Abstract
Soy protein isolate (SPI) has the advantages of low cost, easy processing, stable performance and so on. However, the processing temperature of SPI is high, the processed products are hard and brittle with high water absorption capacity, so it is necessary to modify the soybean protein during processing. Small molecule plasticizer is commonly used globally to plasticize and modify soybean protein. Different plasticizer content has different plasticizing effect on protein, and the properties of materials prepared will also vary greatly. The plasticizer can affect the viscosity of the blends morphology and properties by changing the viscosity of protein phase. In this work, glycerol with the excellent plasticizing effect was selected as plasticizer, and SPI was plasticized with glycerol of different contents, and then the plasticized SPI was blended with poly (butylene adipate-co-terephthalate)(PBAT) to prepare biodegradable blend materials. The influence of plasticizer content on the structure and properties of the blends was explored by observing the microstructure, mechanical and thermal properties of the blends. The results showed that with the increase of glycerol content, the crystallization temperature and melting temperature of the blend system increased, the chain segment was easier to move, and the glass transition temperature(Tg) decreased. At the same time, the viscosity of the blending system decreases, which improves the processing fluidity of the system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the continuous development of the society, environmental pollution and destruction follow, environmental protection and prevention become a social hot spot. With the rise of the field of polymer materials, polymer materials commonly used in nature will cause environmental pollution after being discarded, such as plastic bags and greenhouse film, which will cause white pollution. Therefore, the development and research of biodegradable polymer materials to replace the traditional polymer materials which are difficult to degrade has become a hot topic of social development [1,2,3,4]. Soybean protein-based plastic is a kind of efficiently biodegradable plastic. As a natural polymer, SPI is easily converted into non-polluting carbon dioxide and water by microorganisms in nature. Therefore, the development of soybean protein-based plastic is one of the effective ways to solve the pollution of plastic waste and alleviate the shortage of oil resources in the world [5,6,7,8,9].
Soy protein is the main by-product of soybean oil products. Like other plant proteins, soy protein is a storage protein in soybean. Its monomers are linked by amide bonds to form polypeptide chains, which are intertwined into three-dimensional complex structures by disulfide and hydrogen bonds. Most soy proteins are globulins, containing 20 % basic amino acids, 20 % hydrophobic amino acids, and 25 % acidic amino acids [10,11,12,13]. Although protein has the advantages of degradability, easy processing and low cost, protein plastic products are easy to absorb water and brittle [14, 15]. Therefore, it is necessary to modify the protein before processing. Chemical modification, physical modification and enzyme modification are the main methods of protein modification. These modifications mainly alter the protein structure or conformation without altering the amino acid sequence that forms the polypeptide chain. The denaturation process of protein mainly refers to the modification of the secondary, tertiary or quaternary structures of protein molecules [16,17,18]. After protein denaturation, the spherical tight structure of protein is destroyed, the molecular chain is reorganized, the intermolecular or intramolecular forces are reduced and the molecular chain becomes extended [19, 20].
The methods commonly used to prepare soy protein plastics are hot pressing and extrusion [21, 22]. SPI has good biodegradability, but it is sensitive to water and brittle and easy to break, which limits the application of SPI material, so it needs to be plasticized [23, 24]. Glycerol is the most commonly used plasticizer for protein materials. Glycerol has a high boiling point and can form strong intermolecular hydrogen bonds with protein molecules, so it has a high stability in protein materials. The low strength and high water absorption of SPI plasticized bioplastics still limit the application of SPI. The simplest and most effective way to improve the performance of protein materials is to mix soy protein with other natural or synthetic polymers.
PBAT has the properties of both polybutyl acrylate(PBA) and polybutylene terephthalate(PBT), and is a new biodegradable material. PBAT contains flexible aliphatic chain and rigid aromatic bond, so it has high toughness and high temperature resistance. However, due to the existence of ester bond, it has good ductility and elongation at break, as well as good heat resistance and impact performance [25,26,27,28].
