Abstract
In this research, poly (vinyl alcohol)/polyethyleneimine/Fe3O4 (PVA/PEI/Fe3O4) microfibers nanocomposite was synthesized in order to remove and/or preconcentrate Pb(II) ions. The microfibers nanocomposite was characterized by FT-IR, SEM, and XRD methods. The effects of various parameters such as pH, amount of adsorbent, and contact time on the adsorption efficiency of Pb(II) ions at the adsorbent were fully investigated and the optimum conditions were established. The adsorption capacity was found to be 370.9 mg g− 1 of the adsorbent. The kinetics of metal ions adsorption was evaluated using pseudo-first-order and pseudo-second-order models. The results showed that the adsorption of the investigated metal ion is in agreement with the pseudo first-order kinetics. The adsorption–desorption process was conducted for five cycles and the removal efficiency still kept 97% after three cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the extensive application of heavy metals, more water resources are contaminated which cause noxious effects on the health body and creates environmental problems. Discharging and elimination of heavy metal ions from water samples is very important from an environmental point of view [1]. The concentration of Pb(II) in environmental samples is vital because it is harmful to the health of humans and other living organisms. This metal ion is used in various forms in industries such as electronics and printing industries. Therefore, this element may enter the water sources from the waste of these industries. In addition, one of the main sources of lead-contamination in drinking waters is the old lead linkups and pipes.
Drinking lead-contaminated waters lead to serious damages such as impaired renal function, cancer, loss of immune systems, nervous system disorders, and mental retardation. The legal limitation set by the US Environmental Protection Agency for a lead concentration is 15 ng mL− 1, however, the World Health Organization has reported it below 10 ng mL− 1 [2,3,4]. Therefore, due to the persistent problems and complications caused by contamination and poisoning with Pb(II), various techniques have been undertaken such as membrane separation, ion exchange, precipitation and reverse osmosis [5,6,7]. Among them adsorption has been growing widely because it is more reasonable financially, it is also easy to function and has high efficiency. Nevertheless, the unfriendly environmental solvent or exhausting methods has been used many years for the synthesis of adsorbents. Hence in this article, the researchers have attempted to prepare the electrospun nanofiber adsorbents whose entire route is a green process. Meanwhile, Pb(II) as a cationic ion has demonstrated remarkable treatment, which shows the vast performance of synthesized adsorbent.
Branched polyethyleneimine (PEI) is an important biocompatible polyamine. Due to the plenty of amine groups, which contain primary, secondary and tertiary amine forms, it can adsorb heavy metal ions through chelations, electrostatic interaction and hydrogen bonding [8, 9]. The advancement of nanotechnology in various fields such as medicine, biology, materials and environment has made the significant abilities of this attractive science to many scientists [10]. In addition, electrospinning is a new technique in the formation of nanofibers, which has been considered for its comprehensive advantages such as easy operation using a variety of polymers, low cost, and high surface area to volume ratio [11, 12]. Herein, electrospun microfibers adsorbent was prepared successfully from the mixture of poly (vinyl alcohol) and polyethyleneimine and magnetized in a co-precipitation method (PVA/PEI/Fe3O4). The nanocomposite was then applied to remove Pb(II) ions from aqueous solutions. The concentration of Pb(II) in the feed and the preconcentrated solution was monitored by differential pulse voltammetry [13].
Experimental
Reagent and Apparatus
All chemicals and solvents had the highest purity grade and were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, Missouri, United States) companies and were used without further purification. In all the experiments, the researchers used deionized water with high purification. Standard solutions of Pb(II) ions were prepared from Pb(NO3)2 salts as 1000.0 mg L− 1 and diluted solutions, as per experimental requirements, were prepared from the stocks solution. Glass containers were immersed in a 10% (v/v) nitric acid solution before being used, then washed with deionized water. For measuring the pH, a Metrohm pH meter model 827 (Metrohm, Switzerland) was used. In order to investigate the size and structure of the microfibers composite, a scanning electron microscope (XL30, Netherland Philips, SEM) and XRD instrument (38066 RIVA) was used. In order to prepare the IR spectrum for synthetic adsorbents and to identify functional groups and chemical bonds, FT-IR (Perkin Elmer, Spectrum 100, UK) was applied. Electrospinning device (full option lab2 ESI-II, Nanoazma, Iran) was used in order to produce microfibers. For measuring Pb(II) the polarography (Metrohm 797) technique was used as an electrochemical method. In this technique, three electrodes were used which were reference electrode (Ag/AgCl/KCl with the concentration of 3 mol L− 1 for KCl), platinum electrode as an auxiliary electrode and glassy carbon electrode as a working electrode.
