Abstract
This work utilizes density functional theory (DFT) to analyze the structural, thermodynamic, mechanical, electro-optic, and electron transport characteristics of Rb2CuAsX6 (X = F, Cl, Br) perovskites. The impact of occupancy of different halide ions at the X-site on the thermodynamic, mechanical, optical, and thermoelectric response of studied compounds has also been evaluated. The investigation of the elastic parameters and formation energy has confirmed that the examined perovskites are cubic in structure, stable, and ductile. The thermodynamic characteristics that rely on temperature are estimated using the quasi-harmonic Debye approach. The thermal features such as entropy, heat capacity, and Debye temperature are calculated and analyzed to assess the stability at elevated temperatures and the suitability of compounds for industrial applications. The band structure computations identified Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 are p-type semiconductors with indirect band gaps of 1.25, 1.10, and 0.83 eV, respectively, which decreases while substituting F with Cl and Br. The optical characteristics such as strong optical absorption (> 105 cm−1) and minimal dispersion in the visible and ultraviolet spectrum highlight their suitability for solar energy conversion and optoelectronics. Additionally, thermoelectric characteristics have been determined, showing a higher thermoelectric figure of merit (ZT) value of 0.80, 0.79, and 0.78, respectively, at room temperature. Our research anticipates these perovskite combinations exhibit superior photoelectric and thermoelectric efficiency, rendering them potential prospects for advanced photovoltaic and thermoelectric devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Presently, with social advancements, there is an increasing tendency towards energy-efficient and environmentally friendly solutions. These technologies have the potential to substitute conventional fossil fuels with carbon-containing alternatives while ensuring a continuous supply of electricity [1, 2]. Scientific societies have expressed curiosity regarding technologies such as solar cells, water splitting, and thermal electricity due to their potential to confront energy concerns [3,4,5]. Multiple components are now being explored to enhance such technologies, with a specific emphasis on maximizing efficiency, and functionality, and reducing environmental harm. Currently, experimentally and theoretically evidenced double perovskites are widely regarded as adaptable materials, as a consequence of their diverse range of uses [6,7,8]. Double perovskite compounds have recently attracted significant interest owing to their prospective uses in several fields, such as LEDs, radiation detectors, photovoltaic cells, sensors, fuel cell technology, thermal electricity, catalytic activity, and various other fields [9,10,11,12,13]. Over the last two decades, a notable surge in concern regarding the production of perovskite solar cells has developed because of their excellent performance and cost-effectiveness. The experimentally achieved power conversion efficiency has increased from 3.8% in 2009 to 25.5% in 2021. These unique qualities allowed it to exceed the performance of traditional thin-film solar cells [14,15,16], because of its adequate bandgap, superior absorption coefficient, and almost identical effective masses of charge carriers [17]. The lead-based perovskite combinations are toxic and unstable in long-term exposure to humidity, light, and heat, which hinders their integration into innovative technological applications [18,19,20].
Halide-based double perovskites, represented by the formula A2M+M3+X6, have become a promising alternative to lead-based perovskites for use in photovoltaic cells [21], electronic components [22], and thermoelectric systems [23]. These perovskites offer the capability for improved stability and decreased toxicities while yet retaining high performance. Various experimental and computational investigations are being carried out on Cs-based perovskite materials with the chemical formula Cs2M+M3+X6 (where M+ = Ag+, Ga+, Cu+, etc., M3+ = Bi3+, In3+ and X = Cl−, Br−, I−). McClure et al. researched Cs2AgBiX6 (X = Cl, Br), which had band gaps of 2.7 eV and 1.9 eV, respectively. Nevertheless, these bandgaps are above the Shockley–Queisser band gap threshold for the most efficient solar cells, leading to a decreased efficiency of approximately 2.5%. Furthermore, Cs2AgBiBr6 has been demonstrated to be unstable when subjected to ambient air and light for an extended period [24, 25]. Therefore, it is advantageous to investigate the advancement of double perovskites because of their exceptional potential. Several scientists have done computational research to investigate the electrical, optical, and thermoelectric properties of different perovskite materials [26, 27]. Cs2YAuX6 (X = Cl, Br) [28] and Cs2TIAsX6 (X = Cl, Br, I) [29] have been identified as feasible materials for solar energy and optoelectronic applications due to their appropriate band gaps, low effective masses, strong light absorption, and low reflectivity. Moreover, several combinations Rb2CuBiX6 (Cl, Br) [30], Cs2AgAsX6 (X = Cl, Br, I), Rb2AgAsX6 (X = Cl, Br, I) [31], and Na2AgAsX6 (Cl, Br) [32] have been explored having higher absorbance and higher figure of merit values, which suggest their capability for renewable energy production. These investigations provide valuable insights into the chemical element combinations used in perovskite materials and motivated us to explore novel combinations containing copper (Cu) and arsenic (As) as two monovalent and trivalent cations. This stable combination might have exceptional electro-optic and electron transport features.
