Abstract
In this work, a new series of bio-based poly(ɛ-caprolactone)s (SBO-PCLs) is synthesized through ring-opening polymerization (ROP) of ɛ-caprolactone (ɛ-CL) using stannous octoate as a catalyst and hydroxylated soybean oil (SBO-OH) as a macro-initiator. For this purpose, firstly, epoxy groups of epoxidized soybean oil (ESBO) are converted to hydroxyl functionalities to be used for ROP of ɛ-CL. Then, after the ROP of ɛ-CL using SBO-OH; wettability, biodegradability and thermal properties of the obtained SBO-PCLs are evaluated in terms of loading ratio of ɛ-CL monomer ([OH]/[ɛ-CL] (n/n) = 1:0.5; 1:1 and 1:2). The obtained SBO-PCLs and their intermediates are characterized by Fourier transform infrared spectroscopy (FT-IR), proton nuclear magnetic resonance spectroscopy (1H-NMR), gel permeation chromatography (GPC), water contact angle measurement (WCA), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and enzymatic degradation experiment. SBO-PCL with higher PCL molar ratio shows the lower biodegradability, but higher hydrophobicity and thermal properties compared to others. Thus, it is clear that the successful syntheses of SBO-PCLs encourage the use of these polymers as promising materials for scientists working on PCL applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, accelerated research efforts have been devoted to the development of bio-based materials due to the environmental concerns, and energy and cost saving issues. Bio-based polymers that reduce dependence on petro-chemical-based synthetic polymers (polyethylene, polypropylene, polystyrene, polycarbonate, polyethylene terephthalate, and etc.) provide significant contributions to environmental sustainability in the world [1]. In this regard, there is a growing interest in biomaterials derived from renewable resources, including cellulose, starch, protein, sugar, wood or vegetable oils because they offer the advantages of relatively low cost, readily availability, possible biodegradability, low and toxicity [2,3,4,5,6,7,8,9,10]. Among them, the vegetable oils and their derivatives extracted from various plants are an excellent renewable source for polymer applications, such as synthesis of hybrid magnetic particles [11], terpolymers [12], polyurethanes [13, 14], polymethacrylate coatings [15], polyamides [16], and alkyd resins [17], achieved by using different polymerization techniques. Their chemical structure composed of the triglycerides having different chain lengths and number of double bonds, depending on the growing and the plant conditions [13, 18]. There is a number of vegetable oils in the literature utilized, mainly castor oil, neem oil, olive oil, linseed oil, sunflower oil, rapeseed oil, canola oil, peanut oil, palm kernel oil, soybean oil, and so on for synthesis of various monomers and polymeric materials [8, 19]. Soybean oil is a very promising renewable feedstock for polymer synthesis as either considering the number of C–C double bonds per triglyceride (4.6%) or the annual average production (35.7 million tonnes) [20]. However, unless relatively unreactive double bonds of vegetable oils are transformed into more reactive epoxy, carboxyl or hydroxyl groups, they cannot be served as building blocks for polymers. Especially, hydroxylated vegetable oils having higher reactivity and viscosity compared to others, have a high potential for a wide range of academic and industrial applications such as plastics, coatings, inks, lubricants, waxes, adhesives and cosmetics [21].