Fabrication of PVA/PEI Microfibers
To prepare PVA/PEI electrospun microfibers, a previously reported procedure [14] with some modification was used. First, the powder of poly (vinyl alcohol) was dissolved into the deionized water to reach 10% by weight and stirring for 4 h at 80 °C. Then PVA (10%) and PEI (50 wt%) were mixed together in a ratio of 3:1 and stirred for 12 h to get a uniform solution. The polymer solution was loaded into a 5 mL syringe that equipped with a stainless steel needle with a 0.9 mm diameter and electrospinning was performed on rotating drum aluminum foil collector at 18 kV using high voltage DC power supply with a 0.4 mL h−1 feed rate and the interval of the needle tip to the collector was 20 cm. The polymer solution was electrospun at room temperature and microfibers were collected. Fabricated PVA/PEI microfibers reacted with glutaraldehyde vapor conducive to become stable in water. For this purpose, 20 mL of glutaraldehyde (25%) was poured into the bottom of the desiccator. The aluminum foil containing microfibers was put on the desiccator and was kept under vacuum overnight. Next, the aluminum foil was immersed in the water to separate the microfibers and was washed three times by pure water to remove unreacted glutaraldehyde and then dried under vacuum.
Immobilizing of Fe3O4 Nanoparticles on Fabricated PVA/PEI Microfibers
Magnetite (Fe3O4) nanoparticles were prepared according to the reported method [15]. First, 11.68 g FeCl3·6H2O and 4.3 g FeCl2·4H2O were mixed together and dissolved in a 200 mL pure water with stirring under the nitrogen atmosphere at 85 °C. Then 20 mL ammonia (30% NH3) was dripped during the synthesis, the solution was mixed by a magnetic bar. The black precipitate of Fe3O4 became appeared, instantly PVA/PEI microfibers were added and remained under the nitrogen atmosphere for 60 min. The solution was allowed to stand at room temperature. The obtained nanocomposite was washed several times by a mixture of distilled water and methanol to reach neutral pH. The available nanocomposite was then placed in the vacuum desiccator to dry (Scheme 1).
Batch Adsorption and Desorption Experiments
The study of adsorption was started by adding 0.02 g of PVA/PEI/Fe3O4 into a 150 mL Pb(II) solution at pH 5. The Pb(II) solutions were shaken for 60 min. Then the metal ions loaded PVA/PEI/ Fe3O4 fibers were separated by a magnet from solution easily. The concentration of metal ions decreased with time due to the adsorption at microfibers. The percentage of removal was calculated by the following formula:
In the above equation, C0 and Ct are primary and final concentrations of metal ions, respectively [16]. Desorption studies were carried out to remove metal ions from microfibers substrates by adding 2 mL of 0.1 mol L− 1 HCl solution.
The determination of Pb(II) concentration in the solutions was performed according to a previous report by differential pulse voltammetric procedure and also using a multi-walled carbon nanotubes glassy carbon electrode as the working electrode [13].
Results and Discussion
Characteristics of PVA/PEI/ Fe3O4 Nanocomposite
The morphology and specifications of microfibers were identified before and after immobilizing of magnetite nanoparticles using FTIR spectra, SEM images and XRD patterns which provides information on the dimensions, shape, type of chemical bonds and elements.