The primary aim of the current study is to forecast novel materials with improved optoelectronic and electron transport characteristics using density functional theory for energy technology. This paper examines the structural stability, thermodynamic, elastic, electro-optic, and thermoelectric properties of the Rb2CuAsX6 (X = F, Cl, Br) perovskites. This article also highlights the impact of different halide ion occupancies on the aforementioned properties. The materials Rb2CuAsX6 (X: F, Cl, Br), as far as we are aware, have not been the focus of any theoretical or experimental investigation. We expect that our research will provide essential insights for forecasting lead-free double perovskite materials suitable for upcoming thermoelectric devices and solar energy harvesting.
2 Methodology
The perovskites Rb2CuAsX6 (X = F, Cl, Br) were analyzed using the Wien2k code. This code is based on the principles and concepts of density functional theory, which are implemented using the full potential linearized augmented plane wave (FP-LAPW) approach [33]. The wave function of an electron gas has been determined in relation to the electron density using the Schrödinger wave equation. The spherical and plane wave solutions are allocated to the muffin-tin and interstitial areas by maintaining a constant potential in each zone. The structural analysis used the Perdew–Burke–Ernzerhof generalized gradient approximation (PBE-GGA) to optimize the structures and accurately determine the ground state components [34]. This approach undervalues the band gap and electronic behavior. The Tran–Blaha modified Becke–Johnson (TB-mBJ) potential, known for its versatility and excellent accuracy, was applied over the calculations of PBE-GGA to adjust the band gap [35]. This adjustment is especially beneficial for improving the accuracy of bandgap estimates in materials where there is a significant impact of dominant electron–electron interactions [36]. The Gmax (Gaussian factor) and ℓ (angular momentum) were set at 8.0 and 10, respectively, which are key criteria for software to manage program execution. The energy convergence of the precise Hamiltonian of the structure is achieved by setting the muffin-tin radius (RMT) and wave vector product to 10. The k-mesh order is another feature that might influence convergence. The k-points are chosen in a 20 × 20 × 20 grid to create a dense mesh that converges the energy to within 0.01 mRy. The optical investigation was conducted using the Kramer–Kronig relation [37], whereas the thermoelectric study was performed using the BoltzTraP code [38]. Moreover, thermodynamic aspects were calculated by using the GIBBS2 code [39]. An appropriate RMT radius aims to reduce the overlapping of muffin-tin spheres between neighboring atoms. This gap is essential for accurately representing atom-atom interactions and positioning electrons in close proximity to atoms [26]. The RMT values for constituents Rb, Cu, As, and X (F, Cl, Br) were selected as 2.0, 1.6, 1.9, and 1.8, respectively.
3 Results and Discussion
3.1 Structure and Stability
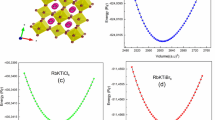
The structural features of Rb2CuAsX6 (X = F, Cl, Br) compounds have been optimized in the cubic phase with space group Fm-3m using the PBE-GGA functional method, which is crucial for improving the long-term stability of the compounds being analyzed [40, 41]. The overall energy of the double perovskite being examined is equal to the system's lowest energy state. The Murnaghan equation of state is a commonly used mathematical model in materials science to explain the relationship between a crystal lattice’s volume and its total energy. It gives a clear and precise explanation of the relation between the equilibrium lattice parameter, volume, energy, and bulk modulus. The lattice parameters are optimized using the Murnaghan equation of state to analyze the anticipated energy curve (ETotal) and determine key parameters including equilibrium lattice parameter, ground state volume (V0), and ground state energy (E0) by the relation [42]:
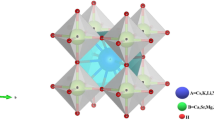
The cubic arrangement of Rb2CuAsX6 (X = F, Cl, Br) with space group Fm-3m is displayed in Fig. 1. Perovskites’ structure and stability may be better understood by the Goldsmith tolerance parameter, which accounts for the effect of different cation sizes at the A and B locations. It allows us to determine if a specific combination of ions may produce a flawless perovskite structure, or whether particular defects and instabilities are more likely to occur. The stability of the structure linked to the Goldsmith tolerance factor (τ) is calculated using the formula [43]:
where rRb, rX, rCu, and rAs represent the ionic radii of Rb, X (F, Cl, Br), Cu, and As atoms, respectively. The material maintains a stable cubic perovskite structure within a tolerance factor range of 0.7 to 1.11 [44]. The optimized double perovskite’s total energy changing with volume is presented in Fig. 2, while Table 1 presents the calculated equilibrium features of investigated materials. The tolerance factor values of Rb2CuAsX6 (X = F, Cl, Br) suggest that their composition is stable, as given in Table 1.
