Abstract
Tooth eruption sequences vary in a non-random way among mammalian species. Several variables have been linked to this, including tooth and jaw shape, adaptations to diet, and food processing. Likewise, changes in eruption patterns correlate with the speed of postnatal growth in some groups, the Schultz’s Rule pattern. Here, the eruption pattern of the permanent dentition in lower jaws from different cervid species have been investigated to discern the effect of these factors and phylogeny as well as to reconstruct the ancestral tooth eruption sequence of cervids. In ruminants, the different patterns of emergence of permanent teeth seem to be best explained by phylogeny. The degree of hypsodonty, age of first molar eruption, and life history parameters such as longevity and age of female sexual maturity do not explain the observed sequential differences in eruption patterns. The Parsimov-based analysis for the ancestral state resulted in a tooth eruption sequence of m1 – m2 – i1 – i2 – i3 – c – m3 – (ppp) for Cervidae; a pattern recorded in Odocoileus, Capreolus, and Hydropotes. The eruption pattern of Caenomeryx filholi, from the Oligocene of Gaimersheim, is identical to the result of the Parsimov-based analysis except for the presence of a first premolar, a tooth lost in cervids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammals replace their teeth only once during their life, possessing milk (deciduous) and adult (permanent) dentitions, but molars have only one generation (e.g., Osborn and Crompton 1973). The relative sequence of permanent tooth eruption is not fixed and varies among species (e.g., Smith 2000). Several hypotheses as to why these sequential differences occur have been postulated (e.g., Slaughter et al. 1974; Tattersall and Schwartz 1974; Simpson et al. 1990; Smith 2000; Godfrey et al. 2005). The aim of this study was to document the patterns of variation in eruption sequences in a clade of mammals and to investigate the variables associated with that variation. For this, the lower jaws of cervids were studied and compared with other members of Ruminantia. The general tooth formula in the lower jaw of cervids as well as ruminants is three incisors, one canine, three premolars, and three molars (Thenius 1989) and few species such as Myotragus, Connochaetes, or Antidorcas have a different dental formula (Rautenbach 1971; Attwell 1980; Jordana et al. 2013). Other shared features are an incisiviform canine followed by a diastema, selenodont tooth morphology, and two distinct sets of functional teeth (van Nievelt and Smith 2005a). Nonetheless, Ruminantia do not share the same eruption pattern (e.g., Smith 2000), which allows testing for different signals such as phylogeny and anatomy as well as ecological and life history variables. In contrast, carnivorans and primates are more variable in the arrangement of teeth within the lower jaw as well as in morphology and the expression of the two sets of teeth (Thenius 1989; van Nievelt and Smith 2005a).
Morphological Constraints, Adaptive Evolution, and Dental Eruption Patterns
The idea that the general facial architecture could influence the pattern of dental eruption was proposed based on the study of ape and hominid remains (Simpson et al. 1990). It was assumed that the human pattern of building and replacing teeth is influenced by three major factors: the reduction of the canine tooth in males, the reduction of prognathism in the hominid lineage, and the peculiarity of the human m3 (Simpson et al. 1990). Tooth morphology and size may also affect the sequential differences in tooth eruption. Slaughter et al. (1974) investigated the eruption sequence of the postcanine teeth in Afrotheria, Carnivora, Eulipotyphla, Leptictida, and Scandentia and concluded that the sequential differences are related to the different morphology of the teeth, especially the carnassials in carnivorans. In Multituberculata, the increasing size of the lower fourth premolar is considered to be causally coupled to several changes within the eruption pattern (Greenwald 1988). Other hypotheses with a more adaptive value have also been discussed in marsupial and placental mammals. One example is the relative delay of development and eruption of certain incisor teeth in marsupials and carnivorans. A late eruption of certain incisors has been linked to suckling, as the resulting open space could allow for a longer preweaning period (Luckett and Wooley 1996; van Nievelt and Smith 2005a, b). Besides that, diet and food processing have been linked with permanent tooth eruption patterns (Godfrey et al. 2001; Dirks 2003; Guthrie and Frost 2011; Forasiepi and Sánchez-Villagra 2014).
Life History and Dental Eruption Patterns
It has been shown in primates that some life history variables such as brain mass and weaning period correlate with the age of first permanent tooth eruption (Smith 1992; Smith et al. 1994; Godfrey et al. 2001). However, not only the time but also the pattern of eruption is variable even in closely related species. To explain this, Smith (2000) postulated Schultz’s Rule based on the work of Adolph Schultz (1956, 1960). According to it, an animal with a slower and longer life history has a tendency to replace deciduous teeth early in the relative sequence of tooth eruption compared to eruption of molar teeth. Therefore, Schultz’s Rule would reflect the dynamics between the decay of the deciduous teeth and the possibility for the jaw to accommodate molar teeth in a slow growing mammal. Fast growing mammals would not have such physical constraints because speed of jaw growth to accommodate the molar teeth would precede the loss of functionality in the deciduous dentition. The validity of Schultz’s Rule has been tested on “ungulates” and primates with a strong support found in primates and “generalized ungulates” such as Suinae but with a weaker support in more “specialized ungulates” such as Ruminantia (Smith 2000). Subsequently, several studies examined this “rule.” Some argued in favor of this hypothesis based on studies on platyrhines (Henderson 2007), pantodonts (McGee and Turnbull 2010), equids (Hellmund 2013), and caprines (Jordana et al. 2013); others have found exceptions in different groups including lemurs (Godfrey et al. 2005; Schwartz et al. 2005), cercopithecines (Jogahara and Natori 2012), and tarsiids (Guthrie and Frost 2011).
Phylogeny and Dental Eruption Patterns
Dental eruption in general has shown to be a reliable marker for relatedness among species. For Multituberculata, the rotative eruption of the fourth premolar, among other characters, has been proposed as synapomorphy for this group (Greenwald 1988). In the Hyaenodontidae, the different tooth eruption patterns between North American and European species have been interpreted as indicating phylogenetic signal (Bastl et al. 2011; Bastl and Nagel 2014). Afrotheria show a common synapomorphy in the development of their dentition; in general, the relative onset of the eruption of the permanent dentition is delayed compared to Archonta and Laurasiatheria (Asher and Lehmann 2008; Asher and Olbricht 2009). This is also found in xenarthrans, where the most basal forms seem to exhibit a relative delayed eruption of permanent dentitions (Ciancio et al. 2012). In primates, several studies have focused on the importance of phylogeny as explanation for differences in the tooth eruption sequence among species (Tattersall and Schwartz 1974; Byrd 1981; Schwartz et al. 2005).
