Abstract
Erythrocebus patas has a short inter-birth interval, juveniles become independent from their mother early, females are young at first birth, and adult females have a high mortality rate. According to Schultz’s rule, the molars of fast-growing and shorter-lived primate species erupt early relative to the replacement teeth. Based on the life history of E. patas, we hypothesized that the molars would erupt before the replacement teeth and/or that the eruption time of its molars would be early. The purpose of the present study was to determine the dental eruption sequence and eruption times for E. patas and to test our hypothesis. The eruption sequence for the permanent teeth of E. patas is \( \frac{{{\text{M1}}\;{\text{I1}}\;{\text{I2}}\;{\text{M2}}\;{\text{P3}}\;{\text{P4}}\;[{\text{C}}\;{\text{M3}}]}}{{{\text{M1}}\;{\text{I1}}\;{\text{I2}}\;{\text{M2}}\;{\text{P4}}\;[{\text{P3}}\;{\text{C}}]{\text{M3}}}} \) in males and \( \frac{{{\text{M1}}\;{\text{I1}}\;{\text{I2}}\;[{\text{M2}}\;{\text{P4}}\;{\text{P3}}\;{\text{C}}]{\text{M3}}}}{{{\text{M1}}\;{\text{I1}}\;{\text{I2}}\;[{\text{M2}}\;{\text{P4}}\;{\text{P3}}\;{\text{C}}]{\text{M3}}}} \) in females. Because these sequences constitute the general pattern seen in cercopithecines, Schultz’s rule could not be applied to E. patas. The emergence time of upper and lower first molar (M1) is earlier in E. patas than in macaques, baboons, and mandrills and is similar to that in Chlorocebus aethiops. The emergence time of deciduous upper and lower fourth premolar (dp4) is similar to that in the above-mentioned cercopithecines but is later than that in Ch. aethiops. The emergence times of upper and lower second molar (M2) and upper and lower third molar (M3) in E. patas are earlier than those in the above-mentioned cercopithecines but later than those in Ch. aethiops. However, the intervals of the emergence time between each permanent molar in E. patas are similar to those of the above-mentioned cercopithecines. The early appearance of M2 and M3 in E. patas is related to the short interval of emergence time between dp4 and M1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The habitat of Erythrocebus patas covers a broad area across central Africa, from the Sahara in the north to the equatorial rain forest in the south (Chism and Rowell 1988). This monkey is classified within the Cercopithecinae along with guenons, mangabeys, macaques, and baboons, among others (Groves 2005) and is most closely related to Chlorocebus aethiops (Tosi et al. 2005). The uniqueness of E. patas among the Cercopithecinae has been reported with regard to certain aspects of its life history. The juvenile E. patas develops rapidly, soon gaining independence from its mother (Chism 1986). The female E. patas is younger at first birth and has a shorter inter-birth interval than the closely related forest guenons (Nakagawa et al. 2003). Mortality rates in the adult females are higher than those in Ch. aethiops (Isbell et al. 2009).
Schultz (1935) proposed that early molar eruption was the most primitive trait in primates and that the eruption of molars was delayed in higher forms. The dental eruption sequence correlates with life history in primates, although not necessarily indicating phylogenetic relationships (Smith 1994, 2000). Smith (2000) reconstructed Schultz’s rule as “the tendency for replacing teeth to come in relatively early in slow-growing, longer-lived species.” Taking the opposite view, fast-growing and shorter-lived species have molars that erupt early relative to the replacement teeth (incisors, canines, and premolars) (see Smith 2000, p. 225). The life history of E. patas suggests that the molars erupt before the replacement teeth. In addition to the dental eruption sequence, E. patas would have molars erupt early relative to the replacement teeth in response to their life history. However, there is little information on the dental eruption sequence in E. patas; only one study, by Bolwig (1963), reported the dental eruption sequence and eruption times of deciduous teeth, based on one living individual. The purpose of the present study was to reveal the dental eruption sequences and eruption times for E. patas. In addition, we tested the hypothesis that the molars of E. patas erupt before the replacement teeth and/or that the eruption time of its molars is early.