Thermal stability has been confirmed by the computation of formation energy (EF) as well as Gibbs free energy (G) [45], EF is determined using the formula [46]:
Moreover, thermal stability is further verified by the negative values of Gibbs free energy resulting from excess energy given up bringing the structure towards equilibrium. The values of EF and G are given in Table 1 which ensures that the studied materials are thermally stable.
3.2 Thermodynamic Properties
The thermodynamic characteristics that rely on temperature are estimated using the quasi-harmonic Debye approach [47,48,49]. Since the quasi-harmonic Debye concept is completely valid throughout the temperature range of 0 to 600 K, Figure 3 displays the volume (V), bulk modulus (B), entropy (S), molar specific heat (CP), and Debye temperature (θD) for Rb2CuAsX6 (X = F, Cl, Br).
The correlation between volume and temperature for Rb2CuAsX6 (X = F, Cl, Br) is justified in Fig. 3a. The volume of the observed three materials varies directly with temperature due to the expansion of material with increasing temperature. Meanwhile, the bulk modulus follows a downward trend with an increase in temperature for the investigated materials. The variation in bulk modulus with temperature may be due to the fact that temperature causes an expansion in the separation between atoms, leading to an increased unit cell volume and therefore a drop in bulk modulus. The inverse correlation of B with temperature is plotted in Fig. 3b. Therefore, based on the B values at 300 K, it is evident that Rb2CuAsF6 would exhibit greater stiffness in comparison to the Rb2CuAsCl6 and Rb2CuAsBr6. Entropy (S) is an inherent attribute that quantifies the degree of disturbance or randomness inside a system's structure [50]. Figure 3c illustrates the relationship between entropy and temperature. It shows a proportional rise in entropy at temperatures above 100 °C, indicating an improvement in structural irregularity. The values of S are found to be higher for Rb2CuAsBr6 than Rb2CuAsF6 and Rb2CuAsCl6 showing greater disorder in Rb2CuAsBr6.
Furthermore, the relationship between specific heat at a fixed pressure (CP) and temperature is illustrated in Fig. 3d. This parameter is crucial in thermodynamics since it offers insights into vibrational characteristics, phase transitions, and other related aspects [51]. The figure illustrates a quick rise in the value of CP at lower temperature values ranging from 0 to 200 K, while beyond 200 K, there is a steady rise in CP. At a temperature of 300 K, the computed specific heat capacity was 241.52 J mol−1 K−1, 253.88 J mol−1 K−1, and 260.45 J mol−1 K−1 for Rb2CuAsF6 and Rb2CuAsCl6, and Rb2CuAsBr6, respectively. Furthermore, Fig. 3e displays the relationship between the thermal expansion coefficient α and temperature. The thermal expansion coefficient offers important information on the interatomic bonding and melting temperature of material [52]. The data shows that the value of α grows as the temperature rises. The rate of increase in α is faster at low temperatures (up to 200 K) and slower at high temperatures (beyond 200 K).
The slow growth of α above 200K is because α becomes saturated at this temperature. The room temperature (300 K) values of α are 10.87 K−1, 12.24 K−1, and 12.98 K−1, respectively, for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6. A lower value of α for Rb2CuAsF6 in comparison to the Rb2CuAsCl6 and Rb2CuAsBr6 indicates stronger bonding, resulting in a greater melting temperature. The Debye temperature (\({\uptheta }_{{\text{D}}}\)) is used to describe the activation of phonons and assess different lattice thermal features and the suitability of compounds for industrial applications. Higher \({\uptheta }_{{\text{D}}}\) leads to increased energy phonon modes by hindering the soft phonon mode lacking radiation relaxation, resulting in increased emission of photons. Conversely, lower \({\uptheta }_{{\text{D}}}\), which typically includes a higher soft phonon mode, can facilitate non-radiative relaxation [52]. Rb2CuAsF6 has a higher \({\uptheta }_{{\text{D}}}\) of 336 K at 300 K, compared to Rb2CuAsCl6 and Rb2CuAsBr6 which have 245.39 K and 184.39 K, as shown in Fig. 3f.