Material and Methods
Specimens
A cross-sectional sampling of different ontogenetic stages of 452 cervid lower jaws representing 15 species was examined. Additionally, 30 lower jaws of Moschus moschiferus as well as 19 lower jaws of two tragulid species, Hyemoschus aquaticus and Tragulus kaInstitut und Museum der Univernchil, were examined (Table 1). The maximum number of lower jaws used per species was 146 (Cervus elaphus) and the minimum number was three (Elaphodus cephalophus). Captive as well as wild-caught animals of both sexes were used. Individuals exhibiting pathological signs in the lower jaw and domestic animals were excluded. Specimens from the following institutes have been studied: MHNG, Muséum d'Histoire Naturelle de la Ville de Genève; NMB, Naturhistorisches Museum Basel; NMBE, Naturhistorisches Museum Bern; PIMUZ, Paläontologisches Institut und Museum der Universität Zürich; MfN, Museum für Naturkunde Berlin; ZMUZH, Zoologisches Museum der Universität Zürich; and ZSM, Zoologische Staatssammlung München. Further, we collected data from the literature (Supplementary Table 1) to compare and analyze the correlations among variables studied.
Additionally, the tooth eruption patterns of seven extinct taxa were investigated: Caenomeryx filholi, Pseudodama s. l. nestii, Eucladoceros giulii, Heteroprox/Euprox, Hoplitomeryx, Cervus philisi, and Procervulus praelucidus (Table 1). Heteroprox/Euprox cannot be distinguished by dental characters and are treated as a unit. For fossil material, the following institutes were visited: IQW, Senckenberg Forschungsstation für Quartär-Paläontologie Weimar; NMB, Naturhistorisches Museum Basel; BSPG, Bayerische Staatssammlung für Paläontologie und Geologie; and NBC, Naturalis Biodiversity Center.
Data Collection
The eruption sequence of the lower jaw was documented. Each tooth was coded as not erupted, erupting, or fully erupted/functional. Teeth that were already visible under the bone but had not begun to erupt were coded as 0. As soon as one cusp or part of the tooth had penetrated through the bone it was coded as 1 and as soon as the tooth reached the functional position it was coded as 2. Nonetheless, due to different speeds of eruption, especially for the third molar, also the status of wear of the dentition and the height above the alveoli were taken into account for the sequences. Not represented stages were reconstructed according to anterior-posterior sequence as it holds true for incisors as well as molars.
Phylogenetic Framework for Cervidae
We used a compound tree based on different sources as phylogenetic framework for the heterochronic analysis. The main arrangement is according to the phylogeny of Gilbert et al. (2006). The position of Ozotoceros bezoarticus follows Barbanti Duarte et al. (2008) and Candiacervus sp. was placed as sister taxon of the fallow deer, Dama dama (de Vos 1984). Most branch lengths were taken from Gilbert et al. (2006). Additional branch lengths were taken from Barbanti Duarte et al. (2008) to include Ozotoceros bezoarticus as well as the split of the two Odocoileus species. The divergence age for the split between the Muntiacus species was taken from Hernández Fernández and Vrba (2005). Splitting age between Candiacervus and Dama was estimated at around 2.15 Ma because the ancestor of Dama dama separated from the ancestor of Cervus elaphus and Rusa unicolor around 4.3 Ma ago (Gilbert et al. 2006).
Heterochrony Analysis for Cervidae
The resolution of the sequences of eruption was completely resolved for 12 cervid species: Axis axis, Capreolus capreolus, Cervus elaphus, Dama dama, Elaphodus cephalophus, Hydropotes inermis, Mazama gouazoubira, Muntiacus muntjak, Odocoileus virginianus, Ozotoceros bezoarticus, Pudu puda, and Rusa unicolor. Four species were added to the analyses based on literature data: Candiacervus sp. (van der Geer et al. 2014), Muntiacus reevesi (Chapman et al. 1985), Odocoileus hemionus (Mosby 1960), and Rangifer tarandus (Miller 1972). Two different methods were used to infer the heterochronic shifts as well as the ancestral sequence of tooth eruption. One was a PGi - Parsimov-based genetic inference (Harrison and Larsson 2008). This method reconstructs sequence heterochronies as well as ancestral states by treating the whole sequence as one character and using a Parsimov-based algorithm for edit-cost optimization. The analysis was conducted using the R software (version 3.0.2) as well as the package pgi 2.0. Included in this analysis were 16 cervid species as well as the eruption of the ten teeth of the adult dentition. Eruption of premolars was coded as one single event due to the significant intraspecific variation of the eruption sequence of premolars in Cervidae. The following parameters were used to conduct eight different PGi-analyses: number of sequences per cycle: 150; number of cycles: 150; and number of sequences retained at each node: 150. The semi-exhaustive search was limited to 10,000 permutations per cycle. All eight analyses were performed independently and the shortest tree was chosen because it represents the most parsimonious scenario for heterochronic shifts. In addition to a PGi-analysis, a continuous analysis (Germain and Laurin 2009) was conducted, resulting in an ancestral sequence, heterochronic shifts, as well as a 95 % confidence interval. This analysis uses squared-change parsimony (Maddison 1991) and independent contrast (Felsenstein 1985), and it is based on a Brownian motion model. The different stages were standardized between 0 and 1 using adjusted ranks formula:
(Laurin and Germain 2011). Continuous analysis was performed using Mesquite (version 3.01) (Maddison and Maddison 2011), together with the module PDAP:PDTREE (version 1.16) (Midford et al. 2011).
Life History
Ruminant life history variables collected from the literature (Pérez-Barbería and Gordon 2005; Tacutu et al. 2013) include longevity, body weight, brain weight, weaning period, and the age of sexual maturity for females (Supplementary Table 2). Information on the age of first molar eruption was taken from different sources: Apeyceros melampus (Roettcher and Hofmann 1970), Alces alces (Peterson 1955), Antidorcas marsupialis (Rautenbach 1971), Antilocapra americana (Lubinski 2001), Bison bonasus (Wegrzyn and Serwatka 1984), Capra ibex (Habermehl 1985), Capra pyrenaica (Vigal and Machordom 1985), Capreolus capreolus (Habermehl 1985), Capricornis crispus (Miura and Yasui 1985), Cervus elaphus (Habermehl 1985), Cervus nippon (Ohtaishi 1980), Connochaetes taurinus (Attwell 1980), Dama dama (Habermehl 1985), Damaliscus lunatus (Mertens 1984), Eudorcas thomsonii (Robinette and Archer 1971), Giraffa camelopardalis (Hall-Martin 1976), Hemitragus jemlahicus (Caughley 1965), Hippotragus niger (Grobler 1980), Muntiacus reevesi (Chapman et al. 1985), Odocoileus hemionus (Mosby 1960), Odocoileus virginianus (Severinghaus 1949), Oryx leucoryx (Ancrenaz and Delhomme 1997), Ovibos moschatus (Henrichson and Grue 1980), Ovis ammon (Habermehl 1985), Ovis canadensis (Mosby 1960), Ovis dalli (Hemming 1969), Ozotoceros bezoarticus (Bianchini and Delupi 1993), Rangifer tarandus (Miller 1972), Rupicapra pyrenaica (Pérez-Barbería and Mutuberría 1996), Rupicapra rupicapra (Habermehl 1985), Saiga tatarica (Bannikov et al. 1961), Sylvicapra grimmia (Wilson et al. 1984), Syncerus caffer (Taylor 1988), and Tragelaphus oryx (Jeffery and Hanks 1981).