At present, the research of SPI/PBAT blend composites has attracted more and more favor and attention, In our previous work, we with SPI and MA-g-PBAT as raw materials, prepared by melt blending method the SPI/MA-g-PBAT biodegradable blend material, explores SPI content and type of plasticizer on the influence of the blending material structure and performance. On the basis of previous work, this study selected glycerol with better plasticizing effect to plasticize SPI by controlling its content, and then blended it with MA-g-PBAT to prepare biodegradable materials. The addition of glycerol as a plasticizer improves the mechanical properties as well as thermal stability of the blends, and improves the fluidity of the system, which provides a feasible strategy for the design and optimization of biodegradable materials in the future.
Experiment
Materials
SPI was purchased from Longyun Protein Food Co., Ltd. PBAT was purchased from Zhejiang Hangzhou Xinfu Pharmaceutical Industry. Glycerol was purchased from was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. All of these materials were directly used without further purification.
Preparation of SPI/ MA-g-PBAT Hot-pressing Films
According to previous studies, pure PBAT and a group of MA-g-PBAT with the highest grafting rate were selected as the raw materials of the blend, and the plasticizer with the best effect was glycerol. Before the experiment, pure PBAT, MA-g-PBAT and SPI (30 %) were dried. The soy protein was plasticized with different content of plasticizer, and the plasticized SPI was put in place for one day, then the MA-g-PBAT was blended with the plasticized SPI by a mixer, and the product was pressed into a film by hot pressing. The preparation process is shown in Fig. 1.
Characterization
In order to study the functional groups and their interactions, the sample was scanned from 4000 to 400 cm-1 by using Fourier transform infrared spectroscopy (FTIR, Nicolet Avater-370, USA). The graft sample was mixed with KBr powder and pressed into tablets. The morphology of the samples was observed using a scanning electron microscope (SEM) (S-4300, Hitachi Ltd, JPN). Firstly, SPI/MA-g-PBAT blend material was vacuum dried at 90 oC for 1 day, then freeze-quenched and freeze-dried in liquid nitrogen. Gold was sprayed on the cross section and the working voltage was set at 15 kV. Differential scanning calorimetry (DSC) analysis was performed by a thermal analyzer (Q100, TA, US). The sample weight was 5–10 mg. In the nitrogen atmosphere, the temperature was raised to 170 oC to eliminate the thermal history. Then the temperature was decreased to − 60 oC at 10 oC/min, and then raised to 170 oC at 10 oC/min. The mechanical properties of the samples were tested by microcomputer controlled electronic universal testing machine (CMT6104, Shenzhen Xinsaini Design & Measurement Company). According to the national standard ISO 1184–1983, the tensile speed was 100mm/min. Each group of samples was tested with five specimens and then the average value was taken. Before the tensile test, the sample was placed in the humidity box for a week, so that the prepared spline maintained the same humidity and stress was completely released. TGA (Q5000, TA Instrument Company) was used to characterize the thermal stability of the samples. Nitrogen was used as the purge gas, and the samples were heated to 800 oC at a heating rate of 20 oC /min.
Results and Discussion
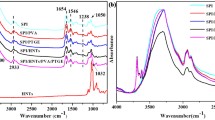
The FTIR spectra of the pure SPI and SPI/MA-g-PBAT blends with different glycerol plasticizers is shown in Fig. 2. The peak near 2800 ~ 2900 cm− 1 corresponds to the stretching vibration of –CH3 or –CH2–. The strong peak corresponding to 1800 ~ 1600 cm− 1 appears to be the stretching vibration of C = O. For pure SPI, the peak near 1650 cm− 1 is the characteristic peak of the amide I band of SPI (C = O stretching vibration in SPI molecule), the peak near 1540 cm− 1 is the characteristic band of amide II band (N – H bending) [29, 30].