3.3 Elastic Properties
3.3.1 Elastic Constants and Mechanical Stability
Elastic constants are physical characteristics used to assess the mechanical stability as well as durability of a solid and to identify its brittle or ductile nature. Knowledge of elastic constants is crucial for evaluating a solid’s suitability for mechanical and engineering purposes [53]. Elastic constants (Cij) for the three double perovskites Rb2CuAsX6 (X = F, Cl, Br) are found using density functional theory (DFT) and are listed in Table 2. The three distinct elastic stiffness coefficients, C11, C12, and C44, obtained by density functional theory (DFT), meet the necessary conditions for mechanically stable cubic structures. The conditions are stated as follows: C11 must exceed the absolute magnitude of C12, C11 + 2C12 must be positive, and C44 should exceed zero according to the Born elastic stability criterion [54,55,56,57]. As a result, the Born criterion states that the double perovskites Rb2CuAsX6 (X = F, Cl, Br) are mechanically stable. Specific mechanical parameters like bulk modulus (B), Young’s modulus (Y), and shear modulus (G) may be determined using the following formulae:
The calculations show that the bulk modulus (B) for the compound Rb2CuAsF6 is 55.77, which is greater than that of Rb2CuAsCl6 (31.43) and Rb2CuAsBr6 (25.46), as reported in Table 2. When these compounds are compressed from each direction, Rb2CuAsF6 shows more resistance to the changes in volume than Rb2CuAsCl6 and Rb2CuAsBr6. The shear modulus (G) is a key parameter that defines the resistance to stress of a material. The shear modulus measurements indicate that Rb2CuAsF6 has a greater shear modulus (20.66) compared to Rb2CuAsCl6 (10.89) and Rb2CuAsBr6 (8.44). The findings show that Rb2CuAsF6 has better hardness and stronger resistance against deformation than Rb2CuAsCl6 and Rb2CuAsBr6. The Young modulus (Y) is a quantity that defines the intrinsic stiffness of a certain material. According to Table 2, the data clearly shows that Rb2CuAsF6 has also a larger value of Y (55.17) compared to Rb2CuAsCl6 (29.28) and Rb2CuAsBr6 (22.77), suggesting that Rb2CuAsF6 has more stiffness. The Poison ratio (ʋ) offers vital information on a material’s mechanical characteristics, including its ductility or brittleness. Materials having a Poisson’s ratio (ʋ) above 0.26 are categorized as ductile, while materials with a Poisson’s ratio less than 0.26 are regarded as brittle. The investigation found that the value of ʋ is above 0.26 for all materials [58]. The results concerning ʋ indicate that the materials being studied display ductile properties. The B/G ratio, often known as Pugh’s criterion, is a measure also used to assess ductility or brittleness. According to Pugh’s criterion, a material is considered ductile if the ratio of bulk modulus (B) to shear modulus (G) surpasses 1.75, and it is classed as brittle if the B/G ratio gets lower than 1.75 [59]. This criterion also verifies the ductile or flexible nature of all compounds since the B/G ratio is greater than 1.75.
3.3.2 Anisotropic Properties
Microscopic fractures in a material may be identified by analyzing its anisotropic characteristics. The anisotropic factor (A) evaluates the structural homogeneity or variation of a material across different orientations and is determined by a formula:
When A = 1, the medium is termed isotropic; however, if 0 < A < 1 or A > 1, then the material is regarded as anisotropic [60]. The values of A indicate that the materials being studied exhibit anisotropic behavior.
To examine anisotropy, Fig. 4 shows three-dimensional visualizations of Young’s modulus of a single crystal along certain crystallographic orientations for Rb2CuAsX6 (X = F, Cl, Br). Materials used for advanced manufacturing and engineering fields need to possess substantial anisotropy. The graphs were created using the SC-EMA approach [61,62,63] and were derived from computations. Both the color scheme and the axes depict Young's modulus in GPa showing a strong association with the direction. All directional surfaces obtained show significant deformations compared to a spherical shape, confirming that mechanical properties vary with direction and indicating the strong elastic anisotropy of the materials studied. For the materials under study, the anisotropy level decreases in the order: Rb2CuAsF6 > Rb2CuAsCl6 > Rb2CuAsBr6. Moreover, the highest Young's modulus value is in the [100], [010], and [001] orientations decreasing in a similar order: Rb2CuAsF6 > Rb2CuAsCl6 > Rb2CuAsBr6, indicating that stiffness is decreasing along with anisotropy. These outcomes suggest greater mechanical strength and anisotropy in Rb2CuAsF6 than in Rb2CuAsCl6 and Rb2CuAsBr6.