We investigated the relationship between hypsodonty index (Janis 1988) and permanent tooth eruption sequence as well as age of first molar eruption (Supplementary Table 2). First, the age of first molar eruption in the lower jaw was tested against the other variables. Second, the tooth eruption sequences of different ruminants were sorted into six different groups as predicted by Schultz’s Rule. Groups are based on relative timing between tooth replacement and molar eruption (Table 2). Kendall’s tau was used to test for statistical support for the correlations. Statistical analyses were performed in PAST software (version 2.17c) (Hammer et al. 2001). Adobe Photoshop CS5 was used to create the artwork for this study.
Results
Tooth Eruption
The recorded eruption sequences are shown in Table 1. Not all relative sequences could be documented to completion. All investigated Cervidae start the eruption sequence with the first molar, and except for the genera Muntiacus and Hippocamelus, all end the sequence with the eruption of the premolar teeth. In Hippocamelus sp. and Muntiacus muntjak, as well as in ruminants Moschus moschiferus and Tragulus kanchil, the last tooth to erupt was the canine. Premolar teeth always erupted simultaneously or closely tight in timing. In all South American cervids, the molar eruption took place before the first incisors were replaced. The only exception might be Blastocerus dichotomus, but the relative eruption sequence of i1 – i2 – m3 could not be resolved. Other related species with the same sequence are Candiacervus sp. and Dama dama with m1 – i1 – m2 – i2 – i3 – c – m3 – p4 – p3 – p2 (van der Geer et al. 2014) as well as both Odocoileus species also with m1 – m2 – i1 – i2 – i3 – c – m3 – (p – p – p) (Mosby 1960). The Odocoileus virginianus sequence differs from the ones described by Severinghaus (1949) and Brokx (1972). Three different premolar sequences were recorded in Capreolus capreolus (Table 3). The relative position of the third molar is not stable; it can erupt either before the third incisor or before or after the canine. Cervus elaphus is the most variable species, with four different tooth eruption sequences and the only documented case where premolar eruption preceded third molar eruption in one case (Table 4). The most common sequence, however, is m1 – m2 – i1 – i2 – m3 – i3 – c – p2 – p3 – p4. The closely related Rusa species do not share a common replacement pattern. Rusa unicolor replaces the teeth in the order of m1 – m2 – i1 – i2 – m3 – i3 – c – (p4 – p3 – p2), whereas Rusa timorensis replaces in a different order: m1 – m2 – i1 – m3 – i2 – (i3 – c – p4 – p3 – p2). Although the final sequence could not conclusively be resolved, the third molar has accelerated its eruption before the second incisor in Rusa timorensis.
In addition to the cervid species, the permanent tooth eruption pattern of one moschid species and two tragulid species were documented. Most basal of these are the tragulids Hyemoschus aquaticus and Tragulus kanchil. For Hyemoschus aquaticus, the sequence is m1 – m2 – m3 – i1 – i2 – i3 – c – p4 – p3 – p2 and for Tragulus kanchil, m1 – m2 – m3 – (i1 – p3 – p4 – p2) – i2 – i3 – c. The resolution of the incisors is based on the constant anterior-posterior replacement of these teeth, a pattern that rarely changes. In these two species the permanent tooth eruption patterns differ. While in one tragulid species, Tragulus kanchil, the eruption of the replacement teeth is premolars before incisors (with overlap), in Hyemoschus aquaticus a replacement pattern from anterior to posterior can be found. In Moschus moschiferus, the sequence is m1 – m2 – m3 – p4 – p3 – p2 – i1 – i2 – i3 – c, comparable to the one from Tragulus kanchil, and not documented in any cervid species. The bovid, Sylvicapra grimmia has a comparable tooth eruption sequence (Wilson et al. 1984).
Heterochrony
The PGi analyses resulted in eight consensus trees of similar length (minimum tree length: 9). This overall consensus in tree length suggests a very stable analysis and a fitting use of parameters. The resulting ancestral sequence for Cervidae is m1 – m2 – i1 – i2 – i3 – c – m3 – (ppp). This sequence is still present or common in Axis, Odocoileus, Capreolus, Hydropotes, and also within the range of variation of Cervus elaphus. Most changes happened in the sequence of the genus Muntiacus. Here, the analysis resulted in an acceleration of the third molar for the genera Muntiacus and Elaphodus (Muntiacini) and also in delayed eruption of the third incisor and the canine for Muntiacus. The uniform tooth eruption sequence of the South American representatives in the dataset originates from an accelerated eruption of the third molar. In general, cervids accelerate the eruption of different tooth positions rather than delay eruption. Details about the heterochronic shifts in the eruption sequence of cervids in phylogeny based on PGi-analysis are depicted in Fig. 1.
Result of the sequence heterochrony analysis using PGi (Harrison and Larsson 2008). The reconstructed ancestral eruption sequence for the lower jaw is listed at the root and species and reconstructed lineages exhibiting this eruption sequence are traced in black. Grey lines indicate a change of this pattern. Lower jaws show the sequential change for a grey line and the affected tooth position. red: tooth position erupts delayed, blue: tooth position erupts accelerated
The continuous method resulted in an ancestral sequence of m1 – m2 – i1 – i2– m3 – i3– c – (ppp). This sequence was documented in Cervus elaphus as well as Rusa timorensis. In general, the continuous method resulted in more heterochronic shifts than the PGi-analysis. All represented species had a heterochronic shift in the second lower incisor, mostly accelerating its eruption (Fig. 2).
Result of the sequence heterochrony analysis using continuous analysis (Germain and Laurin 2009). a The reconstructed ancestral eruption sequence for the lower jaw is listed at the root and species and reconstructed lineages exhibiting this eruption sequence are traced in black. Grey lines indicate a heterochronic shift in this pattern. Lower jaws indicate the sequential change for a line and the affected tooth position. red: tooth position erupts delayed, blue: tooth position erupts accelerated. b 95 % confidence intervals for every single tooth position. The ancestral eruption sequence is represented as midpoint and with extreme points the 95 % confidence intervals are shown
Life History
In the investigated ruminants the age of first molar eruption as well as the pattern of dental eruption were compared with each other as well as with life history and anatomical variables. When comparing the time of first molar eruption with the grouped tooth eruption sequences, no significant correlation was detected using Kendall’s tau. Nonetheless, the trend was positive. The size of the brain was highly correlated with the age of first molar eruption but not with the emergence pattern itself. However, it showed a positive trend for both correlations. Weight showed a positive correlation with the age of first molar eruption but not with the pattern of permanent tooth emergence. Longevity was also highly correlated with the age of first molar eruption and showed a positive trend. It was not correlated with the pattern of tooth eruption. The same is true for the age of female sexual maturity and weaning period. No significant correlation was found between the hypsodonty index and the pattern of permanent tooth emergence (Table 5).