IR spectra for PVA, PVA/PEI and PVA/PEI-Fe3O4 samples were obtained in the range 400–4000 cm− 1. These spectra are shown in Fig. 1. Comparing the spectra for PVA and PVA/PEI microfibers(Fig. 1a, b) showed that the most significant difference between them is the presence of new peaks at 1647 cm− 1 and 1584 cm− 1 correspond to N-H bending of PEI and aldimine between PEI and glutaraldehyde after cross-linking, respectively. Other observed peaks include to the stretching vibration of –OH and –NH at 3337 cm− 1. Stretching vibration peak at 2930 cm− 1 is related to C–H and peaks at 1720 cm− 1 and 1430 cm− 1 are related to C=O and C–H groups, respectively, and final peak at 1093 cm− 1 is related to stretching vibration of ether groups (C–O) after cross-linking between glutaraldehyde and poly (vinyl alcohol). The spectrum for microfibers modified with magnetite nanoparticles (Fig. 1c) showed one peak at 586 cm− 1 corresponds to Fe–O. This method demonstrates the successful production of microfibers composite [17, 18].
The SEM images showed that the original polymer solution is converted to the form of microfibers. The average diameter of the synthesized microfibers was about 270 nm, which then increases to 350 nm after cross-linking by glutaraldehyde. The latest image shows how Fe3O4 magnetite nanoparticles are immobilized on PVA/PEI microfibers composite (Fig. 2a–c).
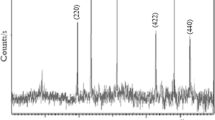
XRD patterns related to microfibers before and after magnetization (Fig. 3) showed a broad band belonging to the amorphous microfibers and the other peaks related to Fe3O4 nanoparticles. From the Scherer equation (Eq. 2) the researchers can determine the crystallite size of nanoparticles of crystals.
In this equation D is the mean crystallite size, K is a constant number equal to 0.9 for magnetite, λ is the X-ray wavelength equal to 1.54 Å, β is the line width at the half-maximum intensity, and θ is the Bragg angle. By calculating these values, the mean crystallite size of Fe3O4 nanoparticles at PVA/PEI fibers was calculated as 16.73 nm [19, 20].
Determination of pHpzc (PVA/PEI-Fe3O4)
pHpzc is one of the most important features of an adsorbent which indicates the electrical neutrality of the adsorbent surface at a specific pH value. The surface of adsorbent in pH greater than pHpzc is negatively charged and at pH lower than pHpzc is positively charged. To justify the elimination mechanism of metals, pHpzc of the absorbent surface must be measured [21]. For this aim, 0.03 g of adsorbent was added into the seven Erlen Meyer with 25 mL volume. Then 25.0 mL of 0.01 mol L− 1 NaCl solution was poured and pH of the solutions was adjusted in the range 3.0 to 9.0 with 0.01 mol L− 1 HCl and/or NaOH and finally stirred for 24 h [22]. The diagram of ∆pH versus pH was plotted. As Fig. 4 shows the related pHpzc of 5.8 for PVA/PEI/Fe3O4 nanocomposite was found.
Effect of Solution pH
As shown in Fig. 5 the pH effect on surface adsorbent for Pb(II) in the range 3.0˗8.0 was studied. The solutions of 0.01 mol L− 1 nitric acid and sodium hydroxide was used for pH adjustment. To carry out this studies 25.0 mL of the mentioned ion solution was used at the concentration of 20.0 mg L− 1. The results of the pH effect on Pb(II) adsorption showed that the maximum adsorption takes place at pH 5.0. There was a dramatic decrease in adsorption efficiency at higher and lower pHs. As a matter of fact, in acidic solution, hydrogen ions compete with Pb(II) to occupy active cites as well as in the higher pH, Pb(II) ions change into Pb(OH)2 form, rendering them unable to adsorb on the surface of microfibers [23, 24].
Effect of the Adsorbent Amount
The dependency of the percentage of Pb(II) adsorption on the amount of adsorbent at room temperature, optimal pH and contact time of 30 min with different amounts of the adsorbent in the range 0.005–0.04 g for 25.0 mL of 20.0 mg L− 1 of metal ion solution was studied. According to the results, showed in Fig. 6, adsorption of approximately 97% Pb(II) was obtained by using 0.02 g of the adsorbent. By increasing adsorbent weight beyond this amount, the quantity of ion removed remained constant. Achieving a high removal efficiency using low adsorbent is due to high activation sites and a large surface ratio to volume of synthetic microfibers.
