Abstract
The present work investigated the degradation of poly(butylene succinate) (PBS) in different aqueous media, as PBS microparticles are intended for use in personal care and cosmetic applications. Degradation tests were performed for the first time at different conditions of salinity, pH and temperature for two types of PBS: microparticles produced through suspension polycondensations and commercial pellets produced through bulk polycondensations. As shown experimentally, rates of PBS degradation were sensitive to modification of degradation conditions, being faster at higher temperatures, at acidic conditions and at alkaline conditions. However, PBS degradation was not very sensitive to the presence of salts, although degradation rates were shown to be higher in real sea water samples. Additionally, rates of PBS degradation were shown to depend significantly on PBS properties and morphology. Based on the obtained experimental data, a model was proposed to evaluate the effects of degradation temperature and particle morphology on the rates of PBS degradation in sea water, providing suitable fits for the available data.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the growing environmental concerns, the industrial production of plastics has been recently focused on sustainability issues. The most important environmental concerns have been related to the increasing demands of space for post-consumption plastic wastes in sanitary landfills [1] and the accumulation of plastics (particularly plastic microparticles) in the oceans [2]. As a matter of fact, the use of biodegradable polymer materials can provide plausible solutions for minimization of solid waste accumulation both in landfills and sea waters [3]. For this reason, there is growing interest in the manufacture and characterization of biodegradable polymers.
Among the many available biodegradable polyesters that can be produced through renewable processes, the use of poly(butylene succinate) (PBS) can be particularly advantageous because this resin presents thermal and physical properties that are similar to thermal and physical properties of polyolefins [4,5,6]. Besides, PBS can be obtained through polycondensation reactions between succinic acid (SA) and 1,4-butanediol (1,4-BD), which can be manufactured from renewable feedstocks [7]. For this reason, the production of green PBS materials is expected to increase in the forthcoming years, with the simultaneous reduction of green PBS prices [7]. Some comparative advantages of PBS can be related to the good biodegradability, easy processability and low cost of this resin, when compared to other biodegradable plastic alternatives, such as poly(lactic acid) and poly(caprolactone) [8].
The production of polymer microparticles through polycondensation processes does not constitute a simple task, as polycondensations are usually performed in bulk and polymer products are almost always pelletized [9]. For this reason, the manufacture of polycondensation polymer microparticles may require the implementation of many distinct additional unit operations, including polymer dissolution, precipitation, separation and drying, among others, using suitable solvents and rendering the operation complex and expensive [10]. On the other hand, polymer microparticles can be obtained more easily if polycondensations are performed in suspension, as recently suggested by Dutra et al. [11, 12].
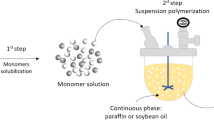
Suspension polycondensation processes are conducted in presence of two immiscible phases: the dispersed phase, which contains the monomers and where polymerization takes place; and the suspending phase, which keeps the reacting phase in suspension, allowing for suitable mixing of the reacting system and for production of polymer microparticles [11, 12]. Besides, polycondensation reactions can be performed in shorter times and without catalysts, leading to products with narrow molecular weight distributions, with weight average molecular weights ranging from 103 to 105 g/mol. Additionally, the final average particle sizes range from 50 to 500 μm and can be controlled through appropriate manipulation of the agitation speed and addition of suitable suspending agents. Comparatively, typical bulk and solution polycondensation processes used to produce PBS resins lead to products with broad molecular weight distributions, with weight average molecular weights ranging from 104 to 105 g/mol, in the form of pellets with average sizes of 2–5 mm and requiring reaction times of 20–50 h [13].
Many biodegradable polyesters are prone to degradation via oxidation (due to oxygen action) or hydrolysis (due to water action) [14]. Oxidizable polymers normally degrade slowly, although polymer materials may become more susceptible to other sorts of degradation when they are initially subject to oxidation [10]. On the other hand, hydrolysable polymers normally degrade faster and are more sensitive to modification of environmental temperatures [15]. It is important to observe that polymer degradation leads to formation of possibly many distinct byproducts, so that it is of utmost relevance to characterize what the degradation products are and how degradation products can affect the environment [16]. Particularly, PBS resins are subject to hydrolysis, which leads to the release of the original SA and 1,4-BD monomers, whose toxicity for marine life is low and do not lead to bioaccumulation in marine organisms [16, 17].