Methods
The present study was based on 107 specimens of E. patas (43 males and 64 females). We selected 95 specimens (38 males and 57 females) from the Japan Monkey Centre (JMC), and 12 specimens (5 males and 7 females) from the Primate Research Institute, Kyoto University (PRIKU). Although the specimens used for the present study were bred in captivity, almost all the individuals from the JMC were bred not in cages but in open enclosures called “patas grassland.” This “patas grassland” was located in Inuyama, Aichi, Japan, on a site within the JMC, from February 1965 to February 1990. The plot size was 7000 m2, and the E. patas were free-range. The colony of “patas grassland” was started with 15 animals in 1965. Three animals (1 male and 2 females) were added in 1986, two (1 male and 1 female) in 1970, and four (2 males and 2 females) in 1990. The colony had a maximum of 25 animals including new born babies. The colony usually kept 10 to 20 animals for mating at the JMC. None of the specimens used in the present study had any skeletal abnormalities. In addition, none of the specimens at the JMC had growth impediment either in their medical history or as a cause of death.
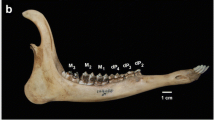
In living mammals, tooth eruption is defined as the point when any part of the crown has pierced the gingiva (Smith 1994). Research on skeletal materials, however, has led some researchers to recognize tooth eruption as the point when the tip of the dental crown is above the alveolar margin (Schultz 1935; Thorington and Vorek 1976), whereas other researchers have restricted this term to the stage of full eruption (Cheverud 1981). Thus, there is no consensus among researchers on the definition of dental eruption with regard to skeletal materials. In the present study, tooth eruption was defined as “emergence” when any part of the dental crown had risen above the alveolar margin, and “full eruption” when the alveolar margin was closed around the base of the dental crown. In each jaw, a tooth was scored as “0” when it was completely buried within the socket or absent, and the score was “1” for full eruption. The dental emergence of a tooth was scored as “1/4”, “1/2”, or “3/4” based on the proportion of dental crown relative to total crown height above the alveolar margin (Schultz 1935). The eruption order was also recognized in accordance with the method of Schultz (1935). For example, if one tooth is scored as “1/2” has erupted and another is scored as “1/4”, it is assumed that the former has appeared before the latter. When the two sides of the dental arcade show different stages of eruption, following Harvati (2000), the most advanced condition for each tooth is scored.
It is known that there are individual variations in the eruption sequences of many primates (Swindler 2002). In the present study, following Harvati (2000), brackets ([]) simply indicate any sequence polymorphism, although these have been used to denote a high level of variation by other workers (Smith 1994). In addition, the equal sign (=) is used when the emergence time of each tooth is identical in all the individuals.
The samples included 22 males and 36 females whose ages were known. Only age in years was inscribed on labels [quotation marks (“and”) are used for such specimens here] for most specimens. Because several teeth erupt within a year in many primates (see Smith 1994), age measured in months should be provided to detect eruption time; however, it is quite difficult to determine the emergence time. The age was provided in years at the JMC. It is meant that both one year and two months animals and one year and ten months animals are counted as one year. Therefore, in this study we calculated “averages of the ranges” with regard to tooth emergence.
