Abstract
A series of renewable resource-based polyurethane networks (NIPUs) with potential application as biodegradable scaffold were prepared through a nonisocyanate methodology. For this purpose, carbonated soybean oil (CSBO) was reacted with amine curing agents composed of 3-aminopropyl-terminated poly (ethylene glycol) (ATPEG) and ethylene diamine (ED) at different weight ratios. CSBO was synthesized from epoxidized soybean oil and carbon dioxide gas at atmospheric pressure using an efficient catalyst system. The chemical identity of NIPUs was confirmed by Fourier transform infrared spectroscopy. Dynamic mechanical analysis of the networks showed single-phase structure for those samples made from ED. Evaluation of tensile property showed widespread behavior from weak plastic up to elastomers with high elongation at break. Hydrolytic degradation profile of NIPUs was increased as ATPEG content increased. Investigation of the L-929 fibroblast cells morphology and evaluation of quantitative tetrazolium dye-based colorimetric assay (MTT assay) confirmed nontoxic behavior and good cytocompatibility of the prepared polyurethanes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodegradable polyurethanes are an important class of biomaterials with a long history of medical applications in areas such as soft-tissue engineering, regenerative medicine and drug delivery systems. These applications are originated from excellent physical properties and relatively good biocompatibility of polyurethanes [1–8]. The most common approach for rendering polyurethanes to be hydrolytically degradable is the introduction of hydrolytically labile polyester soft segments such as polyhydroxyacids, polycaprolactone (PCL), polylactides and their copolymers into the backbone of polyurethanes. The amount of incorporating hydrophilic polyols and its molecular weight significantly affected the mechanical properties, water absorption, calcification and hydrolytic degradation [9–16].
Vegetable oils are one of the cheapest renewable natural resources available in large quantities from various oilseeds. Vegetable oils functionalize by different methods such as oxidation, epoxidation, hydroformylation, ozonolysis and transesterification. Utilization of functionalized vegetable oils as starting materials for the synthesis of polyurethanes is currently receiving increasing attention, since the mode of preparation and the structure of such functional polyols effect considerably on the properties of resulting polyurethanes [17–26].
Most kinds of vegetable oils showed good compatibility under biological condition of human body, therefore, it was emerged that this category of natural material could be good alternative for preparation of polyurethanes for biomedical applications. In fact, vegetable oils are triglycerides of fatty acids. Therefore, it was expected that polyurethanes made from these materials can potentially be biodegradable due to the presence of ester functions of triglycerides. However, due to the hydrophobic nature of triglycerides, the low rate of hydrolytic degradation was detected for vegetable oil-based polyurethanes under biological environment of human body (pH 7.4, 37 °C). Soybean oil-based polyurethane networks as candidate biomaterials were synthesized by ring-opening reaction of epoxidized monoglyceride with lactic acid. The recent developments in the preparation of vegetable oil-based polyurethane, polyester, polyether all of which have potential applications as biomaterials were reviewed by Miao et al. [27–30].
Improving the hydrophilicity of triglyceride-based polyurethanes can increase the hydrolytic degradation rate. This phenomenon is a result of enhancement in water permeability and increase in the surface and bulk hydrophilicity, which provides more water near hydrolytically labile ester groups [31–33].
Meanwhile, an important problem for producing biodegradable polyurethanes has been the toxicity of degradation products, particularly those derived from the general diisocyanate such as 4,4′-methylenediphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) component. Therefore, it is necessary to use other biocompatible aliphatic diiscoyanates like lysine diisocyanate (LDI), hexamethylene diisocyanate (HDI) and 1,4-butane diisocyanate in the formulation of degradable polyurethanes [34–36].
Finding a method to eliminate toxic isocyanate compounds from polyurethane formulations is another interesting approach for increasing merits of polyurethanes for biomaterial purposes. Cyclocarbonates are relatively new classes of compounds attracting research interest to their potential use in the preparation of green polyurethanes, because the highly toxic isocyanates are not involved in the process [37, 38].