3.4 Electronic Properties
This study analyzed the electronic features of Rb2CuAsX6 (X = F, Cl, Br) by computing the band structure, total density of states (TDOS), and partial density of states (PDOS). The band structures have been determined by applying two approaches (PBE-GGA, and TB-mBJ approximation) due to the absence of prior experimental as well as theoretical data for comparison, in order to strengthen the validity of our findings [64].
The band energies are computed using PBE-GGA and TB-mBJ potentials to illustrate the band configuration. The band gap (Eg) values are calculated at the W, X, L, K, and Γ symmetric locations using PBE-GGA, as mentioned in Fig. 5. The Eg values using the PBE-GGA framework are underestimated and found to be 0.97, 0.59, and 0.26 eV. Moreover, the computation has been improved using the TB-mBJ approximation, as shown in Fig. 6, along the high-order symmetry directions W, X, L, K, and Γ for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6. The standard band gap values increased to 1.25 eV for Rb2CuAsF6, 1.10 eV for Rb2CuAsCl6, and 0.83 eV for Rb2CuAsBr6. This boost is due to the TB-mBJ exchange potential pushing the conduction bands composed of specific electronic states, beyond the Fermi level. The band gap values of the current materials drop as the halide ionic size increases from F to Br, as seen in Table 1. Due to the smaller ionic radius of Br compared to Cl and F, replacing Br with Cl and F results in a red shift in the band gap. Therefore, this implies that the examined materials are suitable for application in optoelectronics, photovoltaics, and energy-harvesting devices.
The patterns of TDOS show uniform densities as well as band gaps confirmation of the analyzed perovskites. The total density of states (TDOS) below the Fermi level (EF) is higher than in the conduction band (CB), suggesting a p-type conductivity. Furthermore, the significant variation in the highest valence band along L–Γ–X results in a low effective mass for the holes, leading to strong mobility and exhibiting p-type conductivity. The states in the highest valence band along X–W–K are partially flat, indicating the presence of a low-occupied orbital at the Fermi level. This may also be associated with the comparatively large effective mass of holes [65].
Partial densities of states (PDOS) are analyzed to understand the contributions of Rb, Cu, As, and X elements to the valence bands (VBs) and conduction bands (CBs), aiding in the full assessment of the electronics structure’s impact on device functionality. The outcomes obtained from the TB-mBJ potential are shown in Fig. 7. The PDOS is important in governing the possible electronic transitions among specific energy levels. The PDOS pattern indicates that the valence bands and conduction bands borders are mainly made up of Cu-d and As-p orbitals, respectively. Halides (F, Cl, Br) little contributions were seen in the deep area of the valence band, although they do not affect the electronic behavior. Rb has a non-resistant role in forming the frontier bands by just donating charges to sustain structural stability [65]. The bottom of the conduction band is mostly due to As-p, whereas the top of the valence band includes Cu-d states. Figure 7a–c shows that the influence of the As-p reduces progressively from F to Br. The replacement of Br with I induces a red shift in the I-5p and Sb-5p states in the conduction band, resulting in a red-shifted band gap. Increasing atomic radius decreases the coulombic attraction of the nucleus on valence shell electrons, causing a reduction in the band gap energy. Therefore, the findings of electronic properties provide evidence of the fact that Cu-d and As-p states play vital contributions to electronic transitions, which dictate the optical and thermoelectric attributes of the studied materials.
3.5 Optical Properties
The optical properties of studied materials are mainly determined by the interaction between matter and light and the characteristics of the energy gap. This paper provides a detailed description of the optical characteristics of Rb2CuAsX6 (X = F, Cl, Br) double perovskites in relation to the dielectric function, by employing the TB-mBJ potential. The dielectric function, commonly known as the permittivity of a material, is determined by the relation [66]:
The real portion ε1(ω) represents the generated polarization caused by the interaction between light and the material, whereas the imaginary part ε2(ω) indicates the capacity of attenuation of light that is transmitted via material. The relationship between several associated optical characteristics such as refractive index n (ω), reflectivity R (ω), optical absorption coefficient α (ω), optical energy loss function L (ω), and optical conductivity σ (ω) is analyzed based on ε1(ω) and ε2(ω) [67, 68].