Results
Deciduous teeth
Eruption sequence
Specimen JMC 1562(2) had emerging deciduous incisors (Fig. 1). JMC 1565 had fully erupted deciduous incisors, and emerging deciduous canines and deciduous upper and lower third premolar (dp3). JMC 4324 showed full eruption of deciduous incisors, dc and dp3, and emergence of deciduous upper and lower fourth premolar (dp4). Therefore, it appears that the emergence of the deciduous dentition begins with the incisors, followed by the posterior teeth (Tables 1, 2). The eruption sequence of the deciduous dentition is therefore \( \frac{{{\text{di1 = di2}}\;{\text{dc = dp3}}\;{\text{dp4}}}}{{{\text{di1 = di2}}\;{\text{dc = dp3}}\;{\text{dp4}}}}. \)
Eruption time
One newborn individual (PRIKU 7299) had no teeth. JMC 1562(2), with emerging deciduous incisors was 3 days old. Bolwig (1963) reported that living E. patas, 5–12 days old, had emerging deciduous incisors apart from deciduous lower second incisor (di2), a finding comparable to ours. JMC 4324, with emerging dp4, was 0.42 years old (5 months), while JMC 2384 (0.5 years old) had fully erupted deciduous dentition in the lower jaw (upper dentition was absent). JMC 4934 at 0.399 years old (4 months and 24 days) showed full eruption in the lower jaw (upper dentition was absent). Thus, all deciduous teeth emerged at 0.42 years; and full eruption of deciduous teeth occurred at 0.5 years or even less (Tables 1, 2).
Permanent teeth
Eruption sequence
In both sexes, permanent teeth emerge after full eruption of the deciduous teeth. The timing of tooth emergence is identical in the upper and lower jaws, and full eruption of upper and lower first molar (M1) is achieved before the emergence of other teeth. Among the males, upper and lower first incisor (I1) emerged earlier than upper and lower second incisor (I2) in JMC 2298. Upper and lower second molar (M2) emerged after full eruption of the incisors but before full eruption of the premolars. Upper and lower fourth premolar (P4) emerged later than upper and lower third premolar (P3) in the upper jaw of one specimen and earlier than P3 in the lower jaw of four. Emergence of the upper canines was followed by the upper premolars. Full eruption of the lower canines was followed by the lower premolars. However, the emergence of the lower canines was coincident with that of lower third premolar (P3) in two individuals, with some specimens showing emergence of the lower canines before P3 and others after. Upper third molar (M3) emerged earlier than the canines in two specimens, but later in four. In two specimens, M3 and the upper canines emerged at similar timing. Lower third molar (M3) emerged after the other teeth in the lower jaw (Table 3). In females, I1 either emerged before I2 or fully erupted simultaneously. The emergence of the incisors occurred ahead of or was coincident with that of M2. P4 emerged earlier than P3. The order of emergence of the canines, premolars, and M2 was uncertain, but M2 tended to fully erupt before the canines erupted (six of nine individuals for the upper jaw; six of seven individuals for the lower jaw). Upper and lower third molar (M3) was the last permanent tooth to erupt (Table 4). The eruption sequences of the permanent teeth of E. patas were determined as \( \frac{{{\text{M1}}\;{\text{I1}}\;{\text{I2}}\;{\text{M2}}\;{\text{P3}}\;{\text{P4}}\;[{\text{C}}\;{\text{M3}}]}}{{{\text{M1}}\;{\text{I1}}\;{\text{I2}}\;{\text{M2}}\;{\text{P4}}\;[{\text{P3}}\;{\text{C}}]{\text{M3}}}} \) for males, and \( \frac{{{\text{M1}}\;{\text{I1}}\;{\text{I2}}\;\left[ {{\text{M2}}\;{\text{P4}}\;{\text{P3}}\;{\text{C}}} \right]{\text{M3}}}}{{{\text{M1}}\;{\text{I1}}\;{\text{I2}}\;\left[ {{\text{M2}}\;{\text{P4}}\;{\text{P3}}\;{\text{C}}} \right]{\text{M3}}}} \) for females.
Eruption time
In males, M1 began to emerge in five specimens at age “0 years” and in two individuals at age “1 year”. In JMC 3456, M1 emerged at 0.5 years of age (upper dentition was absent). JMC 2138 showed full eruption of M1 at 1.5 years of age (Table 3). In females, M1 began to emerge at “0 years” in the upper jaw in six specimens and in the lower jaw in seven specimens. One specimen at age “1 year” had an emerging M1 in the upper jaw, and two specimens had an emerging M1 in the lower jaw. In JMC 3746, M1 emerged at 0.5 years of age. In JMC 4192, M1 emerged at age 0.91 years (47 weeks and 4 days) (Table 4). Ranges of averages of dental emergence times were as follows: 0.40 < upper first molar (M1) < 1.40 and 0.29 < lower first molar (M1) < 1.29 in males; 0.22 < M1 < 1.22 and 0.14 < M1 < 1.14 in females (Table 5).