During the aqueous-phase hydrolysis, several studies have pointed out that the amorphous region of semi-crystalline polymers, such as PBS, is more susceptible to degradation than the crystalline region [15, 18]. Kint et al. [19] studied the hydrolytic degradation of films of PBS, poly(ethylene terephthalate) (PET) and PET/PBS in distillated water at 37 °C during 6 months and found that the contents of SA and 1,4-BD in the polymer residual decreased with time, indicating that hydrolysis preferentially occurred in the SA/1,4-BD ester groups. Kint et al. [19] also verified that the rate of polymer degradation increased with the PBS content of the polymer backbone and that the melt temperature and crystallinity of the residuals increased with the degradation extent, suggesting that hydrolysis predominantly took place in the amorphous phases. Zhu et al. [20] investigated the degradation of PBS, poly(ethylene succinate) (PES) and PES/PBS with weight average molecular weights of 2 × 105 g mol−1. Sample films with dimensions of 10 mm × 10 mm × 0.3 mm were prepared and immersed in glass flasks filled with 1 M NaOH aqueous solutions. Zhu et al. [20] concluded that PBS was more resistant to hydrolytic degradation than PES because of the higher crystallinity of PBS. Therefore, the rates of degradation increased with the PES content of the copolymer.
To the best of our knowledge, many studies in literature investigated the degradation of PBS in terrestrial environments [8, 21,22,23,24]; however, only few works focused on the PBS degradation in aqueous environments. Kint et al. [19] and Kanemura et al. [4] reported that PBS and PBS copolymers can exhibit pronounced hydrolytic degradability, which increases with the content of 1,4-butylene succinic units. According to these authors, hydrolysis mainly occurs on the aliphatic ester groups of the polymers. In turn, Kasuya et al. [25] reported that the degradation rates of polyesters is influenced by the presence of salts in water, and thus, is dependent not only on the chemical structure of monomer but also on the used water source.
Based on the previous paragraphs, one can observe that there are incentives for use of PBS microparticles in personal care and cosmetic applications, although studies regarding the hydrolytic degradation of PBS microparticles in aqueous solutions are still lacking. Besides, investigations of PBS degradation in saline solutions that simulate marine environments have yet to be performed. In addition, mathematical models have not been proposed yet to describe the degradation of PBS. For these reasons, the present work investigated the degradation of PBS in different aqueous media at different conditions of salinity, pH and temperature for two types of PBS: microparticles produced through suspension polycondensations and commercial pellets produced through bulk polycondensations. Based on the obtained experimental data, a model was proposed to evaluate the effects of degradation temperature and particle morphology on the rates of PBS degradation in sea water, providing suitable fits for the available data.
Experimental
Materials
SA was provided by Vetec (Rio de Janeiro, Brazil) and used as monomer. 1,4-BD was supplied by Merck (Rio de Janeiro, Brazil) and used as monomer. Span 80 (sorbitan monooleate) was provided by Fluka (Mexico City, Mexico) and used as suspending agent. Paraffin (wax) with melting point of 62 °C was supplied by Vetec (Rio de Janeiro, Brazil) and used as suspending medium. Sodium chloride, calcium chloride, hydrochloric acid was provided by Vetec (Rio de Janeiro, Brazil) while sodium hydroxide was provided by Caledon Laboratory Chemical (Ontario, Canada) used for formulation of the degradation environments. All reagents were obtained as analytic grade materials of high purity and were used as received without further purification. Industrial PBS samples of high molar mass (6.5 × 104 Da) and in the form of pellets with diameter of 4 mm were kindly provided by Braskem (Rio de Janeiro, Brazil).
Preparation of PBS Microparticles
The preparation of PBS microparticles was performed through suspension polycondensations, as described by Dutra et al. [11, 12]. The interested reader should consult the original references for details. However, it is important to emphasize that polycondensations were performed in heterogeneous media for 6 h at 160 °C, using paraffin as the suspending phase and Span 80 as the suspending agent, leading to PBS microparticles with characteristic diameters ranging from 60 to 400 µm (Fig. 1). As described by Dutra et al. [11, 12], at the end of the reaction runs the obtained microparticles were thoroughly washed with acetone in order to remove the residual surfactant and suspension medium.
Hydrolysis Tests
Preparation of Degradation Media
For degradation tests, six different aqueous media were tested. Two alkaline solutions were prepared with sodium hydroxide, with pH values of 10 and 14. Two acidic solutions were prepared with hydrochloric acid, with pH values of 1 and 4. Samples of synthetic saline solutions (prepared in the laboratory with distinct salt concentrations and with sodium chloride:calcium chloride mass ratio of 10:1), and naturally occurring sea water (collected in Recreio dos Bandeirantes beach, Rio de Janeiro, Brazil) were used to simulate the marine degradation environment.