The calculated energy-dependent ε1(ω) of Rb2CuAsX6 (X = F, Cl, Br) in Fig. 8a shows a relatively weak energy dependency at minimal energy. For Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6, the static values of ε1(ω) are 3.40, 4.24, and 5.13, respectively. The rising order of halides from F to Br exhibits an increase in ε1(0), indicating that heavier halogens become increasingly optically polarizable. The observed increase in ε1(0) promotes a reduced recombination rate of energy carriers, enhancing the efficiency of optical components. The value of this parameter is negatively correlated with electronic band gap energy as shown by the Penn model [69]: ε1(0) = 1 + (ħωp/Eg)2. In the visible range, the main peaks of ε1(ω), which are linked to the highest propagation of light, are found at the resonance frequencies. Beyond the resonance limit, the ε1(ω) decreases rapidly with several relaxation peaks associated with electronic inter-band transitions. The relaxation time may be calculated from the peak frequency using the formula: τr = 1/ω = ħ/E. Rb2CuAsBr6 has a higher peak in the visible area compared to Rb2CuAsCl6 and Rb2CuAsF6 due to its smaller band gap energy. Moreover, at the energy values of 2.08 eV, ε1(ω) is less than 0, Rb2CuAsF6 displays metallic properties with complete reflection. Within this energy range, the frequency of the incoming radiation exceeds the plasma frequency. However, Rb2CuAsCl6 and Rb2CuAsBr6 show no reflection throughout the energy range.
Figure 8b demonstrates that ε2(ω) stays at zero for photons with energy below the band gap energy for both substances, suggesting insufficient energy for electronic inter-band transition. Furthermore, the ε2(ω) rises from the threshold levels to the peak value of 7.27 (at 1.80 eV), 6.33 (at 1.94 eV), and 5.53 (at 2.05 eV) for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6, respectively. The multi-peak phenomena seen in the observed energy regions are caused by the varying rates of possible electronic transitions from Cu-d states in the VB to the As-p states in the CB. Moreover, a decrease in peak intensity can be attributed to the decreased PDOS by replacing F with Cl and Br (refer to Fig. 6). Thus, it is evident that Rb2CuAsX6 (X = F, Cl, Br) exhibits varying optical absorption capacities throughout a wide range of electromagnetic frequencies. The large peak magnitudes seen usually facilitate the extension of polarized electrons into conducting states, hence improving the efficiency of photovoltaic conversion. Based on this discussion, the ideal operating areas in the visible and ultraviolet spectrums make the materials very suitable for photovoltaic (PV) conversion and optoelectronic devices.
The transparent nature of Rb2CuAsX6 (X = F, Cl, Br) is primarily dictated by its optical refractive index n(ω). It measures the amount of bending of light as it travels through a substance. Assessing this characteristic is crucial for the precise use of materials in optical devices. Figure 8c shows that the refractive index at zero frequency appears to be 1.84, 2.05, and 2.26, for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6. The rise in n(0) by shifting from F to Br occurs due to the red-shifted band gaps. Furthermore, these values are linked to ε1(0) in the following manner: n2(0) = ε1(0). Additionally, the patterns of n (ω) closely resemble ε1(ω). Rb2CuAsF6 has the maximum refractive index of 3.0 at 1.80 eV, whereas Rb2CuAsCl6 shows a refractive index of 2.84 at 1.94 eV and Rb2CuAsBr6 has 2.88 at 1.60 eV. At an energy of 2.2 eV, the n (ω) value of Rb2CuAsF6 is less than one, suggesting that these materials become optically opaque.
In addition, Fig. 8d shows the correlation between the incoming energetic photons and its extinction coefficient k(ω), determined by the equation 2nk = ε2(ω) [70], representing the ratio of highest to lowest transmitted energy at a certain energy level. This may be verified by referring to Fig. 4d, where the k(ω) exhibited a similar pattern to the ε2(ω) since both parameters are connected by the Kramer-Kronig relation [37]. The greatest values for k(ω) of Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 appeared at 2.24 eV, 2.05 eV, and 2.0 eV, respectively.
The optical absorption coefficient α(ω) indicates the potency of the material to capture a certain amount of photon energy. For photovoltaic conversion, the materials need to have a high optical absorption coefficient (> 105 cm−1) over the whole visible spectrum range. Figure 9a shows the α(ω) spectra of Rb2CuAsX6 (X = F, Cl, Br), which display a wide absorption spectrum mainly in the visible-ultraviolet regions, making it well-suited for photovoltaic and optoelectronic devices. Absorption edges are crucial since they indicate the sites at which absorption begins, which are comparable to the band gaps of materials under study. Additionally, there is a significant increase in peak values of 4.4 × 105 cm−1, 3.85 × 105 cm−1, and 3.4 × 105 cm−1 when transitioning from Cu-d valence states to the unoccupied As-p conduction states in the visible region for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6, respectively. Moreover, the absorption coefficient significantly rises in the UV spectrum for these materials. Based on the band gap energy value and optical absorption spectra, Rb2CuAsX6 (X = F, Cl, Br) are well-suited for photovoltaic applications.