Discussion
This study aimed at investigating the tooth eruption sequence in Cervidae and at demarcating impacting factors thereof. Altogether, 16 lower jaw eruption sequences of cervids were used to infer the ancestral stage of tooth eruption as well as heterochronic shifts. Cainotherium filholi exhibited the same relative eruption sequence of the permanent dentition as the inferred ancestral stage for cervids from the PGi-analysis.
Our analyses show that the eruption patterns in ruminants are not significantly influenced by life history variables or hypsodonty. In general, related species tend to share a common or similar eruption pattern. In cervids, the overall variability of the eruption patterns is restricted due to anatomical constraints such as the ability of the jaw to accommodate all molars and the close timing of eruption of the three premolar teeth.
Sequence of Replacement
In cervids, incisors are replaced sequentially from anterior to posterior. This is also the case in all examined moschids, tragulids, Camelus (Bello et al. 2013), and all documented ruminants from the literature. The lower canine in Ruminantia is incisiviform and increases the size of the incisor shovel (e.g., Thenius 1989). Usually, this tooth is replaced after i3 and therefore follows the anterior-posterior sequential replacement, although eruption of i3 and canine can be closely timed (e.g., Severinghaus 1949; Habermehl 1985; Ancrenaz and Delhomme 1997). The anterior-posterior eruption sequence of the incisors and canines, however, is not generally fixed in placentals (e.g., Kirkpatrick and Sowls 1962; Matschke 1967; Smuts 1974; Habermehl 1975; Smuts et al. 1978; Shigehara 1980; Smith 2000; van Horn et al. 2003; Asher and Olbricht 2009).
The premolar teeth are replaced more or less simultaneously in cervids and any tooth could start the eruption sequence. This is supported by lower jaws where premolar height above the alveoli suggests simultaneous eruption. For Rangifer tarandus and Odocoileus virginianus, a sequence of (p2 – p3) – p4 or p2 – (p3 – p4) has been postulated (Miller 1972; Severinghaus 1949) and for Odocoileus hemionus a sequence of p3 – p4 – p2 (Robinette et al. 1957). Van der Geer et al. (2014) proposed a posterior-anterior replacement pattern for the premolar eruption sequence for Candiacervus. Habermehl (1985) documented an alternate replacement for the roe deer with p3 – p2 – p4. In Bovidae, the premolar eruption pattern is not fixed either. Nonetheless, in many species a pattern of p2 – p3 – p4 has been postulated, although for some the p2 is missing (Supplementary Table 1). Here, no case of reversed sequence has been reported but alternate eruption, either (p3 – p4) – p2 (Mosby 1960; Hemming 1969; Vigal and Machordom 1985) or p3 – (p2 – p4) (Caughley 1965). It has been widely accepted that ruminants share a sequential pattern (p2 – p3 – p4 or p4 – p3 – p2) of replacing teeth usually in the order of p2 – p3 – p4 (Osborn 1970; Smith 2000; Luo et al. 2004), but data presented here show that premolar eruption sequence can vary even in closely related species or within a species. This is also true in other placentals, although premolar eruption might not be as closely timed as in cervids (Slaughter et al. 1974; Tattersall and Schwartz 1974; Smith 1994, 2000; Gingerich and Smith 2010).
Molar eruption in cervids occurs in all recorded cases from anterior to posterior, with no exception. No evidence from other placentals has been found to contradict this (e.g., Slaughter et al. 1974; Habermehl 1985; Smith 2000).
On the Methods of Sequence Heterochrony Analysis
We found differences in the results between PGi-analysis and continuous analysis, as was the case in several other studies (Geiger et al. 2014; Koyabu et al. 2014; Sheil et al. 2014). This is due to the different approach both methods have. PGi-analysis is not based on a time tree and it can deal with ties or simultaneous events in the dataset (Harrison and Larsson 2008). On the other hand, the continuous analysis incorporates branch length data and can be biased due to unresolved sequences (Germain and Laurin 2009; Koyabu et al. 2014). The transformation of the data can also be a source of bias as transformed data have the same intervals between events (Germain and Laurin 2009). Both methods differ in dealing with independence in the dataset. PGi-analysis does not assume independence between events (Harrison and Larsson 2008); continuous analysis, on the other hand, does (Germain and Laurin 2009). In general, the continuous analysis resulted in more heterochronic shifts than the PGi-analysis. Nonetheless, the 95 % confidence interval of the continuous analysis supports the result of the PGi-analysis.
Morphological Constraints, Adaption, and Dental Eruption Patterns
Simpson et al. (1990) hypothesized that the pattern of eruption of permanent dentition can be influenced by the anatomy of the upper or lower jaw. Certain anatomical constraints are also part of Schultz’s Rule (Smith 2000). Cervids and bovids share a very similar dentary shape as well as tooth formula with the four anterior incisiviform teeth followed by a diastema and afterwards three premolars and three molars (e.g., Thenius 1989). Nonetheless, they do not all share the same pattern of tooth replacement. Whereas, e.g., Dama dama and Candiacervus start to replace their first incisor already early, Pudu puda and Mazama gouazoubira replace this tooth after the eruption of all three molars. The stable pattern in the cheek-dentition in cervids, where premolar teeth nearly always erupt after the third molar, indicates that replacement occurs as soon as the mandible is big enough to accommodate the whole permanent dentition. This holds also for Hyemoschus aquaticus, Tragulus kanchil, and Moschus moschiferus. In rare cases (once in Cervus elaphus), the premolar eruption precedes third molar eruption. Only a few Ruminantia such as Ovis ammon (Habermehl 1985) and Myotragus balearicus (Jordana et al. 2013) change the relative sequence between premolar eruption and the third molar one completely.
A simple but effective way to account for the effect of morphology in the ruminant dentition is the hypsodonty index (Janis 1988). Ruminant teeth share general tooth morphology; all are selenodont (e.g., Thenius 1989; Hillson 2005). Not all teeth, however, have the same height and therefore not all teeth need the same space within the jaw. Using Kendall’s tau to analyze the relation between the pattern of tooth emergence and the age of first molar eruption did not result in a correlation between these parameters (Table 5). Although the size of the crown height is definitely different among the species examined, it did not lead to a change in relative eruption sequence. High crowned species such as Antidorcas marsupialis or Eudorcas thomsonii have the same eruption sequence as the low crowned Hyemoschus aquaticus or Elaphodus cephalophus. Hypsodonty does not influence the relative emergence pattern of the permanent dentition in ruminants.