Preparation of PBS Films
In the first step, PBS films were prepared through melt-quenching in Petri dishes. 15 g of PBS particles were initially melted in the mold at 140 °C and then quenched in ice-cold water at 0 °C. The melt-quenched polymer film was used to prepare test pieces with dimensions of 10 mm × 6 mm × 2 mm, with help of precision stylet and sandpaper. All PBS pieces were dried in vacuum oven at 50 °C for 24 h prior to use in degradation tests. These films were prepared in order to standardize the samples exposed to degradation, since commercial PBS was supplied as pellets with average diameter of 4 mm, while the PBS microparticles prepared in suspension presented average diameter of 200 µm.
Hydrolytic Degradation
Hydrolytic degradation tests were carried out in five different aqueous media, at distinct temperature, pH and salinity values, as shown in Table 1. Samples of 150 mg were incubated in 10 mL glass test tubes, with 6 mL of degradation media and maintained in a temperature-controlled water bath under mild shaking. After that, samples were withdrawn at specified sampling times and rinsed thoroughly with distilled water. Then samples were vacuum-dried at 50 °C in an oven until reaching constant weight. Hydrolytic degradation tests were also performed with PBS microparticle sea water, using similar techniques at 25, 40 and 60 °C.
Characterization
Salt Concentration
In order to define the relative salinity of the aqueous media, the conductivities of NaCl solutions prepared with distilled water, with salt concentrations of 2.5, 5.0, 7.5 and 10.0 w/v%, were measured with help of a conductimeter (Gehaka, model CG2000, São Paulo, Brazil) and used to build a calibration curve. Figure 2 shows the obtained calibration curve and the conductivities of the analyzed saline samples.
Gel Permeation Chromatography (GPC)
Gel permeation chromatography (GPC) was used to determine the average molecular weights of PBS. The GPC chromatograph (Viscotek GPCmax, Malvern, Malvern, UK) was equipped with three columns (Shodex GPC HFIP-803, Shodex GPC HFIP-804 and Shodex GPC HFIP-805, Showa Denko, Tokyo, Japan) and analyses were performed at 40 °C in solutions of HFIP (3 mg/mL), used as the mobile phase. The solvent flow rate was kept constant at 1.0 mL/min throughout the analyses. A calibration curve was constructed with poly(methyl methacrylate) standards (American Polymer Standards, Mentor, USA) with molecular weights ranging between 102 and 106 Da.
Scanning Electron Microscopy (SEM–EDS)
The surfaces of virgin and degraded samples were characterized through scanning electron microscopy coupled with energy dispersive spectroscopy (SEM–EDS) (FEI, Inspect S50, Tokyo, Japan) at maximum accelerating voltage of 20 kV under absolute vacuum. Elemental compositions were characterized through energy dispersive X-ray spectrometry, using the EDAX detector (EDAX, New Jersey, USA) for salt content measurement. Prior to analyses, samples were coated with a thin layer of gold (15 nm) through sputtering (Emitech, model K550, Paris, France), in order to prevent electrical discharge.
Model Development
The mathematical model proposed here to simulate the degradation of PBS samples assumes that the analyzed test pieces have the characteristic shape of a parallelepiped, with length w, thickness l, height h and initial mass M0. The important additional model assumptions are:
-
(i)
Hydrolytic degradation is controlled by diffusion and mass transfer at the interface, so that the rate of mass loss is proportional to the area of the piece;
-
(ii)
Hydrolytic degradation is slow and absorption of water is low, so that the density (ρ) of the test piece remains essentially constant;
-
(iii)
The rate of degradation does not depend on the surface direction, so that degradation is isotropic.
Based on these simplifying assumptions, the proposed degradation model can be represented in the form:
where ε is the linear rate of degradation of the characteristic dimensions of the test piece, t is the time of degradation and K is a characteristic kinetic constant. Thus, at a given instant of time t, the mass of the test piece M can be computed as:
so that the relative loss of mass is:
Equation (3) can accommodate for nonlinear rates of degradation if it is written in the form:
where n is a parameter that accounts for the nonlinear behavior of the degradation curve.