The FTIR spectra of the SPI/MA-g-PBAT blends were analyzed by comparing the different content of glycerol plasticizers. It was found that no new peaks were observed, indicating that no new groups were formed after blending. Compared with the pure SPI, the C = O stretching vibration peaks and the N-H bending vibration peaks in the blends are blue shifted, indicating that the C = O of maleic anhydride(MAH) interacts with the amide ll band in SPI by hydrogen bond.
The microstructure of SPI/MA-g-PBAT blend with different glycerol content was shown in Fig. 3. The blends showed a typical Sea Island structure. Among them, SPI is the island structure in the blend. In the blend system, the soy protein is a rigid particle without the addition of plasticizer, the phase size is large, and the two phase interface is obvious. After adding plasticizer, the size of protein phase decreased obviously and the compatibility of two phases was improved obviously. When the plasticizer content is 10 %, the protein phase is lamellar. This is because the plasticizer content is low, the interaction between the protein molecular chains is large, the protein viscosity is high and the fluidity is poor, so the protein is pulled into lamellar by the shear force during the blending. With the increase of the content of plasticizer, the dispersibility of the protein phase in the blend system was improved, and the lamellar structure of the SPI dispersed photo was more and more obvious. In the process of increasing glycerol content, the protein gradually evolved from the rigid particle state to the ductile band structure. Therefore, with the increase of the content of glycerol plasticizer, the compatibility of SPI with MA-g-PBAT was improved, and the dispersibility of protein was improved.
As shown in Fig. 4a, by comparing the glass transition and melting curves of blends prepared under different plasticizer contents, it can be seen that with the increase of glycerol content, the melting peak of the blend moved to high temperature. The added glycerol could penetrate into the SPI molecules, destroying the force between the protein molecular chains, which extends the protein molecular chains that were originally aggregated and folded, increasing the free volume of protein molecules, and reducing the glass transition temperature (Fig. 4c). The increase of glycerol content makes the SPI molecular chain easier to move, so the probability of contact with the PBAT molecular chain is greatly increased, and the interaction between the two phases of the blend is improved. As the molecular chain breaking of the blend material needs to absorb higher energy, the increase of glycerol content increases the melting temperature of the blend material.
Figure 4b shows the change of the crystallization curves of the blend material. With the increase of the content of glycerol plasticizer, the crystallization peaks of the blend moved to the high temperature. This may be because the addition of glycerol makes the molecular chain of the blend material more flexible, and it is easier for the molecular chain to extend and crystallize in the cooling process. Therefore, the addition of glycerol makes the blend material crystallize at higher temperature.
The effect of glycerol plasticizer content on the torque of the blend is shown as Fig. 5a and b. When SPI and MA-g-PBAT blend, the torque increases firstly and then decreases, and finally the torque tends to balance. It can be seen from the Fig. 5a that with the increase of glycerol content, the maximum torque of the blend is 26 Nm from the 36 Nm to the glycerol content of 40 % of the SPI content, and the torque is significantly reduced. It can be seen from the figure that with the increase of glycerol content, the maximum torque of the blend material decreases from 36Nm without plasticizing to 26Nm when glycerol content is 40 %, and the torque is significantly reduced. And the equilibrium torque (shown in Fig. 5b) decreased rapidly after the addition of plasticizer, and then the glycerol content increased and the balance torque decreased less and less. Small molecules of glycerol penetrate into the SPI molecular chain, hydroxyl groups in glycerol form hydrogen bonds with the protein molecules, then cohesion energy of protein molecules decreases and the folded molecular chains stretch, which reduce the interaction between SPI molecules and the internal friction between SPI molecular chains, thus reducing the viscosity and increasing the fluidity of the system. Therefore, with the increase of glycerol content, the torque of the blends decreases.