For a long time, thermoplastic aliphatic polyesters such as poly(dl-lactide) (PDLLA), poly(l-lactide) (PLLA), polyglycolide (PGA), poly(β-hydroxybutyrate) (PHB) and poly(ε-caprolactone) (PCL) have garnered increasing attention, because they present many attractive attributes, including high biodegradation rate in the environment and the human body, good mechanical strength, processability, and etc. Over the past few years, these easily degradable polymers have been a suitable choice for numerous food packaging, controlled drug delivery and tissue engineering applications [22]. Among them, one of the most promising biopolymer is the PCL that composed of hexanoate repeating units obtained from either polycondensation of 6-hydroxyhexanoic acid or ROP of ε-CL [23]. PCL is biodegradable and biocompatible aliphatic polyester which have melting temperature ranging between 56 and 65 °C and is highly or slightly soluble in most solvents, depending on its both molecular weight and degree of crystallinity [24]. With these features as well as thermal and mechanical properties, it meets the requirements of concerning environmental sustainability. Therefore, PCL is the subject of various applications in biomedical fields such as drug delivery systems [25], urethral catheters [26] and resorbable sutures [27], and is proposed as a material for tissue engineering of bone and cartilage [28]. Furthermore, Tsujimoto et al. reported the synthesis of soybean oil based shape memory material from a combination of ESBO and PCL by acid-catalyzed curing reaction [29]. Thus, the aim of this study was to describe a strategy in order to fabricate a series of biodegradable polymeric materials via ring-opening polymerization of ɛ-caprolactone using hydroxylated soybean oil as a macro-initiator. Furthermore, the wettability, thermal and biodegradation properties of the resulting materials were investigated, depending on ε-CL loading ratio in the polymerization medium.
Experimental Section
Materials
Epoxidized soybean oil (Mn = 1000 g/mol, epoxy content = 7% min) was purchased from Parchem (ESBO, New York) and used as received. Chemicals used for both hydroxylation of ESBO and calculation of hydroxyl number of SBO-OH, including hydrochloric acid (HCl, 36.5–38%), magnesium sulfate (MgSO4, 99.9%), ethyl acetate anhydrous (EtAc, 99.98%), acetone (CH3COCH3, ≥ 99.98%), 1,2-dichloroethane anhydrous (C2H4Cl2, 99.8%), acetic anhydride ((CH3CO)2O, ≥ 98%) and potassium hydroxide (KOH, ≥ 85%) were purchased from Sigma-Aldrich (Steinheim, Germany) and used as received. Chemicals used for ring opening polymerization, including monomer ɛ-caprolactone (ɛ-CL, 97%) distilled from calcium hydride before use and catalyst stannous octoate (Sn(Oct)2, 92.5–100%) were purchased from Sigma-Aldrich (Steinheim, Germany) and used as received. Sodium phosphate monobasic (NaH2PO4, Sigma-Aldrich, Germany), disodium hydrogen phosphate (Na2HPO4, Sigma-Aldrich, Germany) and lipase from porcine pancreas (EC 3.1.1.3., Type II, Sigma-Aldrich, Germany) were used for biodegradation experiment and used as received. Rectangular glass plates with dimensions of 76 × 26 mm used for dip-coating were supplied from ISOLAB (Istanbul, Turkey), and all other reagents and solvents were used without further purification or distillation.
Characterization and Measurements
In order to confirm the functional groups and structure of SBO-PCLs and their intermediates, Fourier transform infrared (FT-IR) and proton nuclear magnetic resonance (1H-NMR) spectroscopic analyses were carried out on a Perkin-Elmer brand (Lambda 25, Waltham, USA) FT-IR Spectrum Two Spectrometer equipped with a diamond ATR device at room temperature scanning range covered 400 cm−1 to 4000 cm−1 (24 scans, scan speed of 0.2 cm/s) and a Varian 600 Spectrometer operating at 600 MHz (the chemical shifts were given in ppm units using tetramethylsilane (TMS) as an internal standard, spectral width, 16 ppm; acquisition time, 3.4 s; relaxation delay, 1 s; 90° pulse width, 7.2 µs; time domain, 64 K data points; 32 scans; temperature, 298.15 K), respectively. In order to determine the contact angle between surface of SBO-PCLs and a water drop, water contact angle (WCA) measurements were