The preparation of cyclic carbonates by the addition reaction of carbon dioxide (CO2) to oxiranes is a synthetic route involving safe and cheap starting materials. The production of useful chemicals from CO2 is not only economically beneficial, but also has a positive impact on the global environment [39, 40].
Network or linear nonisocyanate-based polyurethanes (NIPUs) can be formed from the reaction of cyclocarbonate oligomers and primary amines. Petrovic et al. reported reacting carbonated soybean oil with different diamines and the effect of amine structure and carbonate to amine ratio on polyurethane structure and mechanical, physical, and swelling properties was investigated. Since no toxic and/or volatile by-products are produced during preparation of polyurethane via nonisocyanate route, therefore, such nonporous polyurethane seems to be a good candidate for medical applications [41–44].
Application of plant oil-based nonisocyanate polyurethanes as a biomaterial has rarely studied by scientists. The objective of this work was to prepare biodegradable polyurethane elastomers based on CSBO and ATPEG with tunable degradation rates and potential applications in soft-tissue engineering and elastomeric implants.
Experimental
Materials
Epoxidized soybean oil with molecular weight ca. 1000 g/mol and epoxy content 3.3 mol epoxy/kg oil was purchased from PATCHEM, UAE, and used as received. Carbon dioxide gas (CO2) with purity of 99.999 % was supplied from Roham Gas Company, Tehran, Iran. Tetrabutylammonium bromide (TBAB), chloroform, tetrahydrofuran, calcium chloride (CaCl2) and lithium chloride (LiCl) were purchased from Merck Company and used as received. 3-Aminopropyl-terminated poly (ethylene glycol) (ATPEG) with number average molecular weight ca. 1500 g/mol and ethylene diamine (M w = 60.1 g/mol) were obtained from Aldrich and used as received. Mouse L-929 fibroblast cells were supplied from Pasteur Institute of Iran. Phosphate-buffered saline (PBS) was prepared via dissolving NaCl (5.85 g), KH2PO4 (0.6 g), Na2HPO4 (6.4 g) all from Merck in distilled water and the volume adjusted to 1 l. The pH was then correlated to 7.4 by 0.2 M HCl or NaOH solutions. Tris(hydroxymethyl) aminomethane was purchased from Aldrich and used for the preparation of Tris-buffered saline (TBS, 0.05 M Tris, 0.1 M NaCl, pH 7.4) as degradation media.
Synthesis of CSBO
CSBO was prepared and characterized as reported in our previous publication [45].
Synthesis of NIPUs
In a small beaker equipped with magnetic stirrer, CSBO, catalytic amount of LiCl (0.1 wt% of CSBO) and tetrahydrofuran (about 20 wt% of CSBO) were placed. The mixture was stirred at 40 °C until a homogeneous solution was obtained. Equimolar amount of diamine curing agent (based on cyclic carbonate content of CSBO) was added. The reaction content was stirred vigorously and then poured into a Teflon mold. The mold was placed into an air-circulated oven at 70 °C for 10 h. The heating was continued at 100 °C for 3 h. The resulting light brown, transparent and flexible polymeric films were used for subsequent studies. Different formulations are collected at Table 1.
Characterizations
FT-IR spectra were recorded on Bruker instrument (model Aquinox 55, Germany) in the range of 4000–400 cm−1 at a resolution of 0.5 cm−1 and signal averaged over 8 scans. Bandelin Sonopuls HD 2070 ultrasonic homogenizer was used during washing process of CSBO.
Mechanical properties including tensile strength, initial modulus, and elongation at break were determined from stress–strain curves with an Instron 6025 instrument at a strain rate of 5 mm/min. The measurements were performed at 25 °C with a film thickness of about 1 mm and stamped out with an ASTM D638 Die. The presented data are average of five different measurements. Dynamic mechanical testing (DMTA) was carried out on a Tritec 2000 DMTA instrument in temperature range of −100 to 200 °C, heating rate of 3 °C/min and frequency of 1 Hz. The dimension of samples was 10 × 1.5 × 1 mm3.