Blends of different content of glycerol plasticized by the stress-strain curve is shown in Fig. 6a. The stress-strain curves of the blends with plasticized glycerol of different contents are shown in Fig. 6a. Without plasticizer, the rigidity of protein phase was high, and brittle fracture occurred in the blend material. When the plasticizer was added, the rigidity of protein phase was weakened, and the yield phenomenon appeared in the tensile process of the blend material, but the yield phenomenon was not obvious when the plasticizer content was low, and the ductile fracture occurred in the blend material. With the increase of glycerol content, the yield phenomenon of the blends is obvious. As you can see, Fig. 6b shows the tensile properties of SPI/MA-g-PBAT blends with different glycerol content. It can be seen that the tensile strength and elastic modulus of the blends decrease while the elongation at break increases. Protein phase is rigid, as a small molecule polar plasticizer, glycerol can be inserted between the protein molecular chain of glycerol on the molecular chain of hydroxyl and SPI to form strong hydrogen bonds, the SPI aggregation folding of peptide chains at full stretch, orderly, reduce the SPI cohesive energy, protein in blend materials in dispersion increased, which makes the tensile strength of blends decreased and the elongation increased. Therefore, biodegradable products with certain tensile strength, especially the elongation at break, can be prepared by adjusting the content of glycerol plasticizer.
Figure 7 is the decomposition curve (TG) and decomposition rate curve (DTG) of the sample obtained in the nitrogen environment. Thermal decomposition is divided into three stages. The first stage is plasticizer volatilization at 220 ~ 260 oC. In the second stage, the molecular chains of protein phase were broken and decomposed at 320 ~ 350 oC, and in the third stage, the macromolecular skeleton of PBAT phase in the blends was broken and decomposed at 380 ~ 450 oC.
With the increase of glycerol content, blending materials have no significant changes in the thermal stability of 400 oC. Above 450 oC, the carbon residue decreases, because the higher the glycerol content of the same quality test sample, the corresponding blend quality decreases. Although the amount of carbon residue decreases above 450 oC, the thermal decomposition temperature has no significant change.
Conclusions
In summary, using SPI and PBAT as raw materials, we successfully prepared a series of SPI/MA-g-PBAT blend film materials with different glycerol plasticizer contents by melt blending method blend, to explore the impact of different glycerol contents on the structure and properties of the blend materials. The results showed that when glycerol plasticizes the SPI, glycerol can penetrate into the internal structure of the protein and form hydrogen bonds with the molecules of SPI, which weakens the interaction between the molecules of SPI and causes the folded peptide chains in the molecules of SPI to stretch out and arrange in an ordered way. As a result, the crystallization temperature and melting temperature of the blend system increased, and the free volume of protein molecules increased, meanwhile the chain segment moved more easily, and the glass transition Tg decreased. With the increase of glycerol content, the maximum decomposition rate of the blend system decreased, indicating that the interaction between the two phases was enhanced, and the plasticizing effect of glycerol was obvious. It can be seen from the torque test that glycerol can significantly reduce the torque of SPI processing, reduce the viscosity of the blend system and improve the fluidity of the system.