conducted by a KSV Attension Theta Optical Tensiometer (CAM-200, Vastra Frolunda, Sweden) under laboratory conditions using grade water for chromatography. Thermal properties of SBO-PCLs were determined by a TGA and DSC analyses by a SeikoSII TG-DTA 6300 TG/DTA analyzer heated from 20 to 800 °C with a heating speed of 10 °C/min and a DSC analyzer (Mettler Toledo DSC) with the samples (3–5 mg) sealed in aluminum pans using heating rate of 10 °C/min in the temperature ranging from 25 to 100 °C under nitrogen atmosphere, respectively. During the DSC analyses, samples were first heated from room temperature to 100 °C at 20 °C/min and maintained at this temperature for 5 min so as to get rid of the residual solvents. Then, specimens were cooled to − 70 °C before the standard DSC measurement. Data reported were taken from the second scan. Molecular weights and polydispersity indexes (Ð) of obtained SBO-PCLs were determined by gel permeation chromatography (GPC) by a Viscotek GPCmax composed of a refractive index (RI) detector (VE 3580, Viscotek) and a pump module (GPC max, Viscotek, Houston, TX) at 1 mL/min flow rate. An Autosampler system and 50 µL injection volume were used in analyses. The calibration of RI detector was done by narrow molecular weight polystyrene standards. Two columns (LT5000L, Mixed, Medium Organic 300 × 8 mm and 419 LT3000L, Mixed, Ultra-Low Organic 300 × 8 mm) with a guard column (TGuard, Organic Guard Column 10 × 4.6 mm) were used for the tetrahydrofuran eluent at 35 °C. To analyze the data, Viscotek OmniSEC Omni-01 software was used. Thermogravimetric analyses (TGA) of PURs were carried out by a SeikoSII TG–DTA 6300 TG/DTA analyzer heated from 20 to 800 °C with a heating speed of 10 °C/min under dry air atmosphere. The TGA samples were weighed approximately 2–5 mg. The other thermal analysis of PURs was carried out on a DSC analyzer (Perkin Elmer Diamond DSC) with the samples sealed in aluminum pans using heating rate of 20 °C/min in the temperature ranging from 25 to 200 °C under nitrogen atmosphere. Since SBO-PCLs were obtained as a powder product which has not good film forming properties, no mechanical tests were performed on the samples.
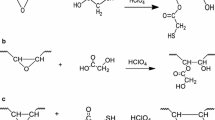
Hydroxylation Procedure of ESBO (SBO-OH)
After the dissolution of ESBO in acetone in a 100 mL flat bottomed flask equipped with a magnetic stir bar placed in an oil bath at 40 °C, hydrochloric acid was slowly added into this solution drop-wise under vigorous stirring for 120 min. After the defined period of time, acetone was evaporated. The resulting residue was dissolved by mixture of ethyl acetate-water (3:1) so as to yield separated two layers and organic phase was washed with large excess of water until neutral pH. Then the final residue was dried over anhydrous MgSO4 and filtered. Finally, after the evaporating of solvent under reduced pressure yellow viscous product was achieved. Hydroxyl number of SBO-OH was calculated as 300 mg KOH/g sample via titrimetric method [30].
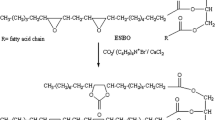
General Procedure for Synthesis of Soybean Oil Based Poly(ɛ-caprolactone)s (SBO-PCLs)
The synthesis procedure for a series of soybean oil based poly(ɛ-caprolactone)s was as follows: Firstly, macro-initiator SBO-OH (0.45 g, 0.008 mol in terms of –OH number) was dissolved in 20 mL one necked flat bottom flask equipped with a magnetic stir bar in 6 mL anhydrous toluene. Then, ɛ-CL (0.38 mL, 0.004 mol) as a monomer and, 0.5 wt% of Sn(Oct)2 catalyst with respect to monomer (for formulation of [OH]/[ɛ-CL] (n/n) = 1:0.5) abbreviated as SBO-PCL-1) was introduced into this solution. After the degassing by using argon gas for 1 min, tightly closed flask having reaction mixture was left under continuous stirring for 24 h at 120 °C. After that, the reaction medium was cooled to room temperature, and precipitated in cold methanol two times. The resulting product was dried in a vacuum for 24 h at r.t. and obtained in about 44% yield gravimetrically as an oily yellow powder. The other polymerizations ([OH]/[ɛ-CL] (n/n) = 1:1 and 1:2) were conducted under similar experimental conditions, and obtained polymers were abbreviated as SBO-PCL-2 and SBO-PCL-3, respectively.