The degradation constant K is expected to be a function of the degradation temperature. For this reason, it is assumed that K follows the Arrhenius equation in the form:
where k0 is the pre-exponential factor, Ea is the activation energy, R is the universal gas constant and T is the absolute temperature. However, it is well known that the mathematical structure of the Arrhenius equation results in high correlation between k0 and Ea, which makes the parameter estimation very difficult [26]. In order to reduce the high correlation between the parameters k0 and Ea, the Arrhenius equation was reparametrized in this form:
where the parameters of the reparameterized equation can be related to the parameters of the traditional Arrhenius equation as:
Parameter Estimation
Parameter estimations were performed with the standard least squares method, through minimization of the sum of the squared differences between measured and calculated data [27]. The estimation procedure was implemented in Mathematica 10.1 [28], using a NelderMead method [29], with relative tolerance of 1.0 × 10−3 and using confidence level of 95% for statistical analyses.
Results
Effects of the Environment on the Hydrolytic Degradation of PBS
Figure 3 shows the weight loss of the analyzed test pieces after 10 days of contact with different aqueous media at 25 and 40 °C. In turn, Fig. 4 shows the average molar mass of polymer samples before and after 60 days of incubation at different conditions.
Although PBS samples produced through suspension polymerizations presented higher rates of mass loss than the commercial PBS (as shown in Fig. 3), significant decrease of the weight average molecular weights was observed only for the commercial sample (Fig. 4).
As the weight average molar masses (Mw) of the polymer produced in suspension were one order of magnitude lower than the Mw values of the commercial sample (Table 2), higher rates of mass loss and lower sensitivity of Mw values might be expected for PBS samples produced in suspension. The decay of average molar masses of hydrolyzed aliphatic polyesters is expected to be due to random chain scission during the hydrolytic degradation [30]; meanwhile, mass loss occurs when chains become sufficient short to dissolve in the aqueous environment [31]. In spite of that, Figs. 4 and 5 shows that the shifts of the molar mass distributions during the analyzed periods were homogeneously small in all cases for PBS samples produced in suspension, indicating that the bulk properties of the test pieces did not change much during the analyses and that chains of all sizes were similarly subject to hydrolytic degradation, as expected in a random process.
From a practical point of view, these results indicate that cosmetic and personal care applications can benefit from using PBS resins with lower molar mass, given the higher rates of degradation in aqueous environments, which must be seriously considered during the product design phase.
Effects of pH on PBS Degradation
Figure 3 shows that degradation of PBS samples is accelerated in acidic and alkaline aqueous media, when compared to degradation in neutral aqueous environment. In the presence of acidic conditions, PBS degradation is catalyzed by protons, forming shorter chains and additional carboxylic groups, which can cause the additional decrease of the pH and lead to autocatalytic degradation conditions [32] and sensitivity to particle sizes [33]. In the presence of alkaline conditions, cleavage of the ester bonds can also be catalyzed by the nucleophilic attack of the hydroxide ion [34]. It is important to note that both acids and bases can act as catalysts for the hydrolysis [35] (as shown in Fig. 6), justifying the lower rates of degradation in neutral media.
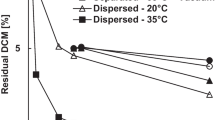
The influence of pH on degradation of the analyzed PBS samples is better visualized in Figs. 7 and 8, where the magnitudes of the effects of temperature and pH variations can be compared. As one can observe, the effect of temperature on the rates of PBS degradation are more pronounced than the effects of pH, when very acidic or alkaline conditions are considered. As shown in Figs. 7 and 8, higher temperatures lead to higher rates of PBS degradation, which can be explained by the higher chemical activities of the species present in the degradation media and the higher absorption of water into the polymer matrix [36].
Figure 8 shows the weight loss of PBS test pieces put in contact with aqueous environments with distinct pH values after 10 days, including the neutral reference (pH 7.0). When compared to the neutral reference, it becomes evident that modification of the pH exerts a significant effect on the rates of PBS degradation, both for acidic and alkaline environments. In all cases, embrittlement of polymer materials could be observed visually after withdrawal. It is important to mention that extreme alkaline media (pH 14) caused the full degradation of the test pieces after 18 h of incubation, which definitely indicates the catalytic effect of the hydroxyl ion. All degradation curves presented similar shapes, with decreasing rates of polymer degradation with time, as shown in Fig. 7. Degradation rates are expected to be higher at lower pH values because of the higher hydrophilicity provided by alcohol and acid ends of the shorter chains, which allow for increase of the water uptake [37]. At alkaline pH, the ionic strength of the aqueous media also increases due to formation of the carboxylate anion, which leads to lower rates of water uptake but higher rates of chain scission [37]. The positive effect of temperature on rates of degradation could be observed in all cases.