Optical conductivity σ(ω) pertains to the bond-breaking mechanism during photon absorption and provides valuable insights into the produced free carriers of energy [71]. The relationship between the energy-dependent σ(ω) pattern and α(ω) is seen in Fig. 9b. In the visible area, Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 have maximum σ(ω) of 1.79 × 103 (Ω cm)−1, 1.62 × 103 (Ω cm)−1, and 1.58 × 103 (Ω cm)−1, respectively. This demonstrates superior absorption properties of Rb2CuAsX6 (X = F, Cl, Br) materials in the visible range. The maximum optical conductivities are reported at the UV region with magnitudes of 2.21 × 103 (Ω cm)−1, 2.62 × 103 (Ω cm)−1, and 1.93 × 103 (Ω cm)−1, respectively. The peak values of Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 in visible and UV ranges are higher, perhaps due to the carrier density of these materials. The current HDPs exhibit optical activity with an energy spectrum that enhances their use in photovoltaic and optoelectronic technologies, even at high energies.
Optical reflectivity R(ω) is the capacity of a material to deflect incoming photons hitting its exterior. Consequently, when fewer influencing photons are captured, the R(ω) increases significantly [71]. Figure 9c shows the reflectivity of Rb2CuAsX6 (X = F, Cl, Br) halide double perovskites. The reflectivity at zero energy R(0) values for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 compounds are 8%, 12%, and 15%, respectively. These convey a glimpse into the surface texture of the materials. The visible spectrum shows peak reflectivity values of around 50% for Rb2CuAsF6 26%, for Rb2CuAsCl6, and 28% for Rb2CuAsBr6. Furthermore, these materials also show weak reflectivity in the UV area, indicating good absorption potency of the materials.
The energy loss factor L(ω) offers valuable insights into the dissipation of optical energy throughout the scattering mechanism when the electron traverses a material or is dissipated by being heated, in addition to absorbance and reflectivity. Figure 9d shows the calculated L(ω) for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6. Scattering is not detected for photonic energy below band gaps, indicating no energy loss. L(ω) is insignificant for Rb2CuAsCl6 and Rb2CuAsBr6, showing peak values of 0.73 and 0.30, respectively, in visible and UV areas, indicating negligible Plasmon losses. However, Rb2CuAsF6 exhibits the highest peak value of 3.38 in the visible region, which is significantly higher than Rb2CuAsCl6, and Rb2CuAsBr6. Thus, reduced R(ω) and L(ω) improve electrical transport and increase the photoelectric yield of the materials used in photovoltaic (PV) and optoelectronic devices.
3.6 Electron Transport Properties
To address ecological and energy challenges, scientists are focusing on developing efficient thermoelectric components to generate electricity through wasted heat [71, 72]. An effective thermoelectric compound is determined by a figure of merit calculated using the formula: ZT = S2σT/k, where ZT is dependent on factors such as the material's electrical conductivity, heat transfer, and the Seebeck coefficient. Thermoelectric factors including power factor, carrier concentration, Hall coefficient, heat capacity, and figure of merit are analyzed between 100 and 600 K and the relaxation period (τ) is 5 × 10−15 s.
Electrons need to transition from the valence band to the conduction band in order to generate free carriers for n-type materials and holes for p-type materials [73]. Therefore, the charge carrier’s mobility is determined by its electrical conductivity (σ/τ), as shown in Fig. 10a. An increase in electrical conductivity is seen in Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 from (3.29, 2.91, and 2.41 × 1018 Ω−1 m−1 s−1) at 100 K to (6.30, 5.30, and 4.94 × 1018 Ω−1 m−1 s−1) at 600 K, respectively. The gradual increase in electrical conductivity indicates that the material exhibits semiconductor properties [74]. Moreover, the electrical conductivity at room temperature, shows that Cs2CuAsCl6 (2.51 × 1018 Ω−1 m−1 s−1) exhibits better conductivity than Rb2CuAsCl6 (1.87 × 1018 Ω−1 m−1 s−1) and Rb2CuAsBr6 (1.64 × 1018 Ω−1 m−1 s−1). This demonstrates that in solar cells, the heat lost through scattering processes may enhance electrical conductivity, potentially boosting the power conversion efficiency of the solar cells.