Surface hydrophilicity of prepared polyurethanes was determined by the measurement of water droplet contact angle. Six different water droplets were placed on the surface at different positions. The contact angle was determined via running Image J 1.44p software on the digital pictures taken from interfaces of films and droplets. The reported values are an average of six measurements.
The bulk hydrophilicity of prepared samples was evaluated based on the measurement of the samples equilibrium water absorption (EWA). The completely dried and accurately weighted films were soaked in distilled water at room temperature until the equilibrium was attained (about 48 h). The weight of swelled sample was determined after gently wiped with filter paper to remove the surface liquid. EWA was calculated using the following equation:
where W d and W s are the weights of dry and swelled samples, respectively.
Biocompatibility of prepared polyurethanes was studied by either microscopic investigation of fibroblast cells morphology after direct contact with samples or tetrazolium dye-based colorimetric assay (MTT assay). Samples were sterilized using an autoclave at 120 °C for 15 min. For the first method, mouse L929 fibroblast cells were pre-cultured in 24-well plates (1 × 104 cells/well) using RPMI-1640 growth medium supplemented 10 % fetal bovine serum (FBS) at 37 °C and atmosphere of 5 % CO2 for 24 h. Then, cells were exposed to the sterilized samples (5 × 5 mm2) which placed in the center of each well and incubated at 37 °C for another 24 h. A well containing cells and growth medium, but no sample, was set up as negative control. The cell growth characteristics and morphological changes as a cytotoxicity sign were recorded using a TMS inverted optical microscope equipped with a Sony DSC-W7 camera. The test was repeated three times for each sample.
The relative viability of the mouse fibroblast cells contacted with either polyurethane films (direct contact) or their leachates in growth medium (indirect contact) was determined by MTT assay. The MTT solution was prepared via dissolving MTT dye (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, Sigma) in phosphate saline buffer (PBS) at a concentration of 0.5 mg/ml. The solution was filtered through a 0.45-μm filter to sterilize and remove small amount of insoluble residues and stored at 2–8 °C for frequent use. Then, pre-cultured cells were seeded into a 96-well plate with a density of 1 × 104 cells/well in RPMI-1640 medium containing 10 % fetal FBS and incubated at 37 °C and atmosphere of 5 % CO2 for 24 h.
The polyurethane films were extracted using RPMI-1640 medium (1 ml/cm2 of film) at 37 °C for 1 week. For direct contact test, cells were exposed to the extracted films (2 × 2 mm2) and incubated at 37 °C for another 24 h. While for indirect contact test, the culture medium of each well was replaced by 180 μl of the films extracts and then 20 μl FBS was added to each well. Incubation at 37 °C was continued for 24 h. For each of direct and indirect methods, the culture medium was discarded and 100 μl of the MTT solution was added into the wells. The cells were incubated at 37 °C for 5 h. Then, the MTT solution was removed and the purple crystals of formazan were dissolved by the addition of 100 μl of isopropanol or DMSO (Merck) per well. The plates were incubated at 37 °C for 15 min prior to absorbance measurements. The optical density (OD) of formazan in the solution was measured at 545 nm using a multiwall micro-plate reader (ELISA reader, Organon Teknika, The Netherlands). A seeded well with no sample and a well containing 100 μl of isopropanol were used as negative and positive controls, respectively. Reported values are the means of five replicates. The percentage of relative cell viability was calculated according to following equation:
Hydrolytic degradation of polyurethanes was evaluated in with Tris-buffered saline (TBS; 0.05 M Tris, 0.1 M NaCl, pH 7.4). Samples were cut into 1 × 1 × 0.1 cm3 cubes and weighed to an accuracy of 0.1 mg. Each sample was placed into an individual vial containing 15 ml of TBS and incubated at 37.8 °C. Four samples of each formulation were removed from the buffer after 3 days, 1, 4 weeks, 3, and 6 months. The degradation medium was refreshed every 1 week. After drying under vacuum at room temperature for 4 days, the samples were reweighed to determine the degradability percentage with the following formula:
where m 0 and m d are the masses of the polymers before and after hydrolysis.