A male that was “2 years” old had an emerging M2, whereas a male that was 2.67 years old (2 years 8 months) had a fully erupted set of M2 (Table 3). In females, two specimens showed M2 emerging at age 2 years, with M2 in one specimen of the same age showing full eruption (Table 4). The emergence times of M2 occurred at age 2 years, i.e., from just 2 years old to the end of age 2 years, in both sexes.
In males, no specimens whose ages were known had emerging M3. The individuals that were 3 years old and aged 3.03 years had no emerging M3, but M3 had fully erupted by age “4 years”. The emergence time of M3 was about 3–4 years in males (Table 5). One female specimen that was “4 years” old (JMC 3092) showed emerging M3, while another of the same age (JMC 3202) had emerging M3 and fully erupted M3. The emergence time of M3 was at age 4 years in females (Table 5).
Discussion
Schultz (1935) noted that the dental eruption sequence was M1, I1, I2, M2, P, P, C, M3 in the cercopithecines Cercopithecus, Macaca, and Papio (individual species were not mentioned, apart from M. fascicularis). Since then, the sequences have been reported for several cercopithecine species, in particular Macaca and Papio (Smith 1994). In the Cercopithecini, although 35 species are classified in the tribe (Groves 2005), there are few reports concerning dental eruption sequences. We are aware of published dental eruption data for Ch. aethiops (Ockerse 1959), Cercopithecus nictitans, Cercopithecus diana, and Cercopithecus mona (Lampel 1962). Table 6 shows the dental eruption sequences in cercopithecines based on these studies. The cercopithecine species are identical with regard to the eruption order of incisors, M1 and M2; namely, M1, I1, I2, M2. M3 is the final tooth to emerge in most species, but is fully erupted before the canines in males of some species where the greater height of their canines requires a much longer time for full eruption (Lampel 1962). E. patas is identical to the cercopithecine species in sequence patterns, i.e., M1, as the first permanent tooth, is followed in order by I1, I2, and M2, and finally M3 emerges. The order of premolars and canines in the sequence differs among species in various ways. E. patas shows sequence polymorphism. Therefore, we could not determine whether any of its sequence patterns of premolars and canines were identical to those of the cercopithecine species.
According to Schultz’s rule, species with a fast life history should exhibit early eruption of molars relative to incisors, canines, and premolars (Smith 2000). E. patas is described as a fast-maturing and quick-breeding monkey with a high female mortality rate, in spite of its large body size for a cercopithecine (Chism et al. 1984; Nakagawa et al. 2003; Isbell et al. 2009). These characteristics, which are indicators of a fast-life-history species (Godfrey et al. 2005), would suggest early eruption of the molars related to the eruption of the replacement teeth, based on Schultz’s rule. Among the living cercopithecids, Ch. aethiops is most closely related to E. patas (Tosi et al. 2005), and its inter-birth interval and age of weaning are the same as those of E. patas (Cheney 1981). However, its age of giving birth for the first time is later than that of E. patas (Cheney 1981), and its body size is smaller (Skinner and Chimimba 2005). Therefore, Ch. aethiops is not a fast-life-history species. The life history of E. patas would suggest that, based on Schultz’s rule, early eruption of the molars would be related to the eruption of the replacement teeth. However, E. patas exhibits the sort of eruption order usually seen in the cercopithecine species.