Tooth Eruption as Indicator for Life History Traits
The time of eruption of the first molar was positively correlated with longevity, age of female sexual maturity, brain mass, weaning period, and body mass. For primates and “ungulates,” positive correlations among some of these traits have also been found, although not always significant (e.g., Smith 1989, 1992, 2000; Godfrey et al. 2001, 2005; Guthrie and Frost 2011). A link among different life history traits in general has been found in Artiodactyla. Brain size, for example, is correlated with body mass, and body mass again is correlated with maximum longevity (Isler and van Schaik 2012). Body size in a broad sense can be a predictor for the pace of life history. However, it might not be the source of differences (e.g., Western 1979; Read and Harvey 1989). Therefore, the age of first molar eruption can be seen as a predictor for the pace of life history in the investigated ruminants. The pattern of eruption is not linked to these life history and anatomical traits (Table 5). Schultz’s Rule fails to predict the life history of Ruminantia in this case. An outstanding example in this regard is the emergence sequence of the two taxa Muntiacus and Giraffa. Both genera share the same tooth eruption pattern, but all life history variables investigated differ considerably. For example, the age of first molar eruption is 10 months in Giraffa camelopardalis but only 0.23 months in Muntiacus reevesi (Supplementary Table 2).
Phylogeny and Dental Eruption Patterns
The tooth eruption sequence in cervids, as in other mammals, is largely conserved in phylogeny (e.g., Byrd 1981; Greenwald 1988; Luo et al. 2004; Asher and Lehmann 2008; Ciancio et al. 2012), as for example seen in the genera Capreolus and Hydropotes as well as Muntiacus and Elaphodus. The divergence splits of both groups have been estimated with around 7.3 Ma for Muntiacini and 5.6 Ma for Capreolini (Gilbert et al. 2006). Nonetheless, Capreolus still shows the eruption sequence of Hydropotes within its variability, and within Muntiacini, the eruption pattern of Elaphodus cephalophus falls within the range of variation of Muntiacus muntjak (Table 4). Other examined taxa also have similar eruption sequences such as Pudu puda, Mazama gouazoubira, and Ozotoceros bezoarticus; Dama dama and Candiacervus sp.; as well as Rusa unicolor and Cervus elaphus (Table 1). The genus Odocoileus is more variable than is recorded in this study. Patterns from literature differ from the pattern observed here. Odocoileus virginianus for example has been documented with the same tooth eruption sequence as Rangifer tarandus (Severinghaus 1949; Miller 1972).
In Bovidae, some closely related species have similar sequences such as Ovibos and Capricornis as well as Eudorcas and Antidorcas (Supplementary Table 1). The emergence patterns of bovids in general, however, are less well resolved and many species exhibit unique eruption patterns. An explanation for this might be that the taxa represented are usually not closely related, having divergence times of more than 5 – 10 Ma (Bibi 2013).
Comparisons with Fossil Artiodactyls
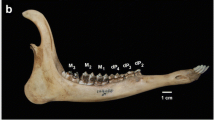
One extinct taxon studied preserved a complete permanent eruption pattern in the lower jaw, the caenotherid Caenomeryx filholi, from the Oligocene of Gaimersheim (Fig. 3). Cainotheriidae are a sister taxon to ruminants (Theodor 2010). The sequence represented in this artiodactyl is in concordance with the result of the PGi-analysis (Fig. 1). Lower first premolars have been lost in modern ruminants (Thenius 1989). Caenomeryx filholi still possesses the complete ancestral tooth formula 3143/3143 of placental mammals (Thenius 1989) and therefore preservation of ancestral characteristics in replacement order of permanent teeth is possible.
All examined fossils from extinct taxa in this study have a constant pattern in premolar-molar eruption. This persistence of premolar eruption after molar eruption stands in contrast to Schultz’s Rule, because body size range, a proxy for life history, among these species is large (e.g., Western 1979; Read and Harvey 1989).
From the literature, the ruminant Myotragus balearicus follows Schultz’s Rule scenario (Jordana et al. 2013). This extinct island bovid has an eruption sequence of m1 – i1 – m2 – (p3 – p4) – m3 and the first molar emerged at around nine months (Jordana et al. 2013). It is one of the rare cases where premolar eruption accelerated the third molar one and studies showed that this species had a slow life history as well as a long life span (Marín-Moratalla et al. 2011; Jordana et al. 2012, 2013). The next living relative to the genus Myotragus, however, is the genus Ovis (Lalueza-Fox et al. 2005). Ovis ammon also accelerates premolar eruption before the third molar one (Habermehl 1985). Therefore, the eruption pattern of Myotragus might be influenced by phylogeny as well.
Conclusion
Tooth eruption in cervids, as commonly in extant Ruminantia, shows that there is variation in the permanent tooth eruption sequences even among closely related species. According to our results, these sequential differences are not influenced by brain size, body mass, hypsodonty, or the life history factors tested for but rather by their phylogeny. Closely related species often share their tooth eruption sequences and differences in patterns can be explained by observed intraspecific and interspecific variations. These results stand in contrast to the proposed Schultz’s Rule-hypothesis because here, the speed of growth should have had a bigger impact on the permanent tooth eruption sequence than any other factor (Smith 2000). Nonetheless, Smith (2000) stated that the predictions of this rule are not as strong for ruminant artiodactyls, which she included in so called “specialized ungulates.” These “ungulates” would only slowly adapt to changes in pace of life history.
The age of first molar eruption, however, was correlated with life history variables such as longevity, age of female sexual maturity, and weaning period. Both brain weight and weight in general were highly correlated with age of first molar eruption. This agrees with the literature on primates as well as “ungulates” (e.g., Smith 1989, 2000; Smith et al. 1994; Godfrey et al. 2001, 2005; Guthrie and Frost 2011). The fossil record as well as heterochrony methods allow for reconstruction of the ancestral emergence pattern. A pattern of m1 – m2 –i1 – i2 – i3 – c – m3 – (ppp) holds true for the common ancestor of today’s cervids, based on results from PGi-analysis, as well as for Caenomeryx filholi.
Premolar eruption remains unresolved due to the variability of relative eruption sequence in these teeth. Sequential as well as alternate replacement was documented even within one species, which makes phylogenetic statements based on premolar replacement problematic.