Polymeric Coating Procedure
The different formulations were coated onto rectangular glass plate substrates with dimensions of 76 × 26 mm by means of a precise homemade mechanical dip-coater. Typically, coatings were prepared by dissolving the SBO-PCLs in THF at a concentration of ∼ 30 mg mL−1. During the coating process, the glass plates, which are cleaned with chromic acid solution, and distilled water, were immersed into SBO-PCLs solutions with withdraw rate 80 mm min−1 for 2 min of waiting duration. The coated plates were stored in a desiccator to remove residual solvents for 48 h at room temperature prior to WCA measurements.
Contact Angle Measurement
The contact angle between sample surfaces and 5 µL of LC–MS grade water drop was determined through the sessile drop technique using a micro-syringe with a stainless steel needle. To ensure the reproducibility of the data, the images of the water drops on the samples were captured on different locations, taking image of five times with a conventional digital camera. The contact angle values were reported as average of these measurements.
Biodegradation Experiment
Biodegradation experiments were carried out on SBO-PCL specimens by enzymatic degradation in 0.01 M phosphate buffer solution (PBS, pH 7.40) in the presence of 1 mg/mL lipase (or without lipase for blank test). Lipase is used in enzymatic degradation experiments in the literature because its degradation effect of ester bonds in a material is well known [31]. Biodegradation procedure for the specimens was as follows [32, 33]: Each sample was placed into an individual vial containing 8 mL of buffer solution and incubated constant shaking at 37 °C in a water bath. The samples were removed within specified periods, cleaned with deionized water, and dried to constant weight in a vacuum oven at 50 °C for 24 h and reweighed. The residual weight percentages (RW) of specimens were calculated using the following formula (Eq. 1):
where W0 and W1 are weight of dry samples before and after degradation days, respectively.
Results and Discussion
As the mentioned in the introduction part, there are several vegetable oils to be used for synthesis of macromolecular architectures, depending on their content of carbon–carbon double bond numbers ranging from 0 to 5 and carbon numbers from 8 to 24 [20]. The presented carbon–carbon double bonds in the structure of vegetable oils are utilized in olefin metathesis, free radical, or cationic polymerizations in order to achieve various polymers [34]. On the other hand, unsaturated double bonds of the triglyceride chain of vegetable oil ought to be transformed into the required functional groups by epoxidation, hydroxylation, acrylation, transesterification, or maleination reactions to create the polymeric materials from aforementioned oils. In this regards, the SBO-PCLs were obtained from ESBO by two step reactions. Firstly, epoxy groups of ESBO were converted to hydroxyl functionalities by ring-opening reaction in the presence of hydrochloric acid catalyst, and OH number of obtained SBO-OH was determined as 300 mg KOH/g sample by titration method. Secondly, SBO-OH was utilized in the ring-opening polymerization of different loading ratio of ɛ-CL in the presence of Sn(Oct)2 performed at 120 °C in toluene for 24 h so as to achieve the corresponding SBO-PCLs (Scheme 1).
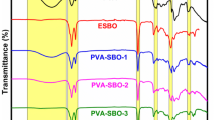
Figure 1 shows the FT-IR spectra of ESBO, SBO-OH and SBO-PCLs. The structure of the ESBO was characterized by the bands located at 820, 1175, 1725 and 2915 cm−1 corresponded to epoxy group stretching, C–O stretching, > C=O stretching and C-H stretching vibrations, respectively. After the hydroxylation reaction of ESBO, the loss of the epoxy group stretching vibration band assigned at 820 cm−1 as well as the appearance of the new broad –OH stretching vibration band at 3440 cm−1 showed the formation of SBO-OH. As can be seen in the spectra of SBO-PCLs, the disappearance of –OH stretching vibrations, the intensified C–O and > C=O stretching vibrations due to the repeating units of PCL, and all other bands protected coming from starters indicated the success of ring opening polymerizations.