In primates, the age at eruption of M1 is highly correlated with life history variables, such as inter-birth interval, age at first breeding, and age at weaning (Smith 1989, 1992). It is possible to assume that M1 of E. patas erupt early, because this guenon is also characterized as a species with short inter-birth interval, young age at first breeding, and early independence from the mother (Chism et al. 1984; Nakagawa et al. 2003). Indeed, E. patas shows shorter molar crown formation relative to body size than other primates (Macho 2001). Figures 2 and 3 (and Appendix Table 1) show the averages or the medians of emergence times of dp4 and molars for selected cercopithecines. Emergence times of M1 for Macaca, Papio, and Mandrillus fall outside the upper limits of ranges of averages for E. patas, but those for Ch. aethiops are in the same range as those for E. patas. This indicates that the emergence time of M1 in E. patas is earlier than that in the above-mentioned cercopithecines and is similar to that in Ch. aethiops. On the other hand, the emergence time of dp4 in E. patas is similar to that in Macaca, Papio, and Mandrillus but is later than that in Ch. aethiops. Intervals between the emergence of dp4 and M1 are around 1 year in most cercopithecine species, the longest being 1.80 years in the lower jaw of the male Mandrillus sphinx, and the shortest being 0.66 years in the upper jaw of Ch. aethiops. The interval between dp4 and M1 is less than 0.5 years in E. patas, a statistic obtained simply by calculating the difference between the median of ranges of averages for M1 and the emergence time of dp4 (0.42 years). Furthermore, in our study, one specimen showed M1 emerging at 0.5 years (6 months), representing a difference from 0.42 years of only 0.08 years (1 month). The emergence times of M1 of E. patas have a large average ranges than those of other cercopithecines. The upper limit of the interval between emergence times of dp4 and M1 were 0.98 years in upper dentition and were 0.87 years in lower dentition of male E. patas (Table 5). This time is relatively short. In E. patas, therefore, in comparison with other cercopithecines, M1 tends to emerge immediately after the eruption of dp4.
In E. patas, the emergence times for M2 and M3 were 2 and 4 years, respectively, which is earlier than that for Macaca, Papio, and Mandrillus, and later than that for Ch. aetiops (Figs. 2, 3). However, E. patas is similar to the above-mentioned cercopithecines regarding intervals of emergence times between permanent molars (Figs. 2, 3). In Ch. aethiops, M1 appears earlier than in other cercopithecines, excluding E. patas, and the intervals of eruption time between molars are less than those of the other cercopithecines, including E. patas, especially the interval between M1 and M2 (Figs. 2, 3). Thus, all molars erupt early in Ch. aethiops, whereas only the interval between dp4 and M1 is short in E. patas. The early appearance of M2 and M3 in E. patas occurs because of the short interval in emergence times between dp4 and M1.
References
Bolwig N (1963) Bringing up a young monkey (Erythrocebus patas). Behaviour 21:300–330
Cheney DL (1981) Intergroup encounters among free-ranging vervet monkeys. Folia Primatol 35:124–146
Cheverud JM (1981) Epiphyseal union and dental eruption in Macaca mulatta. Am J Phys Anthropol 56:157–167
Chism J (1986) Development and mother-infant relations among captive patas monkey. Int J Primatol 7:49–61
Chism J, Rowell TE (1988) The natural history of patas monkeys. In: Gautier-Hion A, Bouliere F, Gautier J (eds) A primate radiation: evolutionary biology of the African guenons. Cambridge University Press, New York, pp 412–438
Chism J, Rowell TE, Olson DK (1984) Life history patterns of female patas monkeys. In: Small M (ed) Female primates: studies by women primatologists. Alan R Liss, New York, pp 175–190
Godfrey LR, Samonds KE, Wright PC, King SJ (2005) Schultz’s unruly rule: dental developmental sequences and schedules in small-bodied, folivorous Lemurs. Folia Primatol 76:77–99
Groves CP (2005) Order primates. In: Wilson DE, Reeder DM (eds) Mammal species of the world. Smithsonian Institution, Washington, pp 111–184