Effect of Contact Time and Adsorption Kinetics
The effect of contact time on the adsorption was investigated by studying the time needed to completely remove the ions present in 25.0 mL of solution at a concentration of 20.0 mg L− 1 at optimum pH and adsorbent value (Fig. 7). The concentration of ions was reduced in the solution due to the removal of ions on the synthetic microfibers and with passing time almost all ions were removed. In order to study the kinetics of the adsorption process, pseudo˗first˗order and pseudo˗second˗order models were considered. The pseudo˗first˗order kinetic equation is generally presented as the following equation:
where qe and q are, respectively, the amount of adsorption per unit mass of the adsorbent in equilibrium and at the instant (t), k1 (min− 1) is the pseudo-first-order model rate constant.
The pseudo˗second˗order kinetic equation for an adsorption system is expressed as follow:
k2 (g·min − 1·mg− 1) is the pseudo˗second˗order adsorption rate constant. In recent years, kinetic models have been widely used for the adsorption in aqueous solutions. Also, the Chi˗square test calculates the difference between the experimental data and results obtained with kinetic models. The smaller the Chi-square value will be more compliance with that model. The value of Chi-square is obtained from the following equation:
qe, exp and qe, cal are the equilibrium capacity from experimental data and kinetic models, respectively [25, 26]. The data of two kinetic models were analyzed using Matlab R 2012a (7.14.0.739) software. The results are summarized in Table 1. As the results show the squared regression coefficient (r2) is higher for pseudo-first-order kinetic model and also the Chi˗square (χ2) is lower for this kinetic model. Furthermore, another parameter called the root mean square (RMS) which shows the error value is smaller in the pseudo-first-order kinetic model. Therefore it can be concluded that the adsorption of Pb(II) is followed by the pseudo-first-order model.
Adsorption Isotherms
Equilibrium adsorption isotherms are necessary for the design of adsorption systems and provide information on the adsorption capacity. To determine the adsorption capacity, experimental data were matched by Langmuir, Freundlich and Sips isotherm models. The linear formula of the single-layer Langmuir isotherm is as follows:
qe (mg g− 1) is the amount of adsorbed analyte per unit mass of the adsorbent substance, Ce (mg L− 1) the concentration of the analyte in the solution at equilibrium, KL(L g− 1) the Langmuir equilibrium constant and qmax is the maximum adsorption value in a single adsorbent layer. The Langmuir isotherm expresses single layer adsorption on a uniform surface with a limited number of adsorbed sites.
The linear formula of Freundlich multilayer isotherm model is shown below:
Kf (mg1 − 1/nL1/ng−1) is the Freundlich constant; 1/n is the constant that corresponds to the heterogeneity of the surface.
Sips isotherm (Langmuir and Freundlich) presented as follows:
KS (L mg− 1) the Sips equilibrium constant, this isotherm is converted to Freundlich isotherm at low concentrations of the adsorbate. Also when the adsorbent surface is homogeneous (n = 1), it is converted to Langmuir isotherm [26, 27]. The equilibrium adsorption data were fitted by three mentioned isotherm models with nonlinear or linear regression, the parameter values were obtained as listed in Table 2. The squared correlation coefficient is more consistent with the Langmuir isotherm model. Indeed the high surface to volume ratio of microfibers causes ions become adsorbed to a single layer on surface as long as available sites exist. Surface adsorption has been increased by increasing the concentration of the analyte. However after the occupation of active sites, adsorption capacity remains constant. The high adsorption capacity of the synthesized microfibers and simply separation of it from solution based on its magnetic properties, make PVA/PEI/Fe3O4 microfibers as a good adsorbent in comparison with various adsorbents previously used to remove Pb(II) ion (Table 3).
As shown in Table 3, the proposed adsorbent provides adsorption capacities for the adsorption Pb(II) that is higher than that reports for other methods.
Desorption and Regeneration Process
Desorption of adsorbed Pb(II) ions was studied by various eluents including HCl, NaCl, EDTA and, acetic acid. The best results for the desorption of Pb(II) from microfibers were obtained using 2.0 mL of 0.1 mol L− 1 HCl solution. These conclusions are also in agreement with the results of the pH effect. The effect of desorption time on the desorption of the adsorbed ions was also studied. As Fig. 8 shows, the time of 40 min was chosen for Pb(II) as the optimal time of desorption. Recovery of the adsorbent is economically important. That is why the ability of nanofiber recovery in five consecutive cycles was investigated. As it is clear from Fig. 9, it is possible to recover the adsorbent up to 3 periods without worrying about reducing removal efficiency with 97% recovery power.