Result and discussion
The synthetic route for preparation of CSBO is depicted in Fig. 1. Spectroscopic assignment of the CSBO and evaluation of their cyclic carbonate content is reported in our previous publication [45]. The synthetic route leading to intended polyurethane networks (NIPUs) through ring-opening reaction of cyclic carbonate groups of CSBO with amine groups of curing agent is shown in Fig. 2.
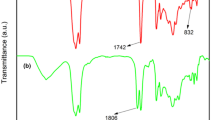
Because of relatively stable nature of five-membered cyclic carbonate moieties, their reaction with amines for production of hydroxyurethanes is slow. The accelerating effect of inorganic salts on his reaction was reported by Endo. Based on results obtained from previous works, the reaction of CSBO with ATPEG was conducted in the presence of LiCl salt under mild heating [46]. The structure of prepared polyurethane networks was confirmed by examination of their FT-IR spectra in Fig. 3. Under applied reaction condition complete disappearance of cyclic carbonate band at 1805 cm−1 was observed, meanwhile new bands at 1695, 1544, and 3296 cm−1 due to the urethane and hydroxyl groups were generated.
The viscoelastic properties of the prepared samples were characterized by means of DMTA. This technique allows different types of transitions and relaxations to be detected and related to the structure and morphology of the samples. The variations of the storage modulus (È) and loss tangent (tan δ) vs. the temperature are shown in Figs. 4 and 5.
Sample NIPU1 made from ethylene diamine curing agent showed single transition, while NIPU2 made from ATPEG exhibited two thermal transitions. The single transition of NIPU1 and the lower temperature transition of NIPU2 were attributed to the α-type transition (glass transition, T g) of soft segment consisting hydrocarbon backbone of soybean oil moieties. The second thermal transition (ca. 49 °C) of NIPU2 was related to melting of crystalline domain composed from aggregation of ethylene glycol moieties of curing agent, since the position of this thermal transition was not changed upon alteration of frequency. For NIPU3 sample with major contribution of ATPEG, the thermal behavior as observed from DMTA curves resembles to NIPU2 sample; however, the thermal behavior of NIPU4 and NIPU5 with a single glass transition temperature was more similar to NIPU1 sample. At glassy state, the ATPEG-rich samples with higher crystallinity exhibited high modulus (higher stiffness). However, the values of modulus at rubbery plateau (i.e., after melting of crystalline domain) for NIPU1 and other samples with higher concentration of ethylene diamine higher modulus were showed.
Investigation of loss tangent curve in Fig. 5 showed that by increasing the mass ratio of ethylene diamine, the tan δ peak associated with glass transition temperature slightly shifted to higher temperature and approached to T g of pure ethylene diamine-based sample. Meanwhile, the height of tan δ peaks becomes smaller as contribution of ethylene diamine component decreased. This phenomenon was attributed to the decrease in degree of freedom for segmental mobility of the polymer chain as a result of increase in chemically effective crosslinking.
The evaluation of mechanical properties from stress–strain curves showed the same trend. The stress–strain curves of crosslinked sample films are shown in Fig. 6 and the mechanical property data are collected in Table 2. The synthesized polymers showed widespread mechanical properties depending on the composition of curing agent. Except NIPU2 sample, all the other samples display a smooth transition in the stress–strain behavior from the elastic to plastic deformation regions similarly to lightly crosslinked rubbers. NIPU2 sample made solely from ATPEG shows typical behavior of low crystalline plastic. In fact, due to low crosslink density of these polymers, their tensile behaviors were mainly determined by crystalline content of networks. By increasing ATPEG content in the blends modulus and tensile strength increased. The data of modulus in the table were calculated based on DMTA curves (Fig. 4) at 80 °C.