Harvati K (2000) Dental eruption sequence among colobine primates. Am J Phys Anthropol 112:69–85
Honjo S, Cho F (1977) Macaques (genus Macaca). In: Tazima Y (ed) Laboratory animal science, vol 3. Asakura Publishing Co. Ltd, Tokyo, pp 312–346 (in Japanese)
Hurme VO, van Wagenen G (1961) Basic data on the emergence of permanent teeth in the rhesus monkey. Proc Am Phil Sot 105:105–140
Isbell LA, Young TP, Jaffe KE, Carlson AA, Cahcellor RL (2009) Demography and life histories of sympatric patas monkeys, Erythrocebus patas, and vervets, Cercopithecus aethiops, in Laikipia, Kenya. Int J Primatol 30:103–124
Iwamoto M, Hamada Y, Watanabe T (1984) Eruption of deciduous teeth in Japanese monkeys (Macaca fuscata). J Anthrop Soc Nippon 92:273–279
Iwamoto M, Watanabe T, Hamada Y (1987) Eruption of permanent teeth in Japanese monkeys (Macaca fuscata). Primate Res 3:18–28
Kahumbu P, Eley RM (1991) Teeth emergence in wild olive baboons in Kenya and formulation of a dental schedule for aging wild baboon populations. Am J Primatol 23:1–9
Lampel G (1962) Variationsstatistische und morphologische uutersuchungen am gebiss der cerecopithecinen. Acta Anat 49 (Suppl 45). S Karger, Basel
Macho GA (2001) Primate molar crown formation times and life history evolution revisited. Am J Primatol 55:189–201
Nakagawa N, Ohsawa H, Muroyama Y (2003) Life-history parameters of a wild group of West African patas monkeys (Erythrocebus patas patas). Primates 44:281–290
Ockerse T (1959) The eruption sequence and eruption times of the teeth of the vervet monkey. J Dental Assoc S Afr 14:422–424
Phillips-Conroy JH, Jolly CJ (1988) Dental eruption schedules of wild and captive baboons. Am J Primatol 15:17–29
Schultz AH (1935) Eruption and decay of the permanent teeth in primates. Am J Phys Anthropol 19:489–581
Seier JV (1986) Breeding vervet monkeys in a closed environment. J Med Primatol 15:339–349
Setchell JM, Wickings EJ (2004) Sequences and timing of dental eruption in semi-free-ranging mandrills (Mandrillus sphinx). Folia Primatol 75:121–132
Sirianni JE, Swindler DR (1985) Growth and development of the pigtailed macaque. CRC Press, Inc, Boca Raton
Skinner JD, Chimimba CT (2005) The mammals of the Southern Africa sub-region, 3rd edn. Cambridge University Press, New York
Smith BH (1989) Dental development as a measure of life history in primates. Evolution 43:683–688
Smith BH (1992) Life history and the evolution of human maturation. Evol Anthropol 1:134–142
Smith BH (1994) Ages of eruption of primate teeth: a compendium for aging individuals and comparing life histories. Am J Phys Anthropol 37:177–231
Smith BH (2000) “Schultz’s rule” and the evolution of tooth emergence and replacement patterns in primates and ungulates. In: Teaford MF, Smith MM, Ferguson MWJ (eds) Development, function, and evolution of teeth. Cambridge University Press, New York, pp 212–227
Swindler DR (2002) Primate dentition: an introduction to the teeth of non-human primates. Cambridge University Press, Cambridge
Thorington RW, Vorek RE (1976) Observations on the geographic variation and skeletal development of Aotus. Lab Anim Sci 26:1006–1021
Tosi AJ, Detwiler KM, Disotell TR (2005) X-chromosomal window into the evolutionary history of the guenons (Primates: Cercopithecini). Mol Phylogenet Evol 36:58–66
Acknowledgments
We would like to thank Dr. Daisuke Shimizu of the Japan Monkey Centre and Professor Masanaru Takai of the Primate Research Institute, Kyoto University, for granting permission to examine the materials used in this study and giving helpful comments. We would also like to thank the staff at the Japan Monkey Centre, and the staff at the Primate Research Institute, Kyoto University. This study was supported by the Cooperation Research Program of Primate Research Institute, Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Jogahara, Y.O., Natori, M. Dental eruption sequence and eruption times in Erythrocebus patas . Primates 53, 193–204 (2012). https://doi.org/10.1007/s10329-011-0286-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-011-0286-y