Prior to ring-opening polymerization of ɛ-CL, 1H-NMR spectroscopy was used to confirm the successful transformation from ESBO to SBO-OH (Fig. 2). In this spectrum, the methine and methylene protons around the epoxy rings appeared ranging between δ 2.8 and 3.2 and δ 1.4 and 1.7 ppm were assigned as g, f, h and j. On the other hand, while protons of triglyceride chains detected ranging around δ 1.8 to 2.0 ppm were labelled as d, e and k, methine and methylene protons of them found ranging around δ 5.1 to 5.3 and δ 4.1 to 4.4 ppm were labelled as a and b. Methylene protons that attached with the ester group of fatty acid at position c appeared at δ 2.3 ppm. In addition, l found at around δ 0.8 ppm indicated the methyl protons of aliphatic group in SBO. These signed protons proved the structure of ESBO. Besides, characteristic multiple peaks of epoxy groups disappeared, the appearance of new CH–OH protons at around δ 3.5–3.7 ppm and the protection of the characteristic protons from other groups clearly confirmed the cleavage of the epoxy groups after the epoxy ring opening reaction [7, 35,36,37].
After the ring-opening polymerization of ɛ-CL, 1H-NMR analyses of obtained SBO-PCLs were conducted and the overlaid spectra of them were also given in Fig. 3. The obtained spectra after the ring-opening polymerization of different ɛ-CL mole ratios in the presence of ESBO-OH as a macro-initiator confirmed the structure of SBO-PCLs. As depicted in Fig. 3, all SBO-PCLs had fully similar spectrum. For example, in the case of SBO-PCL-1, the characteristic signals of PCL repeating unit at around δ 4.1 and δ 2.3 ppm were detected and labelled as t and o, respectively. Moreover, it should also be noted that peaks from the repeating units of the PCL (o, p, r, s and t) and the peaks from SBO-OH (a, b, c, d, e, f, g, m, h, j, k and l) as the macro-initiator were found in the same spectrum, indicating the success of the ring-opening polymerization reactions. These spectral findings combined with FT-IR analysis clearly confirmed the structure of intermediates and final products before and after the polymerizations.
In order to determine the molecular weights and molecular weight characteristics of SBO-PCLs, GPC measurements were conducted (Fig. 4). In all cases the GPC traces were narrow and unimodal, demonstrating that no important side reactions occurred. As can be seen in the Fig. 4, GPC trace of the SBO-PCL-1 having lower monomer loading exhibited molecular weight of Mn = 14,200 g mol−1, whereas SBO-PCL-2 and SBO-PCL-3 displayed molecular weight of Mn = 16,800 and 18,800 g mol−1, respectively. In addition, the molecular weight distributions of SBO-PCLs obtained were very close to each other. The combined FT-IR, 1H-NMR and GPC results clearly confirmed the successful ring-opening polymerization of ɛ-CL without detectable free homopolymer formation. The molecular weights and molecular weight distributions of SBO-PCLs were also summarized in Table 1.
The most common used descriptions in surface science are hydrophilicity and hydrophobicity. These terms definite the interaction of the boundary layer of a solid phase with liquid or vapor water and provide an idea of the surface properties of the solid [38]. In this regards, the surface properties of achieved SBO-PCL samples were determined by WCA measurements which is a measure of the wettability. Figure 5 shows the contact angles between the sample surfaces and a water drop, as well as the shape of water droplets on the sample surfaces. The experimental results obtained indicated when ɛ-CL loading was increased; the WCA of PURs enhanced from 57° ± 1 to 66° ± 1 and 76° ± 2.1 for SBO-PCL-1, SBO-PCL-2 and SBO-PCL-3, respectively, denoting lower wetting property. The increasing trend of hydrophobicity for the SBO-PCLs may be attributed to the increasing molecular weight of the samples [39].