Effect of the Initial Sample Volume on Recovery
To obtain a high preconcentration factor, a high volume of the sample solution is required. In addition, collecting analyte in the small amounts of eluent will help to raise this factor. Hence solutions containing 1.0 mg L− 1 Pb(II) were diluted by deionized water to 25.0–250.0 mL and calculated their recovery percentage by desorption in 2.0 mL of eluent in optimum time. The results of Fig. 10 show that a 150.0 mL sample solution is the optimal initial volume. Since the adsorbed ion became desorbed in 2.0 mL eluent, the preconcentration factor for this ion was obtained as 75.
Coexisting Ions Interference
In order to determine the selectivity of the method, the effect of various anionic and cationic species must be investigated. The existence of other chemical species leads to their competition with Pb(II) ions for occupying active sites on the microfibers. In this study, the tolerance limit is considered as the concentration of interference species to cause a less than ± 5% error in response to 20.0 ng mL− 1 of Pb(II) ions. As Table 4 shows, most of the investigated ions did not interfere with the preconcentration and determination of Pb(II) even when present in 500- to 1000-fold excess over the analyte Therefore it can be concluded that the proposed method has a high tolerance limit versus matrix interference.
Analytical Figures of Merit
Under optimum conditions, the calibration curve was obtained to determine the linear range of Pb(II) ions concentration. The Pb(II) ions calibration curve was obtained from electrochemical measurements in the range 4.0–100.0 ng mL−1 and the calibration equation was I = 3597C + 0.2422 with a correlation coefficient of 0.9928. The detection limit of the method was found to be 1.37 ng mL−1. The RSD values for three concentrations of 5.0, 10.0 and 100.0 ng mL−1 of the analyte were determined out. In each experiment, three measurements were performed. The RSD values for the determination of Pb(II) ions were found to be 4.3%, 2.8% and 1.2% (n = 3), respectively. Analytical parameters are summarized in Table 5. Also, the proposed method was compared with costly and in some cases inaccessible methods that have been reported in Table 6.
Analysis of the Real Samples
In order to evaluate the ability of the proposed method for separation and recovery of Pb(II) ions in real samples, the proposed adsorbent was also applied to the preconcentration of trace amounts of Pb(II) in water and waste-water samples prior to their determination. The samples were also analyzed after spiking with different concentrations of the analyte. The results (Table 7) show that the obtained recoveries are in the range 95.7–105.8%. This indicates the applicability of the proposed method for the determination Pb(II) in such samples has good accuracy.
Conclusion
A simple procedure was proposed for removing and/or preconcentration of Pb(II) ion from aqueous solutions. The newly synthesized PVA/PEI/Fe3O4 microfibers composite was highly efficient for the simple and rapid removal and preconcentration of Pb(II) from aqueous solutions. While synthesis of the adsorbent is simple and economically cost-effective, its high adsorption capacity is recognized as a unique feature of this adsorbent. Organic solvents were not used in the extraction process, therefore the method can be discussed as an environmentally friendly method. On the other hand, the easy separation of the synthesized nanofiber, due to magnetization, makes it a good candidate for preconcentration and determination of metal ions. Therefore a new, safe, simple, environmentally friendly and cost-effective method is suggested. The satisfactory results from the measurement of real samples indicate the real sample matrix has no interfering effect in measuring these metal ions.