Effects of Salinity on PBS Degradation
Figure 3 shows that degradation of PBS samples is not very sensitive to presence of salts in the aqueous media, indicating that rates of degradation in marine media should not be very different from rates of degradation in pure water. The obtained weight loss profiles of test pieces are shown in Fig. 9. As observed previously, degradation curves presented similar shapes, with decreasing rates of polymer degradation with time. It is important to observe that test pieces prepared with the commercial PBS sample presented the lowest rates of degradation rate in different media (although a single run is shown in Fig. 9), confirming the previous analyses. Once more, the positive effect of temperature on rates of degradation might be observed in all cases.
It is expected that water is preferentially absorbed in the amorphous regions of polymer samples during the initial stages of degradation, causing significant degradation of crystalline domains only when most (or all) of the amorphous polymer region vanishes [38]. This can possibly explain the decreasing rates of mass loss with degradation time. Moreover, the significant increase of degradation rates with temperature indicates that the rates of PBS degradation can be very different in distinct marine environments. The increase of the salt concentration causes the reduction of the water activity and might lead to slightly lower rates of polymer degradation [39], as observed in the present study. In spite of that, the effect of the salt concentration on observed rates of PBS degradation was negligible when compared to the effect of temperature and pH values.
Morphology
Figure 10 shows micrographs of test pieces prepared with PBS microparticles and treated under sea water after 60 days at 40 °C. It can be observed that PBS test pieces presented smooth surfaces before the degradation process. However, after the degradation test, PBS samples exhibited rough surfaces, filled with radially oriented cracks. This behaviour is due to the initial swelling of PBS samples, followed by superficial erosion and formation of micro-cracks [40, 41]. In general, similar phenomena have been reported for the degradation of polyesters in different aqueous media [40], however, previous works have focused mainly on the influence of the material on the degradation process, using different PBS copolymers as samples, and not on the influence of the degradation media.
Unlike other works found in the literature, the present study evaluated the influence of different aqueous media on the degradation of PBS. As reported previously, different degradation media were studied and, after 60 days at 40 °C, PBS samples lost 10.1 wt% in sea water, 12.52 wt% in distilled water and 9.0 wt% in saline water. However, the obtained micrographs indicated that similar degradation processes took place, regardless the particular compositions of the analyzed aqueous media, with formation of micro-cracks, as shown in Fig. 10 for samples partially degraded in sea water. Although the degradation process seems to be similar, the intensity of degradation clearly depends on the salt concentration. For instance, as shown in Figs. 3 and 9, the rate of degradation is lower in the presence of salts, as also described in other works for other materials [37].
Modelling of the Polymer Degradation
Using the degradation model described in Eq. 4 and the available experimental mass loss data (Figs. 7, 9), it was possible to estimate the model parameters for each analyzed aqueous medium. As shown in Table 3, the objective functions achieved values that were in accordance with the expected confidence intervals, as calculated with the Fisher (F) distribution with confidence level of 95% and respective NE-NP degrees of freedom [42]. Parameter estimated and the respective confidence regions are shown in Table 3.
Although most estimated parameters can be regarded as statistically similar, parameter estimates presented in Table 3 confirm that degradation occurs faster in sea water than in the other aqueous media, indicating that the more complex chemical composition and unavoidable presence of microorganisms can enhance the rates of PBS degradation [15, 16, 25]. It is important to emphasize that in the present work two types of saline media were considered: a natural saline medium (collected directly from the sea) and synthetic saline media (prepared in the lab at different concentrations with sodium chloride : calcium chloride mass ratio of 10:1). According to the obtained results, the saline solutions prepared in the lab cannot fully simulate the aqueous sea environment during the degradation tests, given the distinct rates of PBS degradation observed in both cases (faster in the sea samples). This is not exactly surprising, given the much more complex composition (naturally occurring sea water can contain a larger number of distinct cations and anions) and the unavoidable presence of microorganisms in sea water. Besides, the salt concentration in the synthetic saline solution was higher than in the naturally occurring sea water, as shown in Fig. 2, confirming that addition of salts tends to retard the degradation of PBS samples.
The quality of proposed model is shown in Figs. 11, 12 and 13, which present the predicted and experimental mass loss data in sea water. As one can see, the model was able to represent the available experimental data satisfactorily in all conditions. It must be observed that the parameter n indicates that the PBS degradation does not follow a linear profile, probably due to the increased crystallinity of the residual polymer, as well documented in the literature [43,44,45,46].
Figure 14 shows the simulation of the time required for total degradation of PBS test pieces in sea water at the studied temperatures. As shown, the PBS specimens degrade much faster than usual plastics, but the degradation times can still be regarded as excessively long.