One of the parameters that may influence on blood compatibility of biomaterials is their surface hydrophilicity. Theoretically, a decrease in contact angle of water droplets on a biomaterial surface shows higher surface wettability (or polarity) of a material. According to experimental results, which are tabulated in Table 2, NIPU1 showed the highest static contact angle. Increasing the content of ATPEG led to surface hydrophilicity increase, as demonstrated by a decrease in the water contact angle. Water absorption was also measured to determine the polymer bulk hydrophilicity, since this parameter was expected to have a substantial impact on hydrolytic degradation rate. The water uptake as a function of the time and sample type is presented in Table 2. The polarity of a sample is the main factor that can control the amount of absorbed water. With increasing the content of hydrophilic moieties derived from ATPEG component the overall water uptake amount of the final networks is also increased.
The results of in vitro degradation for NIPU1–NIPU5 samples in TBS solution at 37 °C are presented in Fig. 7. Presence of ester groups of triglyceride in the structure of carbonated soybean oil (CSBO) renders prepared polyurethanes to be hydrolytically degradable. Due to inherent hydrophobic nature of fatty acid and the presence of crosslinked domains in the structure of NIPUs, low rate of hydrolytic degradation is expected of these materials. Introducing hydrophilic polyols such as PEG can enhance the degradation rate, because PEG can enhance water permeability and cause an increase in surface and bulk hydrophilicity which provide more water in vicinity of hydrolytically labile ester groups. Therefore, the extent of degradation increased when the bulk hydrophilicity of polymeric film increased. In fact, the rate of hydrolytic degradation of NIPU1–NIPU5 was directly related to the water uptake value of networks, with faster degradation rate found in the more absorbent polymers. The margin of error in the each step is almost four percent.
The response of polymers surface to platelets was considered as criterion of blood compatibility. Cell culture tests were used to evaluate both cytotoxicity and cytocompatibility of the specimens. In the cell culture method, the performance of a cell is investigated by comparing it with a negative control. A negative control, tissue cell culture polystyrene in this experiment, is a sample thoroughly compatible with cells and is cultured with the main samples. A material is considered to be biocompatible, if it supports cell attachment and growth. Figure 8 shows optical photomicrographs of L-929 fibroblast cell interaction with the negative control, NIPU1, NIPU2, and NIPU3. The evaluation of cell morphology shows that cells survived and grew on the bottom of all wells with spindle-shaped morphology, for full 7 days and none of the polymers appeared to give off any toxic, since cells grew to confluence in all wells. Fortunately, the same conclusion was attained by MTT assay of prepared polymers. For all of these polymers cells survived in either direct contact of samples or upon treatment with leachates extracted from samples after 48 h. Results are collected in Table 3.
Conclusion
In this work, for the first time, the potential of NIPUs for application as biomaterial was examined through evaluation of their biodegradability, biocompatibility and hemocompatibility. Non-cytotoxicity, acceptable biocompatibility, non-hemolytic behavior and tunable biodegradability are fascinating features of prepared NIPUs. It was expected that these compounds take advantage of the different mechanical properties and degradation rates based on aminated PEG.