References
Ancrenaz M, Delhomme A (1997) Teeth eruption as a means of age determination in captive Arabian oryx, Oryx leucoryx (Bovidae, Hippotraginae). Mammalia 61:135–138. doi: 10.1515/mamm.1997.61.1.109
Asher RJ, Lehmann T (2008) Dental eruption in afrotherian mammals. BMC Biol 6:1–11. doi:10.1186/1741-7007-6-14
Asher RJ, Olbricht G (2009) Dental ontogeny in Macroscelides proboscideus (Afrotheria) and Erinaceus europaeus (Lipotyphla). J Mammal Evol 16:99–115. doi: 10.1007/s10914-009-9105-2
Attwell CAM (1980) Age determination of the blue wildebeest Connochaetes taurinus in Zululand. S Afr J Zool 15:121–130
Bannikov AG, Zhirnov LV, Lebedeva LS, Fandeev AA (1961) Biologiya saigaka [Biology of the Saiga Antelope]. Selskokhozyaistvennaya Literatura, Moscow
Barbanti Duarte JM, González S, Maldonado JE (2008) The surprising evolutionary history of South American deer. Mol Phylogenet Evol 49:17–22. doi: 10.1016/j.ympev.2008.07.009
Bastl K, Nagel D (2014) First evidence of the tooth eruption sequence of the upper jaw in Hyaenodon (Hyaenodontidae, Mammalia) and new information on the ontogenetic development of its dentition. Palaeontol Z 88:481–494. doi: 10.1007/s12542-013-0207-z
Bastl K, Morlo M, Nagel D, Heizmann E (2011) Differences in the tooth eruption sequence in Hyaenodon (‘Creodonta’: Mammalia) and implications for the systematics of the genus. J Vertebr Paleontol 31:181–192. doi: 10.1080/02724634.2011.540052
Bello A, Sonfada ML, Umar AA, Umaru MA, Shehu SA, Hena SA, Onu JE, Fatima OO (2013). Age estimation of camel in Nigeria using rostral dentition. Sci J Anim Sci 2:9–14
Bianchini JJ, Delupi LH (1993) Determinación de la edad en ciervos de las pampas (Odocoileus bezoarticus) mediante el estudio comparado del desarrollo y desgaste de los dientes. PHYSIS (Buenos Aires), Secc. C, 48:27–40
Bibi F (2013) A multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record to systematics. BMC Evol Biol 13:166. doi: 10.1186/1471-2148-13-166
Brokx PA (1972) Age determination of Venezuelan White-Tailed deer. J Wildl Manage 36:1060–1067. doi: 10.2307/3799233
Byrd KE (1981) Sequences of dental ontogeny and callitrichid taxonomy. Primates 22:103–118. doi: 10.1007/BF02382561
Caughley G (1965) Horn rings and tooth eruption as criteria of age in the Himalayan thar, Hemitragus jemlahicus. New Zealand J Sci 8:333–351
Chapman DI, Chapman NG, Colles CM (1985). Tooth eruption in Reeves’ muntjac (Muntiacus reevesi) and its use as a method of age estimates (Mammalia: Cervidae). J Zool 205:205–221. doi: 10.1111/j.1469-7998.1985.tb03529.x
Ciancio MR, Castro MC, Galliari FC, Carlini AA, Asher RJ (2012) Evolutionary implications of dental eruption in Dasypus (Xenarthra). J Mammal Evol 19:1–8. doi: 10.1007/s10914-011-9177-7
de Vos J (1984) The Endemic Pleistocene Deer of Crete. North-Holland Publishing Company, Amsterdam
Dirks W (2003) Effect of diet on dental development in four species of catarrhine primates. Am J Primatol 61:29–40. doi: 10.1002/ajp.10106
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Forasiepi AM, Sánchez-Villagra MR (2014) Heterochrony, dental ontogenetic diversity, and the circumvention of constraints in marsupial mammals and extinct relatives. Paleobiology 40:222–237. doi: 10.1666/13034
Geiger M, Forasiepi AM, Koyabu D, Sánchez-Villagra MR (2014) Heterochrony and post-natal growth in mammals—an examination of growth plates in limbs. J Evol Biol 27:98–115. doi: 10.1111/jeb.12279
Germain D, Laurin M (2009) Evolution of ossification sequences in salamanders and urodele origins assessed through event-pairing and new methods. Evol Dev 11:170–190. doi: 10.1111/j.1525-142X.2009.00318.x.
Gilbert C, Ropiquet A, Hassanin A (2006) Mitochondrial and nuclear phylogenies of Cervidae (Mammalia, Ruminantia): systematics, morphology, and biogeography. Mol Phylogenet Evol 40:101–117. doi:10.1016/j.ympev.2006.02.017
Gingerich PD, Smith BH (2010) Premolar development and eruption in the early Eocene adapoids Cantius ralstoni and Cantius abditus (Mammalia, Primates). Contrib Mus Paleontol Univ Mich 32:41–47
Godfrey LR, Samonds KE, Jungers WL, Sutherland MR (2001) Teeth, brains and primate life histories. Am J Phys Anthropol 114:192–214. doi: 10.1002/1096-8644(200103)114:3<192::AID-AJPA1020>3.0.CO;2-Q
Godfrey LR, Samonds KE, Wright PC, King SJ (2005) Schultz’s unruly rule: dental development sequences and schedules in small-bodied, folivorous lemurs. Folia Primatol 76:77–99. doi: 10.1159/000083615
Greenwald NS (1988) Patterns of tooth eruption and replacement in multituberculate mammals. J Vertebr Paleontol 8:265–277. doi: 10.1080/02724634.1988.10011709
Grobler JH (1980) Body growth and age determination of the sable Hippotragus niger niger (Harris, 1838). Koedoe 23:131–156. doi: 10.4102/koedoe.v23i1.641
Guthrie EH, Frost FR (2011) Pattern and pace of dental eruption in Tarsius. Am J Phys Anthropol 145:446–451. doi: 10.1002/ajpa.21525
Habermehl K-H (1975) Altersbestimmung bei Haus- und Labortieren, 2nd edn. Verlag Paul Parey, Hamburg
Habermehl K-H (1985) Altersbestimmung bei Wild- und Pelztieren – Möglichkeiten und Methoden – ein praktischer Leitfaden für Jäger, Biologen und Tierärzte, 2nd edn. Verlag Paul Parey, Hamburg
Hall-Martin AJ (1976) Dentition and age determination of the giraffe Giraffa camelopardalis. J Zool 180:263–289. doi: 10.1111/j.1469-7998.1976.tb04678.x
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9 pp
Harrison LB, Larsson HC (2008) Estimating evolution of temporal sequence changes: a practical approach to inferring ancestral developmental sequences and sequence heterochrony. Syst Biol 57:378–387. doi: 10.1080/10635150802164421