References
Battu Deeksha VS, Hariram N, Varada Rajulu Anumakonda (2021) Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J Bioresour Bioproduct 6(1):75–81. https://doi.org/10.1016/j.jobab.2021.01.003
Henry C, Oyeoka CME, Iheoma C, Nwuzor CM, Obele JT, Nwabanne (2021) Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J Bioresour Bioproduct. https://doi.org/10.1016/j.jobab.2021.02.009
Demirbas A (2007) Biodegradable plastics from renewable resources. Ener Sour Part A-Recovery Util Environ Eff 29(5):419–424. https://doi.org/10.1080/009083190965820
Zubair M, Ullah A (2020) Recent advances in protein derived bionanocomposites for food packaging applications. Crit Rev Food Sci Nutr 60(3):406–434. https://doi.org/10.1080/10408398.2018.1534800
Zhao F, Zhang D, Li X, Dong H (2018) High-pressure homogenization pretreatment before enzymolysis of soy protein isolate: the effect of pressure level on aggregation and structural conformations of the protein. Molecules 23 (7). doi:https://doi.org/10.3390/molecules23071775
Zhao F, Liu X, Ding X, Dong H, Wang W (2019) Effects of high-intensity ultrasound pretreatment on structure, properties, and enzymolysis of soy protein isolate. Molecules 24 (20). doi:https://doi.org/10.3390/molecules24203637
Tian HF, Guo GP, Xiang AM, Zhong WH (2018) Intermolecular interactions and microstructure of glycerol-plasticized soy protein materials at molecular and nanometer levels. Polym Test 67:197–204. doi:https://doi.org/10.1016/j.polymertesting.2018.03.002
Wang Z, Kang HJ, Zhao SJ, Zhang W, Zhang SF, Li JZ (2018) Polyphenol-induced cellulose nanofibrils anchored graphene oxide as nanohybrids for strong yet tough soy protein nanocomposites. Carbohydr Polym 180:354–364. doi:https://doi.org/10.1016/j.carbpol.2017.09.102
Xu B, Wu X, Ma W, Qian L, Xin F, Qiu Y (2018) Synthesis and characterization of a novel organic-inorganic hybrid char-forming agent and its flame-retardant application in polypropylene composites. J Anal Appl Pyrol 134:231–242. doi:https://doi.org/10.1016/j.jaap.2018.06.013
Guo G, Tian H, Wu Q (2019) Influence of pH on the structure and properties of soy protein/montmorillonite nanocomposite prepared by aqueous solution intercalating. Appl Clay Sci 171:14–19. doi:https://doi.org/10.1016/j.clay.2019.01.020
Ferreira LF, de Oliveira ACS, Begali DD, Neto ARD, Martins MA, de Oliveira JE, Borges SV, Yoshida MI, Tonoli GHD, Dias MV (2021) Characterization of cassava starch/soy protein isolate blends obtained by extrusion and thermocompression. Ind Crop Prod 160:11. doi:https://doi.org/10.1016/j.indcrop.2020.113092
Kumar R, Anjum KN, Rani S, Sharma K, Tiwary KP, Kumar KD (2019) Material properties of ZnS nanoparticles incorporated soy protein isolate biopolymeric film. Plastics Rubber Composites 48(10):448–455. doi:https://doi.org/10.1080/14658011.2019.1651125
Guo G, Tian H, Wu Q (2019) Nanoclay incorporation into soy protein/polyvinyl alcohol blends to enhance the mechanical and barrier properties. Polym Compos 40(9):3768–3776. doi:https://doi.org/10.1002/pc.25238
Cao N, Fu YH, He JH (2007) Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocolloids 21(7):1153–1162. doi:https://doi.org/10.1016/j.foodhyd.2006.09.001
Xi DL, Yang C, Liu XY, Chen MQ, Sun C, Xu YL (2005) Graft polymerization of styrene on soy protein isolate. J Appl Polym Sci 98(3):1457–1461. doi:https://doi.org/10.1002/app.22278
Yufei H, Kuang L, Hui C, Jianzhang L (2017) Properties of soy protein isolate biopolymer film modified by graphene. Polymers 9(8):312–312. doi:https://doi.org/10.3390/polym9080312
Martelli-Tosi M, Assis OBG, Silva NC, Esposto BS, Martins MA, Tapia-Blacido DR (2017) Chemical treatment and characterization of soybean straw and soybean protein isolate/straw composite films. Carbohydr Polym 157:512–520. doi:https://doi.org/10.1016/j.carbpol.2016.10.013