References
Chen LF, Liang HW, Lu Y, Cui CH, Yu SH (2011) Langmuir 27:8998
Maratta A, Vázquez S, Lopez A, Augusto M, Pacheco PH (2016) Microchem J 128:166
Li B, Zhou F, Huang K, Wang Y, Mei S, Zhou Y, Jing T (2017) Sci Rep 7:43082
Zhao G, Ren X, Gao X, Tan X, Li J, Chen C, Huang Y, Wang X (2011) Dalton Trans 40:10945
Fu F, Wang Q (2011) J Environ Manage 92:407
Gohari RJ, Lau WJ, Matsuura T, Halakoo E, Ismail AF (2013) Sep Purif Technol 120:59
Soylak M, Unsal YE, Kizil N, Aydin A (2010) Food Chem Toxicol 48:517
Wang Y, Wu D, Wei Q, Wei D, Yan T, Yan L, Hu L, Du B (2017) Sci Rep 7:10264
Liu Z, Liu Y, Ho CH, Liu Y, Jing XY, Cheng A (2018) J Environ Eng 144:04018004
Morillo Martín D, Magdi Ahmed M, Rodríguez M, García M, Faccini M (2017) Materials 10:1352
Habiba U, Siddique TA, Talebian S, Lee JJL, Salleh A, Ang BC, Afifi AM (2017) Carbohydr Polym 177:32
Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S (2003) Compos Sci Technol 63:2223
Li X, Hou S, Xie C, Fan G (2018) Int J Electrochem Sci 13:3161
Fang X, Xiao S, Shen M, Guo R, Wang S, Shi X (2011) New J Chem 35:360
Liu X, Ma Z, Xing J, Liu H (2004) J Magn Magn Mater 270:1
Sharma R, Singh N, Tiwari S, Tiwari SK, Dhakate SR (2015) RSC Adv 5:16622
Huang Y, Ma H, Wang S, Shen M, Guo R, Cao X, Zhu M, Shi X (2012) ACS Appl Mater Interfaces 4:3054
Hu D, Xiao Y, Liu H, Wang H, Li J, Zhou B, Liu P, Shen M, Shi X (2018) Colloids Surf A 552:9
Pan W, Han R, Chi X, Liu Q, Wang J (2013) J Alloys Compd 577:192
Wang P, Zhang M, Cai Y, Cai S, Du M, Zhu H, Boa S, Xie Q (2014) Soft Mater 12:387
Bagheri H, Afkhami A, Saber-Tehrani M, Khoshsafar H (2012) Talanta 97:87
Panneerselvam P, Morad N, Tan KA (2011) J Hazard Mater 186:160
Lee MY, Lee JH, Chung JW, Kwak SY (2018) J Environ Manage 206:740
Afkhami A, Saber-Tehrani M, Bagheri H, Madrakian T (2011) Microchim Acta 172:125
Duran C, Ozdes D, Gundogdu A, Imamoglu M, Senturk HB (2011) Anal Chim Acta 688:75
Bahiraei A, Afkhami A, Madrakian T, Gheitaran R (2018) Ultrason Sonochem 40:1049
Afkhami A, Moosavi R (2010) J Hazard Mater 174:398
Wang J, Lu X, Ng PF, Lee KI, Fei B, Xin JH, Wu JY (2015) J Colloid Interface Sci 440:32
Li B, Zhou F, Huang K, Wang Y, Mei S, Zhou Y, Jing T (2016) Sci Rep 6:33573
Liu D, Li Z, Zhu Y, Li Z, Kumar R (2014) Carbohydr Polym 111:469
Tan Z, Peng H, Liu H, Wang L, Chen J, Lu X (2015) J Appl Polym Sci 132:42384
Saeed K, Haider S, Oh TJ, Park SY (2008) J Membr Sci 322:400
Saçmacı Ş, Kartal Ş (2010) Microchim Acta 170:75
Parham H, Pourreza N, Rahbar N (2009) J Hazard Mater 163:588
Silva EL, dos Santos Roldan P (2009) J Hazard Mater 161:142
Ozcan SG, Satiroglu N, Soylak M (2010) Food Chem Toxicol 48:2401
Mendil D, Karatas M, Tuzen M (2015) Food Chem 177:320
Ghanemi K, Nikpour Y, Omidvar O, Maryamabadi A (2011) Talanta 85:763
Yilmaz E, Soylak M (2013) Talanta 116:882
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bavel, E., Afkhami, A. & Madrakian, T. Removal and Preconcentration of Pb(II) Heavy Metal Ion from Water and Waste-Water Samples onto Poly (vinyl alcohol)/polyethyleneimine/Fe3O4 Microfibers Nanocomposite. J Polym Environ 28, 614–623 (2020). https://doi.org/10.1007/s10924-019-01632-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01632-7