Hellmund M (2013) Odontological and osteological investigations on propalaeotheriids (Mammalia, Equidae) from the Eocene Geiseltal Fossillagerstätte (Central Germany)—a full range of extraordinary phenomena. Neues Jahrb Geol P-A 267:127–154. doi: 10.1127/0077-7749/2013/0300
Hemming JE (1969) Cemental deposition, tooth succession, and horn development as criteria of age in Dall sheep. J Wildl Manage 33:552–558. doi: 10.2307/3799377
Henderson E (2007) Platyrrhine dental eruption sequences. Am J Phys Anthropol 134:226–239. doi: 10.1002/ajpa.20658
Henrichson P, Grue H (1980) Age criteria in the muskox (Ovibos moschatus) from Greenland. Danish Rev Game Biol 11:3–18
Hernández Fernández M, Vrba ES (2005) A complete estimate of the phylogenetic relationships in Ruminantia: a dated species-level supertree of the extant ruminants. Biol Rev 80:269–302. doi: 10.1017/S1464793104006670
Hillson S (2005) Teeth, 2nd edn. Cambridge Manuals in Archaeology. Cambridge University Press, New York
Isler K, van Schaik CP (2012) Allomaternal care, life history and brain size evolution in mammals. J Hum Evol 63:52–63. doi:10.1016/j.jhevol.2012.03.009
Janis CM (1988) An estimation of tooth volume and hypsodonty indices in ungulate mammals, and the correlation of these factors with dietary preference. In: Russell DE, Santoro J-P, Sigogneau-Russell D (eds) Teeth Revisited: Proceedings of the VIIth International Symposium on Dental Morphology. Mémoires du Muséum national d’Histoire naturelle, Science de la Terre (Série C), Tome 53, Éditions du Muséum national d’Histoire naturelle, Paris, pp 367–387
Jeffery RCV, Hanks J (1981) Age determination of eland Taurotragus oryx (Pallas, 1766) in the Natal highveld. S Afr J Zool 16:113–122
Jogahara YO, Natori M (2012) Dental eruption sequence and eruption times in Erythrocebus pata. Primates 53:193–204. doi: 10.1007/s10329-011-0286-y
Jordana X, Marín-Moratalla N, DeMiguel D, Kaiser TM, Köhler M (2012) Evidence of correlated evolution of hypsodonty and exceptional longevity in endemic insular mammals. Proc Roy Soc B 279:3339–3346. doi: 10.1098/rspb.2012.0689
Jordana X, Marín-Moratalla N, Moncunill-Solé B, Bover P, Alcover JA, Köhler M (2013) First fossil evidence for the advance of replacement teeth coupled with life history evolution along an anagenetic mammalian lineage. PLoS ONE 8(7): e70743. doi: 10.1371/journal.pone.0070743
Kerr MA, Roth HH (1970) Studies on the agricultural utilization of semi-domesticated eland (Taurotragus oryx) in Rhodesia. Rhod J Agr Res 8:149–155
Kirkpatrick RD, Sowls LK (1962) Age determination of the collared peccary by the tooth-replacement pattern. J Wildl Manage 26:214–217. doi: 10.2307/3798608
Koyabu D, Werneburg I, Morimoto M, Zollikofer CPE, Forasiepi AM, Endo H, Kimura J, Ohdachi SD, Truong Son N, Sánchez-Villagra MR (2014) Mammalian skull heterochrony reveals modular evolution and a link between cranial development and brain size. Nat Commun 5:3625. doi:10.1038/ncomms4625
Lalueza-Fox C, Castresana J, Sampietro L, Marquès-Bonet T, Alcover JA, Bertranpetit J (2005) Molecular dating of caprines using ancient DNA sequences of Myotragus balearicus, an extinct endemic Balearic mammal. BMC Evol Biol 5:70. doi:10.1186/1471-2148-5-70
Laurin M, Germain D (2011) Developmental characters in phylogenetic inference and their absolute timing information. Syst Biol 60:630–644. doi: 10.1093/sysbio/syr024
Lubinski PM (2001) Estimating age and season of death of pronghorn antelope (Antilocapra americana Ord) by means of tooth eruption and wear. Int J Osteoarchaeol 11:218–230. doi: 10.1002/oa.536
Luckett WP, Wooley PA (1996) Ontogeny and homology of the dentition in dasyurid marsupials: development in Sminthopsis virginiae. J Mammal Evol 3:327–364. doi: 10.1007/BF02077449
Luo Z-X, Kielan-Jaworowska Z, Cifelli RL (2004) Evolution of dental replacement in mammals. Bull Carnegie Mus Nat Hist 36:159–175. doi: 10.2992/0145-9058(2004)36[159:EODRIM]2.0.CO;2
Maddison WP (1991) Squared-change parsimony reconstructions of ancestral states for continuous-valued characters on a phylogenetic tree. Syst Zool 40:304–314. doi: 10.2307/2992324
Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis. Version 3.01
Marín-Moratalla N, Jordana X, García-Martínez R, Köhler M (2011) Tracing the evolution of fitness components in fossil bovids under different selective regimes. CR Palevol 10:469–478. doi: 10.1016/j.crpv.2011.03.007
Matschke GH (1967) Aging European wild hogs by dentition. J Wildl Manage 31:109–113. doi: 10.2307/3798365
McGee EM, Turnbull WD (2010) A paleopopulation of Coryphodon lobatus (Mammalia: Pantodonta) from Deardorff hill Coryphodon quarry, Piceance Creek Basin, Colorado. Fieldiana Geol 52:1–12. doi: 10.3158/0096-2651-52.1.1
Mertens H (1984) Détermination de l’âge chez le topi (Damaliscus korrigum Ogilby) au Parc National des Virunga (Zaïre). Mammalia 48:425–435. doi: 10.1515/mamm.1984.48.3.425
Midford PE, Garland T Jr, Maddison WP (2011) PDAP Package of Mesquite. Version 1.16
Miller FL (1972) Eruption and attrition of mandibular teeth in barren-ground caribou. J Wildl Manage 36:606–612. doi: 10.2307/3799093
Miura S, Yasui K (1985) Validity of tooth eruption-wear patterns as age criteria in the Japanese serow, Capricornis crispus. J Mammal Soc Jpn 10:169–178. doi: 10.11238/jmammsocjapan1952.10.169
Mosby HS (1960) Manual of Game Investigational Techniques. The Wildlife Society, Ann Arbor
Ohtaishi N (1980) Estimation of sex, age, and season of death using mandibles of Cervus nippon excavated from an archaeological site. Arch Nat Sci 13:51–74 (in Japanese).