References
Beauty D, Pronobesh C, Manabendra M, Brigitte V, Niranjan K (2013) Bio-based biodegradable and biocompatible hyperbranched polyurethane: a scaffold for tissue engineering. Macromol Biosci 13(1):126–139
Zhang Q, Liu Y, Chen KC, Zhang G, Shi X, Chen H (2013) Surface biocompatible modification of polyurethane by entrapment of a macromolecular modifier. Colloid Surf B 102:354–360
Pavlova M, Draganova M (1993) Biocompatible and biodegradable polyurethane polymers. Biomaterials 14:1024–1029
Yang Chin W, Cherng J, Shau M (2004) Synthesis of novel biodegradable cationic polymer: N, N-diethylethylenediamine polyurethane as a gene carrier. Biomacromolecules 5:1926–1932
Gogolewski S, Gorna K (2007) Biodegradable polyurethane cancellous bone graft substitutes in the treatment of iliac crest defects. J Biomed Mater Res 80:94–101
Fromstein JD, Woodhouse KA (2002) Elastomeric biodegradable polyurethane blends for soft tissue applications. J Biomat Sci Polym E 13(4):391–406
Grada S, Kupcsika L, Gornab K, Gogolewskib S, Alini M (2003) The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials 24:5163–5171
Zhang C, Zhang N, Wen X (2006) Improving the elasticity and cytophilicity of biodegradable polyurethane by changing chain extender. J Biomed Mater Res 79:335–344
Kloss J, de Souza F, da Silva E, Dionísio J, Akcelrud L, Zawadzki S (2006) Polyurethanes elastomers based on poly (ε-caprolactone) diol: biodegradation evaluation. Macromol Symp 245:651–656
Wang SH, Silva LF, Kloss J, Munaro M, Wada MA (2003) Polycaprolactone based biodegradable polyurethanes. Macromol Symp 197:255–264
Yeganeh H, Lakouraj M, Jamshidi S (2005) Synthesis and characterization of novel biodegradable epoxy-modified polyurethane elastomers. J Polym Sci Chem 43:2985–2996
Gorna K, Gogolewski S (2002) In vitro degradation of novel medical biodegradable aliphatic polyurethanes based on ε-caprolactone and Pluronics with various hydrophilicities. Polym Degrad Stab 75:113–122
Lim DI, Park HS, Park JH, Knowles JC, Gong MS (2013) Application of high-strength biodegradable polyurethanes containing different ratios of biobased isomannide and poly(ε-caprolactone) diol. J Bioact Compat Pol 28(3):274–288
Mei T, Zhu Y, Ma T, He T, Li L, Wei C (2014) Synthesis, characterization, and biocompatibility of alternating block polyurethanes based on PLA and PEG. J Biomed Mater Res 102(9):3243–3254
Oh JK (2011) Polylactide (PLA)-based amphiphilic block copolymers: synthesis, self-assembly, and biomedical applications. Soft Matter 7:5096–5108
Han JJ, Huang HX (2011) Preparation and characterization of biodegradable polylactide/thermoplastic polyurethane elastomer blends. J Appl Poly Sci 120:3217–3223
Petrovic ZS (2008) Polyurethanes from vegetable oils. Polym Rev 48:109–155
Tu Y, Kiatsimkul P, Suppes G, Hsieh F (2007) Physical properties of water-blown rigid polyurethane foams from vegetable oil-based polyols. J Appl Polym Sci 105:453–459
Hill K (2000) Fats and oils as oleochemical raw materials. Pure Appl Chem 72:1255–1264
Petrović ZS, Yang L, Zlatanić A, Zhang W, Javni I (2007) Network structure and properties of polyurethanes from soybean oil. J Appl Polym Sci 105:2717–2727
Lligadas G, Ronda JC, Galia M (2010) Plant oils as platform chemicals for polyurethane synthesis: current state-of-the-art. Biomacromolecules 11:2825–2835
Radojčić D, Ionescu M, Zoran S, Petrović ZS (2013) Novel potentially biodegradable polyurethanes from bio-based polyols. Contemporary Materials 4:9–21
Desroches M, Benyahya S,Besse V,Auvergne R, Boutevin B, Caillol S (2014) Synthesis of bio-based building blocks from vegetable oils: a platform chemicals approach.Lipid Technol 26(2):35–38
Nohra B, Candy L, Blanco JF, Guerin C, Raoul Y (2013) From petrochemical polyurethanes to biobased polyhydroxyurethanes. Macromolecules 46:3771–3792