Chen Y, Wang W, Qiu Y, Li L, Qian L, Xin F (2017) Terminal group effects of phosphazene-triazine bi-group flame retardant additives in flame retardant polylactic acid composites. Polym Degrad Stab 140:166–175. doi:https://doi.org/10.1016/j.polymdegradstab.2017.04.024
Guan M, Chen X, Zhang Y, Tang X, Du K, Yang S, Yong C (2020) Alkali- and glutaraldehyde-modified pig blood as renewable adhesives for engineered bamboo products. J Adhes Sci Technol. doi:https://doi.org/10.1080/01694243.2020.1824364
Coltelli MB, Aliotta L, Gigante V, Bellusci M, Cinelli P, Bugnicourt E, Schmid M, Staebler A, Lazzeri A (2020) Preparation and compatibilization of PBS/Whey protein isolate based blends. Molecules. doi:https://doi.org/10.3390/molecules25143313
Zhang J, Mungara P, Jane J (2001) Mechanical and thermal properties of extruded soy protein sheets. Polymer 42(6):2569–2578. doi:https://doi.org/10.1016/s0032-3861(00)00624-8
Ma W, Xu B, Shao L, Liu Y, Chen Y, Qian L (2019) Synthesis of (1,4-Methylenephenylphosphinic acid) piperazine and its application as a flame retardant in epoxy thermosets. Macromol Mater Eng. doi:https://doi.org/10.1002/mame.201900419
Mungara P, Chang T, Zhu J, Jane J (2002) Processing and physical properties of plastics made from soy protein polyester blends. J Polym Environ 10(1–2):31–37. doi:https://doi.org/10.1023/a:1021018022824
Das O, Capezza AJ, Martensson J, Dong Y, Neisiany RE, Pelcastre L, Jiang L, Xu Q, Olsson RT, Hedenqvist MS (2020) The effect of carbon black on the properties of plasticised wheat gluten biopolymer. Molecules. doi:https://doi.org/10.3390/molecules25102279
Costa ARD, Crocitti A, de Carvalho LH, Carroccio SC, Cerruti P, Santagata G (2020) Properties of biodegradable films based on poly(butylene Succinate) (PBS) and poly(butylene Adipate-co-Terephthalate) (PBAT) blends. Polymers 12(10):17. doi:https://doi.org/10.3390/polym12102317
Sangroniz A, Gonzalez A, Martin L, Irusta L, Iriarte M, Etxeberria A (2018) Miscibility and degradation of polymer blends based on biodegradable poly (butylene adipate-co-terephthalate). Polym Degrad Stab 151:25–35. doi:https://doi.org/10.1016/j.polymdegradstab.2018.01.023
Xu Z, Qiao XY, Sun K (2020) Environmental-friendly corn stover/poly(butylene adipate-co-terephthalate) biocomposites. Mater Today Commun 25:9. doi:https://doi.org/10.1016/j.mtcomm.2020.101541
Chen Y, Xu L, Wu X, Xu B (2019) The influence of nano ZnO coated by phosphazene/triazine bi-group molecular on the flame retardant property and mechanical property of intumescent flame retardant poly (lactic acid) composites. Thermochim Acta. doi:https://doi.org/10.1016/j.tca.2019.178336
Tian HF, Guo GP, Fu XW, Yao YY, Yuan L, Xiang AM (2018) Fabrication, properties and applications of soy-protein-based materials: A review. Int J Biol Macromol 120:475–490. doi:https://doi.org/10.1016/j.ijbiomac.2018.08.110
Wang Z, Kang HJ, Zhang W, Zhang SF, Li JZ (2017) Improvement of interfacial interactions using natural polyphenol-inspired tannic acid-coated nanoclay enhancement of soy protein isolate biofilms. Appl Surf Sci 401:271–282. doi:https://doi.org/10.1016/j.apsusc.2017.01.015
Acknowledgements
This work was supported by Natural Science Foundation of Beijing Municipality (2202014), School Level Cultivation Fund of Beijing Technology and Business University for Distinguished and Excellent Young Scholars (BTBUYP2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, P., Zhao, Y., Li, K. et al. Effect of Plasticizer Content on the Structure and Properties of SPI/MA-g-PBAT Blend Films. J Polym Environ 30, 562–568 (2022). https://doi.org/10.1007/s10924-021-02223-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02223-1