 2009;64(1):65–72.
Sridharan K, Qader AM, Hammad M, Jassim A, Diab DE, Abraham B, Hasan HMSN, Pasha SAA, Shah S. Evaluation of the association between single nucleotide polymorphisms of metabolizing enzymes with the serum concentration of paracetamol and its metabolites. Metabolites. 2022;12(12):1235.
Diab DE, Sridharan K. Development of urinary assay methods for the estimation of paracetamol glucuronide and paracetamol sulfate in preterm neonates with patent ductus arteriosus. Curr Chromat. 2022;9:1–5.
Monolix version 2021R2. Antony, France: Lixoft SAS, 2022. http://lixoft.com/products/monolix/. Accessed 9 October 2022.
Package ‘Rsmlx’. https://cran.r-project.org/web/packages/Rsmlx/Rsmlx.pdf. Accessed 9 Oct 2022.
Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 2002;40(1):3–20.
Rawlins MD, Henderson DB, Hijab AR. Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol. 1977;11(4):283–6.
Acetaminophen injection. Product monograph. Baxter corporation. https://www.baxter.ca/sites/g/files/ebysai1431/files/2021-08/EN_ACETAMINOPHEN%20INJECTION.pdf. Accessed 13 Oct 2022.
Allegaert K, Olkkola KT, Owens KH, Van de Velde M, de Maat MM, Anderson BJ; PACIA study group. Covariates of intravenous paracetamol pharmacokinetics in adults. BMC Anesthesiol 2014; 14: 77.
de Maat MM, Tijssen TA, Brüggemann RJ, Ponssen HH. Paracetamol for intravenous use in medium–and intensive care patients: pharmacokinetics and tolerance. Eur J Clin Pharmacol. 2010;66(7):713–9.
Kluge M, Tacke F. Liver impairment in critical illness and sepsis: the dawn of new biomarkers? Ann Transl Med. 2019;7(Suppl 8):S258.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received to conduct this study.
Data Availability Statement
The data is available with the corresponding author and shall be shared upon a reasonable request.
Conflict of Interest
Kannan Sridharan, Mwila Mulubwa, and Ali Mohamed Qader do not have any conflict of interest.
Ethics Approval
The study was approved by the Institutional Ethics Committee of Arabian Gulf University (E032-PI-9/21 on 17th October 2021), and Salmaniya Medical Complex (126081121 on 8th November 2021), Kingdom of Bahrain.
Consent to participate
Consent to participate in this research study was obtained from the patients or their legally acceptable representatives.
Consent for publication
Not applicable.
Code availability
Not applicable.
Authors' contributions
KS: Conceived the study idea, wrote proposal, and obtained ethics approval; KS, AMQ: Data collection; MW: Model development; KS, MW: Wrote the first draft of the manuscript; KS, MW, AMQ: revised the drafts and final approval of the final version.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sridharan, K., Mulubwa, M. & Qader, A.M. Population Pharmacokinetic Modeling and Dose Optimization of Acetaminophen and its Metabolites Following Intravenous Infusion in Critically ill Adults. Eur J Drug Metab Pharmacokinet 48, 531–540 (2023). https://doi.org/10.1007/s13318-023-00841-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00841-9