Osborn JW (1970) New approach to Zahnreihen. Nature 225:343–346. doi: 10.1038/225343a0
Osborn JW, Crompton AW (1973) The evolution of mammalian from reptilian dentitions. Breviora 399:1–18
Pérez-Barbería FJ, Gordon IJ (2005) Gregariousness increases brain size in ungulates. Oecologia 145:41–52. doi: 10.1007/s00442-005-0067-7
Pérez-Barbería FJ, Mutuberría G (1996) Teeth eruption pattern in Cantabrian chamois Rupicapra pyrenaica parva. Acta Theriol 41:217–221
Peterson RL (1955) North American Moose. University of Toronto Press, Toronto
Rautenbach IL (1971) Ageing criteria in the springbok, Antidorcas marsupialis (Zimmermann, 1780) (Artiodactyla: Bovidae). Ann Transv Mus 27:83–133
Read AF, Harvey PH (1989) Life history differences among the eutherian radiations. J Zool 219:329–353. doi: 10.1111/j.1469-7998.1989.tb02584.x
Robinette WL, Archer AL (1971) Notes on ageing criteria and reproduction of Thomson’s gazelle. E Afr Wildl J 9:83–98. doi: 10.1111/j.1365-2028.1971.tb00222.x
Robinette WL, Jones DA, Rogers G, Gashwiler JS (1957) Notes on tooth development and wear for Rocky Mountain mule deer. J Wildl Manage 21:134–153. doi: 10.2307/3797579
Roettcher D, Hofmann RR (1970) The ageing of impala from a population in the Kenya Rift Valley. E Afr Wildl J 8:37–42. doi: 10.1111/j.1365-2028.1970.tb00828.x
Schultz AH (1956) Postembryonic age changes. In: Hofer H, Schultz AH, Starck D (eds) Primatologia, 1st vol. Karger, Basel, pp 887–964
Schultz AH (1960) Age changes in primates and their modification in man. In: Tanner JM (ed) Human Growth, 3rd vol. Pergamon Press, Oxford, pp 1–20
Schwartz GT, Mahoney P, Godfrey LR, Cuozzo FP, Jungers WL, Randria GFN (2005) Dental development in Megaladapis edwardsi (Primates, Lemuriformes): implications for understanding life history variation in subfossil lemurs. J Hum Evol 49:702–721. doi: 10.1016/j.jhevol.2005.08.006
Severinghaus CW (1949) Tooth development and wear as criteria of age in white-tailed deer. J Wildl Manage 13:195–216. doi: 10.2307/3796089
Sheil CA, Jorgensen M, Tulenko F, Harrington S (2014) Variation in timing of ossification affects inferred heterochrony of cranial bones in Lissamphibia. Evol Dev 16:292–305. doi: 10.1111/ede.12092
Shigehara N (1980) Epiphyseal union, tooth eruption, and sexual maturation in the common tree shrew, with reference to its systematic problem. Primates 21:1–19. doi: 10.1007/BF02383820
Simpson SW, Lovejoy CO, Meindl RS (1990) Hominoid dental maturation. J Hum Evol 19:285–297. doi: 10.1016/0047-2484(90)90070-R
Slaughter BH, Ronald PH, Nobuko EP (1974) Eruption of cheek teeth in Insectivora and Carnivora. J Mammal 55:115–125. doi: 10.2307/1379261
Smith BH (1989) Dental development as a measure of life history in primates. Evolution 43:683–688. doi: 10.2307/2409073
Smith BH (1992) Life history and the evolution of human maturation. Evol Anthropol 1:134–142. doi: 10.1002/evan.1360010406
Smith BH (1994) Sequence of emergence of the permanent teeth in Macaca, Pan, Homo, and Australopithecus: its evolutionary significance. Am J Hum Biol 6:61–76. doi: 10.1002/ajhb.1310060110
Smith BH (2000) ‘Schulz’s rule’ and the evolution of tooth emergence and replacement patterns in primates and ungulates. In: Teaford MF, Smith MM, Ferguson MWJ (eds) Development, Function and Evolution of Teeth. Cambridge University Press, Cambridge, pp 212–228
Smith BH, Crummet TL, Bradt KL (1994) Ages of eruption of primate teeth: a compendium for aging individuals and comparing life histories. Yearb Phys Anthropol 37:177–231. doi: 10.1002/ajpa.1330370608
Smuts GL (1974) Age determination in Burchell’s zebra (Equus burchelli antiquorum) from the Kruger National Park. S Afr J Wildl Res 4:103–115
Smuts GL, Anderson JL, Austin JC (1978) Age determination of the African lion (Panthera leo). J Zool 185:115–146. doi: 10.1111/j.1469-7998.1978.tb03317.x
Tacutu R, Craig T, Budovsky A, Wuttke D, Lehmann G, Taranukha D, Costa J, Fraifeld VE, de Magalhaes JP (2013) Human ageing genomic resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res 41(D1):D1027-D1033. doi: 10.1093/nar/gks1155
Tattersall I, Schwartz JH (1974) Craniodental morphology and the systematics of the Malagasy lemurs (Primates, Prosimii). Anthropol Pap Am Mus 52:139–192
Taylor RD (1988) Age determination of the African buffalo, Syncerus caffer (Sparrman) in Zimbabwe. Afr J Ecol 26:207–220. doi: 10.1111/j.1365-2028.1988.tb00972.x
Thenius E (1989) Zähne und Gebiß der Säugetiere. In: Niethammer J, Schliemann H, Starck D (eds) Handbuch der Zoologie, VIII Mammalia. Walter de Gruyer, Berlin
Theodor JM (2010) Micro–computed tomographic scanning of the ear region of Cainotherium: character analysis and implications. J Vertebr Paleontol 30:236–243. doi: 10.1080/02724630903415979
van der Geer AAE, Lyras GA, MacPhee RDE, Lomolino M, Drinia H (2014) Mortality in a predator-free insular environment: the dwarf deer of Crete. Am Mus Novitates 3807:1–26. doi: 10.1206/3807.1
van Horn RC, McElhinny TL, Holekamp KE (2003) Age estimation and dispersal in the spotted Hyena (Crocuta crocuta). J Mammal 84:1019–1030
van Nievelt AFH, Smith KK (2005a) To replace or not to replace: the significance of reduced functional tooth replacement in marsupial and placental mammals. Paleobiology 31:324-346. doi: 10.1666/0094-8373(2005)031[0324:TRONTR]2.0.CO;2
van Nievelt AFH, Smith KK (2005b) Tooth eruption in Monodelphis domestica and its significance for phylogeny and natural history. J Mammal 86:333-341. doi: 10.1644/BWG-224.1
Vigal CR, Machordom A (1985) Tooth eruption and replacement in the Spanish wild goat. Acta Theriol 19:305-320
Wegrzyn M, Serwatka S (1984) Teeth eruption in the European bison. Acta Theriol 29:111-121.
Western D (1979) Size, life history and ecology in mammals. Afr J Ecol 17:185-204. doi: 10.1111/j.1365-2028.1979.tb00256.x
Wilson VJ, Schmidt JL, Hanks J (1984) Age determination and body growth of the common duiker Sylvicapra grimmia (Mammalia). J Zool 202:283-297. doi: 10.1111/j.1469-7998.1984.tb05955.x
Acknowledgments
We thank the following colleagues for access to collections: Loïc Costeur (NMB), Christiane Funk (MfN), Alexandra van der Geer (NBC), Marianne Haffner (ZMUZH_MAMM), Stefan T. Hertwig (NMBE), Michael Hiermeier (ZSM), Lars van den Hoek Ostende (NBC), Ralf-Dietrich Kahlke (IQW), Barbara Oberholzer (ZMUZH_MAMM), Itatí A. Olivares (MLPA), Gertrud Rössner (BSPG), Manuel Ruedi (MHNG), Manuel Schweizer (NMBE), and Diego H. Verzi (MLPA). Our gratitude goes also to Madeleine Geiger (PIMUZ), Robert Asher (UMZC), Daisuke Koyabu (UMUTZ), and the anonymous reviewer for their helpful advice with methodological questions as well as for discussion and to Ashley Latimer (PIMUZ) for reviewing the English. This research was funded by the Swiss National Science Foundation (SNF) grant 31003A_149605 to Marcelo R. Sánchez-Villagra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(PDF 374 kb)
Supplementary Table 2
(PDF 372 kb)
Rights and permissions
About this article
Cite this article
Veitschegger, K., Sánchez-Villagra, M.R. Tooth Eruption Sequences in Cervids and the Effect of Morphology, Life History, and Phylogeny. J Mammal Evol 23, 251–263 (2016). https://doi.org/10.1007/s10914-015-9315-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-015-9315-8