PCL is known as a semi-crystalline, biocompatible and biodegradable polymer and can biodegrade at different times ranging from several months to several years according as its molecular weight, hydrophilicity or hydrophobicity, degree of crystallinity, and environmental conditions [40]. On the other hand, biodegradation behavior of polymers obtained by reacting vegetable oil with synthetic monomers or polyurethanes obtained by reacting vegetable oil-based polyol with diisocyanates has been extensively investigated in the literature [41]. In our case, we proposed a way to synthesize SBO-PCLs under mild conditions, starting from soybean oil based polyol as a macro-initiator and ɛ-CL as a monomer. Then we investigated the effect of monomer loading ratio in reaction medium on biodegradation behavior of final products. For this purpose, enzymatic degradation studies were carried out using phosphate buffer solution containing porcine pancreas lipase or without porcine pancreas lipase, and the results were reported as a function of the residual weights at the end of every one day for 9 days (Fig. 6a, b). In Fig. 6a, the residual weights of SBO-PCL-1, SBO-PCL-2 and SBO-PCL-3 samples treated with PBS solutions without lipase enzyme were 93.1, 94.6 and 96.8%, respectively. On the other hand, the residual weights of 48.7, 57.6 and 62.5% were observed for SBO-PCL-1, SBO-PCL-2 and SBO-PCL-3 samples, respectively (Fig. 6b). Biodegradation studies demonstrated that SBO-PCL with a higher molecular weight or longer PCL chain had lower biodegradability. This behavior probably due to the inhibition effect of long polymer chains in the fatty acid part of the triglyceride on binding and activity of lipase which cleave the glycerol ester bonds [41]. Moreover, surface hydrophobicity can also be evaluated as a relative criterion to acquire an idea of the biodegradability of SBO-PCLs.
The thermal properties of SBO-PCLs were studied by TGA and DSC analyses, and the results in terms of initial decomposition temperature (Ton), maximum degradation temperature (Tmax) glass transition temperature (Tg) and melting point (Tm) were summarized in Table 1. The TGA curves of obtained SBO-PCLs were shown in Fig. 7. In this figure, one may notice that all samples displayed two degradation stages. As can be seen easily from TGA curves of samples, the initial and maximum degradation temperatures of SBO-PCL-3 higher than those of other samples probably due to the higher inter or intra-molecular attractions of polymer chains, resulting from higher PCL content of mentioned sample.
On the other hand, DSC thermograms of SBO-PCLs were depicted in Fig. 8. After the ring-opening polymerization reactions, the obtained SBO-PCLs with different molar ratio had a single glass transition temperature (− 40.5, − 37.5 and − 31.5 °C for SBO-PCL-1, SBO-PCL-2 and SBO-PCL-3, respectively) and melting point 39.2, 40.1 and 43.7 °C for SBO-PCL-1, SBO-PCL-2 and SBO-PCL-3, respectively) due to the semi-crystalline characteristic of PCL. Compared to the transition temperatures and melting point of the specimens, SBO-PCL-3 exhibited higher Tg and Tm values probably because of its higher molar ratio of PCL which led to decreased chain mobility or increased molecular attractions [42].
Conclusions
Here, we have demonstrated the ability of hydroxylated soybean oil to act as a macro-initiator for the ring-opening reaction of the ɛ-caprolactone monomer. We have shown that when ɛ-CL loading ratio is increased in the polymerization medium, molar ratio of PCL side chains is increased and thus increased surface hydrophobicity and thermal properties of the SBO-PCL obtained. These properties have been supported by WCA, TGA and DSC analyses, and it has been shown that if the molar ratio of PCL is increased, a possible inhibition effect of long polymer chains occurs, which induces a decrease in the binding and activity of lipase, with a consequent decrease in the biodegradation behaviour of the final SBO-PCLs. These properties make them good candidates for use in bio-applications such as drug delivery, either as blend component or copolymer of biomaterials, and allow them to be used in place of non-environmentally friendly materials.