Li X, Fang Z, Li X, Tang S, Zhang K, Guo K (2014) Synthesis and application of a novel bio-based polyol for preparation of polyurethane foams. New J Chem 38:3874–3878
Desroches M, Escouvios M, Auvergne R, Caillol S, Boutevin B (2012) From vegetable oils to polyurethanes: synthetic routes to polyols and main industrial products. Polym Rev 52:38–79
Miao S, Sun L, Wang P, Liu R, Su Z, Zhang S (2012) Soybean oil-based polyurethane networks as candidate biomaterials: synthesis and biocompatibility. Eur J Lipid Sci Technol 114:1165–1174
Miao S, Wang P, Su Z, Zhang S (2014) Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater 10:1692–1704
Dutta S, Karak N, Saikia JP, Konwar BK (2009) Biocompatible epoxy modified bio-based polyurethane nanocomposites: mechanical property, cytotoxicity and biodegradation. Bioresour Technol 100(24):6391–6397
Tseng S, Tang S, Shau M, Zeng Y, Cherng J, Shih M (2005) Structural characterization and buffering capacity in relation to the transfection efficiency of biodegradable polyurethane. Bioconjug Chem 16:1375–1381
Yeganeh H, Hojati-Talemi P (2006) Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly (ethylene glycol). Polym Degrad Stab 92:480–489
Yeganeh H, Jamshidi H, Jamshidi S (2007) Synthesis and properties of novel biodegradable poly (ε-caprolactone)/poly (ethylene glycol)-based polyurethane elastomers. Polym Int 56:41–49
Fromstein JD, Woodhouse KA (2002) Elastomeric biodegradable polyurethane blends for soft tissue applications. J Biomat Sci Polym E 13:391–406
Chia S, Gorna K, Gogolewski S, Alini M (2006) Biodegradable elastomeric polyurethane membranes as chondrocyte carriers for cartilage repair. Tissue Eng 12:1945–1953
Skarja GA, Woodhouse KA (1998) Synthesis and characterization of degradable polyurethane elastomers containing an amino acid-based chain extender. J Biomat Sci Polym E 9:271–295
Skarja GA, Woodhouse KA (2000) Structure-property relationships of degradable polyurethane elastomers containing an amino acid-based chain extender. J Appl Polym Sci 75:1522–1534
Diakoumakos C, Kotzev D (2004) Non-isocyanate-based polyurethanes derived upon the reaction of amines with cyclocarbonate resins. Macromol Symp 216:37–46
Figovsky O, Shapovalov L (2002) Features of reaction amino-cyclocarbonate for production of new type nonisocyanate polyurethane coatings. Macromol Symp 187:325–332
Xiao L, Su D, Yue C, Wu W (2014) Protic ionic liquids: a highly efficient catalyst for synthesis of cyclic carbonate from carbon dioxide and epoxides. J CO2 Util 6:1–6
Song B, Guo L, Zhang R, Zhao X, Gan H, Chen C, Chen J, Zhu W, Hou Z (2014) The polymeric quaternary ammonium salt supported on silica gel as catalyst for the efficient synthesis of cyclic carbonate. JCO2 Util 6:62–68
Bahr M, Mulhaupt R (2012) Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion. Green Chem 14:483
Kim M, Kim H, Ha C, Park D, Lee J (2001) Syntheses and thermal properties of poly (hydroxy) urethanes by polyaddition reaction of bis (cyclic carbonate) and diamines. J Appl Polym Sci 81:2735–2743
Kihara N, Endo T (1993) Synthesis and properties of poly (hydroxyurethane) s. J Polym Sci Chem 31:2765–2773
Kihara N, Kushida Y, Endo T (1996) Optically active poly (hydroxyurethane)s derived from cyclic carbonate and l-lysine derivatives. J Polym Sci Chem 34:2173–2179
Jalilian M, Yeganeh H, Nekoomanesh HM (2008) Synthesis and properties of polyurethane networks derived from new soybean oil-based polyol and a bulky blocked polyisocyanate. Polym Int 57:1385–1394
Ochiai B, Inoue S, Endo T (2005) One-pot non-isocyanate synthesis of polyurethanes from bisepoxide, carbon dioxide, and diamine. J Polym Sci Chem 43:6613–6618
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalilian, S., Yeganeh, H. Preparation and properties of biodegradable polyurethane networks from carbonated soybean oil. Polym. Bull. 72, 1379–1392 (2015). https://doi.org/10.1007/s00289-015-1342-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1342-3