Influence of Geometry on the Degradation Process
The proposed model was used to predict the mass loss for pieces with different shapes, but with similar characteristic dimension of 2 mm. As one might already expect and shown in Fig. 15, parallelepipeds are expected to degrade faster and spheres are expected to degrade more slowly because of the respective higher and smaller specific areas, as suggested by Grizzi et al. [47].
Based on Fig. 15 and considering that polymer particles find widespread use in the cosmetic and personal care industries, it seems appropriate to perform degradation experiments also with the PBS microparticles prepared through suspension polymerizations (“Preparation of PBS Microparticles” section) [48]. Obtained results are presented in Fig. 16. As one might already expect, the rates of PBS degradation were higher for microparticles, given the much higher specific surfaces of the microparticles, when compared to test pieces. Figure 17 presents the expected total degradation time in sea water as a function of the particle size, as predicted with the proposed model. It is worth mentioning that PBS microparticles with characteristic diameter smaller than 1 µm (as particles added to exfoliators) are expected to degrade in aqueous media in less than 1 day, as predicted by the model. Figure 18 shows micrographs of PBS microparticles after distinct degradation times, highlighting the occurrence of particle degradation as indicated by the proposed model.
Conclusions
The present work investigated the degradation of PBS in different aqueous media. Degradation tests were performed for the first time at different conditions of salinity, pH and temperature for two types of PBS: microparticles produced through suspension polycondensations and commercial pellets produced through bulk polycondensations. As shown experimentally, rates of PBS degradation were sensitive to modification of degradation conditions, being faster at higher temperatures, at acidic conditions and at alkaline conditions. However, PBS degradation was not very sensitive to the presence of salts, although degradation rates were shown to be higher in real sea water samples. Additionally, rates of PBS degradation were shown to depend significantly on PBS properties and morphology and to decrease with the degradation time. Particularly, it was shown that the rates of PBS degradation depend on the specific area of analyzed pieces, being faster for microparticles because of the much larger specific area of powders, when compared to test pieces. Besides, the rates of mass loss were shown to decrease with the molecular weight, encouraging the use of PBS resins of lower molecular weights in cosmetic and personal care applications. Based on the obtained experimental data, a model was proposed to evaluate the effects of degradation temperature and particle morphology on the rates of PBS degradation in sea water, providing suitable fits for the available data. According to the obtained fits, PBS microparticles are expected to degrade completely in sea water in < 1 year, if they have characteristic diameter of 100 µm, and in < 1 day, if they have characteristic diameter of 1 µm.
References
Bergmann M, Gutow L, Klages M (2015) Marine anthropogenic litter. Springer International Publishing, Cham
Setälä O, Norkko J, Lehtiniemi M (2016) Feeding type affects microplastic ingestion in a coastal invertebrate community. Mar Pollut Bull 102:95–101. https://doi.org/10.1016/j.marpolbul.2015.11.053
Kuruppalil Z (2011) Green plastics: an emerging alternative for petroleum-based plastics? Int J Eng Res Innov 3:59–64
Kanemura C, Nakashima S, Hotta A (2012) Mechanical properties and chemical structures of biodegradable poly(butylene-succinate) for material reprocessing. Polym Degrad Stab 97:972–980. https://doi.org/10.1016/j.polymdegradstab.2012.03.015
Ren M, Song J, Song C et al (2005) Crystallization kinetics and morphology of poly(butylene succinate-co-adipate). J Polym Sci B 43:3231–3241. https://doi.org/10.1002/polb.20539
Zhang Y, Lu B, Lv F et al (2012) Effect of processing conditions on poly(butylene succinate) foam materials. J Polym Sci B 126:756–761. https://doi.org/10.1002/app