References
Babu RP, O’connor K, Seeram R (2013) Progr Biomater 2:8
de Mesquita JP, Donnici CL, Pereira FV (2010) Biomacromol 11:473
Wu R-L, Wang X-L, Li F, Li H-Z, Wang Y-Z (2009) Bioresour Technol 100:2569
Cuq B, Gontard N, Guilbert S (1998) Cereal Chem 75:1
Acik G, Yildiran S, Kok G, Salman Y, Tasdelen M (2017) Express Polym Lett 11:799
Weiss M, Haufe J, Carus M, Brandão M, Bringezu S, Hermann B, Patel MK (2012) J Ind Ecol 16:S169
Uysal N, Acik G, Tasdelen MA (2017) Polym Int 66:999
Xia Y, Larock RC (2010) Green Chem 12:1893
Allı S, Aydın RST, Allı A, Hazer B (2015) J Am Oil Chem Soc 92:449
Allı A, Allı S, Becer CR, Hazer B (2014) J Am Oil Chem Soc 91:849
Medeiros AM, Machado F, Bourgeat‐Lami E, Rubim JC, McKenna TF (2018) Macromol Mater Eng 1800449
Neves J, Valadares L, Machado F (2018) Colloids Interfaces 2:46
Zlatanić A, Lava C, Zhang W, Petrović ZS (2004) J Polym Sci Part B 42:809
Zhao M, Wang Y, Liu L, Liu L, Chen M, Zhang C, Lu Q (2018) Polym Compos 39:4355
Decostanzi M, Lomège J, Ecochard Y, Mora A-S, Negrell C, Caillol S (2018) Progr Org Coat 124:147
Magami SM, Oldring PK, Castle L, Guthrie JT (2015) Progr Org Coat 78:325
Igwe I, Ogbobe O (2000) J Appl Polym Sci 75:1441
Meier MA, Metzger JO, Schubert US (2007) Chem Soc Rev 36:1788
Sawpan MA (2018) J Polym Res 25:184
Pfister DP, Xia Y, Larock RC (2011) Chemsuschem 4:703
Goodrum JW, Geller DP (2005) Biores Technol 96:851
Zhao H (2018) J Chem Technol Biotechnol 93:9
Labet M, Thielemans W (2009) Chem Soc Rev 38:3484
Xu Q, Ren X, Chang Y, Wang J, Yu L, Dean K (2004) J Appl Polym Sci 94:593
Coombes A, Rizzi S, Williamson M, Barralet J, Downes S, Wallace W (2004) Biomaterials 25:315
Jones DS, McLaughlin DW, McCoy CP, Gorman SP (2005) Biomaterials 26:1761
Kweon H, Yoo MK, Park IK, Kim TH, Lee HC, Lee H-S, Oh J-S, Akaike T, Cho C-S (2003) Biomaterials 24:801
Reddi AH (2000) Tissue Eng 6:351
Tsujimoto T, Takayama T, Uyama H (2015) Polymers 7:2165
Lligadas G, Ronda J, Galia M, Biermann U, Metzger J (2006) Journal of Polymer Science Part A 44:634
Arutchelvi J, Sudhakar M, Arkatkar A, Doble M, Bhaduri S, Uppara PV (2008)
Acik G, Altinkok C, Tasdelen MA (2018) Journal of Polymer Science Part A 56:2595
Acik G, Kamaci M, Altinkok C, Karabulut HF, Tasdelen MA (2018) Prog Org Coat 123:261
Henna P, Larock RC (2009) J Appl Polym Sci 112:1788
Adhvaryu A, Liu Z, Erhan S (2005) Ind Crops Prod 21:113
Adhvaryu A, Erhan S (2002) Ind Crops Prod 15:247
Dai H, Yang L, Lin B, Wang C, Shi G (2009) J Am Oil Chem Soc 86:261
Acik G, Cansoy CE, Kamaci M (2019) Colloid Polym Sci 297:77
Vlad S, Spiridon I, Grigoras CV, Drobota M, Nistor A (2009) Polymers. doi:10.1515/epoly.2009.9.1.37
Leja K, Lewandowicz G (2010) Polish J Environ Stud 19:255–266
Shogren RL, Petrovic Z, Liu Z, Erhan SZ (2004) J Polym Environ 12:173
Wang Y-H, Wang W-H, Zhang Z, Xu L, Li P (2016) Eur Polym J 75:36
Acknowledgements
This research did not received any specific grant from funding agencies in the public, commercial or not for profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication.
Rights and permissions
About this article
Cite this article
Acik, G. Bio-based Poly(ɛ-caprolactone) from Soybean-Oil Derived Polyol via Ring-Opening Polymerization. J Polym Environ 28, 668–675 (2020). https://doi.org/10.1007/s10924-019-01597-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01597-7