Aeschelmann F, Carus M (2015) Biobased Building blocks and polymers in the world: capacities, production, and applications-status quo and trends towards 2020. Ind Biotechnol 11:154–159. https://doi.org/10.1089/ind.2015.28999.fae
Zhao JH, Wang XQ, Zeng J et al (2005) Biodegradation of poly(butylene succinate) in compost. J Appl Polym Sci 97:2273–2278. https://doi.org/10.1002/app.22009
MORGAN PW (1962) Low-temperature polycondensation processes. American Chemical Society, Washington, DC, pp 191–199
Vroman I, Tighzert L (2009) Biodegradable polymers. Materials (Basel) 2:307–344. https://doi.org/10.3390/ma2020307
Dutra L, Nele S, Pinto M JC (2018) A novel approach for the preparation of poly(butylene succinate) microparticles. Macromol Symp 381(1):1800118
Dutra L, Nele S, Pinto M JC (2018) Preparation of polymer microparticles through non-aqueous suspension polycondensations. Part IIeffects of operating variables on properties of poly(butylene succinate). Macromol React Eng. https://doi.org/10.1002/mren.201800039
Achmad F, Yamane K, Quan S, Kokugan T (2009) Synthesis of polylactic acid by direct polycondensation under vacuum without catalysts, solvents and initiators. Chem Eng J 151:342–350. https://doi.org/10.1016/j.cej.2009.04.014
Amass W, Amass A, Tighe B (1998) A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym Int 47:89–144. https://doi.org/10.1002/(SICI)1097-0126(1998100)47:2%3C89::AID-PI86%3E3.0.CO;2-F
Goujard L, Roumanet P-J, Barea B et al (2016) Evaluation of the effect of chemical or enzymatic synthesis methods on biodegradability of polyesters. J Polym Environ 24:64–71. https://doi.org/10.1007/s10924-015-0742-7
Lindström A, Albertsson AC, Hakkarainen M (2004) Quantitative determination of degradation products an effective means to study early stages of degradation in linear and branched poly(butylene adipate) and poly(butylene succinate). Polym Degrad Stab 83:487–493. https://doi.org/10.1016/j.polymdegradstab.2003.07.001
Pohanish RP (2015) Sittig’s handbook of pesticides and agricultural chemicals. Elsevier, Amsterdam, pp 738–768
Ahn BD, Kim SH, Kim YH, Yang JS (2001) Synthesis and characterization of the biodegradable copolymers from succinic acid and adipic acid with 1,4-butanediol. J Appl Polym Sci 82:2808–2826
Kint DPR, Alla A, Deloret E et al (2003) Synthesis, characterization, and properties of poly(ethylene terephthalate)/poly(1,4-butylene succinate) block copolymers. Polymer 44:1321–1330. https://doi.org/10.1080/00380768.2001.10408366
Zhu QY, He YS, Zeng JB et al (2011) Synthesis and characterization of a novel multiblock copolyester containing poly(ethylene succinate) and poly(butylene succinate). Mater Chem Phys 130:943–949. https://doi.org/10.1016/j.matchemphys.2011.08.012
Terzopoulou ZN, Papageorgiou GZ, Papadopoulou E et al (2016) Development and study of fully biodegradable composite materials based on poly(butylene succinate) and hemp fibers or hemp shives. Polym Compos 37:407–421. https://doi.org/10.1002/pc.23194
Kim HS, Kim HJ, Lee JW, Choi IG (2006) Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym Degrad Stab 91:1117–1127. https://doi.org/10.1016/j.polymdegradstab.2005.07.002
Phua YJ, Lau NS, Sudesh K et al (2012) Biodegradability studies of poly(butylene succinate)/organo-montmorillonite nanocomposites under controlled compost soil conditions: effects of clay loading and compatibiliser. Polym Degrad Stab 97:1345–1354. https://doi.org/10.1016/j.polymdegradstab.2012.05.024
Hoshino A, Sawada H, Yokota M et al (2001) Influence of weather conditions and soil properties on degradation of biodegradable plastics in soil. Soil Sci Plant Nutr 47:35–43. https://doi.org/10.1080/00380768.2001.10408366
Kasuya K, Takagi K, Ishiwatari S et al (1998) Biodegradabilities of various aliphatic polyesters in natural waters. Polym Degrad Stab 59:327–332. https://doi.org/10.1016/S0141-3910(97)00155-9
Schwaab M, Pinto JC (2007) Optimum reference temperature for reparameterization of the Arrhenius equation. Part 1: problems involving one kinetic constant. Chem Eng Sci 62:2750–2764. https://doi.org/10.1016/j.ces.2007.02.020
Chen S, Billings SA, Luo W (1989) Orthogonal least squares methods and their application to non-linear system identification. Int J Control 50:1873–1896. https://doi.org/10.1080/00207178908953472
Wolfram Research, Inc. (2018) Wolfram Programming Lab, ver 10. Wolfram Research, Inc., Champaign, IL. http://www.wolfram.com/
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7:308–313. https://doi.org/10.1093/comjnl/7.4.308
Xu J, Guo BH (2010) Poly(butylene succinate) and its copolymers: research, development and industrialization. Biotechnol J 5:1149–1163. https://doi.org/10.1002/biot.201000136
Kim HS, Yang HS, Kim HJ (2005) Biodegradability and mechanical properties of agro-flour-filled polybutylene succinate biocomposites. J Appl Polym Sci 97:1513–1521. https://doi.org/10.1002/app.21905
Antheunis H, Van Meer JC, Der, De Geus M et al (2009) Improved mathematical model for the hydrolytic degradation of aliphatic polyesters. Macromolecules 42:2462–2471. https://doi.org/10.1021/ma802222m
Siepmann J, Elkharraz K, Siepmann F, Klose D (2005) How autocatalysis accelerates drug release from PLGA-based microparticles: a quantitative treatment. Biomacromol 6:2312–2319. https://doi.org/10.1021/bm050228k
Solomons TWG, Fryhle CB (2007) Organic chemistry, 9th edn. Wiley India Pvt, Limited
Lucas N, Bienaime C, Belloy C et al (2008) Polymer biodegradation: mechanisms and estimation techniquesa review. Chemosphere 73:429–442. https://doi.org/10.1016/j.chemosphere.2008.06.064
Fukushima K, Tabuani D, Dottori M et al (2011) Effect of temperature and nanoparticle type on hydrolytic degradation of poly(lactic acid) nanocomposites. Polym Degrad Stab 96:2120–2129. https://doi.org/10.1016/j.polymdegradstab.2011.09.018
Sailema-Palate GP, Vidaurre A, Campillo AF, Castilla-Cortázar I (2016) A comparative study on poly(ϵ-caprolactone) film degradation at extreme pH values. Polym Degrad Stab 130:118–125. https://doi.org/10.1016/j.polymdegradstab.2016.06.005
Hakkarainen M, Albertsson AC, Karlsson S (1996) Weight losses and molecular weight changes correlated with the evolution of hydroxyacids in simulated in vivo degradation of homo- and copolymers of PLA and PGA. Polym Degrad Stab 52:283–291. https://doi.org/10.1016/0141-3910(96)00009-2
Mouzakis DE, Zoga H, Galiotis C (2008) Accelerated environmental ageing study of polyester/glass fiber reinforced composites (GFRPCs). Composites 39:467–475. https://doi.org/10.1016/j.compositesb.2006.10.004
Cho K, Lee J, Kwon K (2001) Hydrolytic degradation behavior of poly(butylene succinate)s with different crystalline morphologies. J Appl Polym Sci 79:1025–1033. https://doi.org/10.1002/1097-4628(20010207)79:6%3C1025::AID-APP50%3E3.0.CO;2-7
Huang T, Du X, Duan J et al (2017) Poly(ethylene oxide) induced microstructure and hydrolytic degradation behavior changes of poly(butylene succinate). Polym Test 61:8–16. https://doi.org/10.1016/j.polymertesting.2017.05.001
Schwaab M, Pinto JC (2007) Análise de Dados Experimentais I – Fundamentos de Estatística e Estimação de Parâmetros, 1st edn. e-papers, Rio de Janeiro
Wang Y, Pan J, Han X et al (2008) A phenomenological model for the degradation of biodegradable polymers. Biomaterials 29:3393–3401. https://doi.org/10.1016/j.biomaterials.2008.04.042
Heljak MK, Swieszkowski W, Kurzydlowski KJ (2014) Modeling of the degradation kinetics of biodegradable scaffolds: the effects of the environmental conditions. J Appl Polym Sci 131:1–7. https://doi.org/10.1002/app.40280
Kobayashi S, Naito K (2011) Biodegradation of poly(lactic acid)/ poly(butylene succinate) polymer blends. J Environ Eng 6:861–868. https://doi.org/10.1299/jee.6.861
Partini M, Pantani R (2007) FTIR analysis of hydrolysis in aliphatic polyesters. Polym Degrad Stab 92:1491–1497. https://doi.org/10.1016/j.polymdegradstab.2007.05.009
Grizzi I, Garreau H, Li S, Vert M (1995) Hydrolytic degradation of devices based on poly(DL-lactic acid) size dependence. Biomaterials 16:305–311. https://doi.org/10.1016/0142-9612(95)93258-F
Chang M (2015) Reducing microplastics from facial exfoliating cleansers in wastewater through treatment versus consumer product decisions. Mar Pollut Bull 101:330–333. https://doi.org/10.1016/j.marpolbul.2015.10.074
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva Dutra, L., de Souza Belan Costa, T., Lobo, V.T.V. et al. Preparation of Polymer Microparticles Through Non-aqueous Suspension Polycondensations: Part III—Degradation of PBS Microparticles in Different Aqueous Environments. J Polym Environ 27, 176–188 (2019). https://doi.org/10.1007/s10924-018-1329-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1329-x