The Seebeck coefficient (S) quantifies the voltage generated resulting from the junction of the two distinct materials having different temperatures and influences thermoelectric efficiency [75]. Figure 10c shows that the values of the Seebeck coefficient decrease as temperature increases because of the disruption of atomic bonding. The positive number implies that a large proportion of carriers are holes, categorizing the researched compounds as p-type semiconductors. Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 compounds have Seebeck values of 271.7 µV K−1, 254.6 µV K−1, and 272.4 µV K−1 at 100 K. This value decreases to 209.1 µV K−1, 226.7 µV K−1, and 234.6 µV K−1 at 600 K. Subsequently, decrease in S as temperature rises, indicating a steady reduction in the diffusion of agitating charges with increasing temperature [71]. The Seebeck coefficient value of Rb2CuAsBr6 (263.55 µV K−1) at room temperature is higher than Rb2CuAsCl6 (259.3 µV K−1) Rb2CuAsF6 (250.6 µV K−1).
The thermal conductivity determined by considering just the electrical portion is shown in Fig. 10c. A linear increase in thermal conductivity (κe/τ) from 100 to 600 K is observed for all materials. The κe/τ for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 increases from (3.22, 2.40, and 2.17 × 1014 Ω−1 m−1 s−1) at 100 K to (2.04, 2.06, and 2.05 × 1014 Ω−1 m−1 s−1) at 600 K, respectively. Moreover, Rb2CuAsF6 exhibits the highest thermal conductivity of 5.88 × 1014 W mks−1 at room temperature, followed by Rb2CuAsCl6 (4.78 × 1014 W mks−1), and Rb2CuAsBr6 (4.36 × 1014 W mks−1). These compounds are ideal for thermoelectric devices because of the thermal conductivity differential factor of 10−5 from electrical conductivity.
The power factor of a material, crucial for determining its thermoelectric effectiveness, is calculated using the formula PF = σS2, where σ represents electrical conductivity and S is the Seebeck coefficient of materials. The power factor increases with temperature, ranging from (0.24,0.19, and 0.18) × 1011 W mK2s−1 at 100 K to (2.76, 2.73, and 2.72) × 1011 W mK2s−1 at 800 K in the sequence of Rb2CuAsF6 > Rb2CuAsCl6 > Rb2CuAsBr6, as seen in Fig. 10d. It is clear that the compounds are sustainable when used at high temperatures since their power factor increases as the temperature rises.
The specific heat capacity (Cv) of materials is formed by charged carriers as well as some phonon contribution. The specific heat capacity for both compounds exhibits a sharp rise with increasing temperature. This rise is in accordance with the Debye model since Cv is proportional to T3. The value of Cv rises from 0.22 J molK−1, 0.09 J molK−1, and 0.09 J molK−1 to 7.51 J molK−1, 2.34 J molK−1, and 1.92 J molK−1 for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6. The value of Cv is significantly higher for Rb2CuAsF6 than Rb2CuAsCl6 and Rb2CuAsBr6 which demonstrates that Rb2CuAsF6 has greater capacity of heat absorption.
The carrier concentration (n) is a key parameter to govern the thermal conductivity of a material. Materials having greater carrier concentration might have enhanced electrical conductivity. Figure 11b illustrates a rise in charge concentration from 0.0036 to 0.14 e μc−1 for Rb2CuAsF6, from 0.0021 to 0.055 e μc−1 for Rb2CuAsCl6, and from 0.0017 to 0.047 e μc−1 for Rb2CuAsBr6 as the temperature rises from 100 to 600 K. This increase in carrier concentration with temperature is attributed to the excitation of charge carriers at elevated temperatures.
The computed Hall coefficient (RH) at various temperatures for Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 is displayed in Fig. 11c. This shows that the RH value is highest at lower temperatures and declines rapidly as the temperature increases, eventually approaching zero for all materials. The Hall Effect is the creation of a current in a semiconductor caused by the presence of opposing charge densities at both sides. Higher temperatures lead to an increase in thermal vibrations which results in increased dispersion and a drop in the values of the Hall coefficient. The positive values of RH further indicate a p-type semiconductor character of Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 perovskites.
3.6.1 Figure of Merit
The figure of merit is a crucial characteristic that dictates the effectiveness of thermoelectric systems. Figure 11d illustrates the variation of the figure of merit with temperature up to 600 K for Rb2CuAsX6 (X = F, Cl, Br). The ZT = 1 is regarded as the ideal efficiency provided by any material [76]. Throughout the studied temperature range (100–600 K), the ZT value of Rb2CuAsF6 and Rb2CuAsCl6 increases from 0.75 to 0.81 and 0.78 to 0.79, respectively, whereas for Rb2CuAsBr6, slightly decreases from 0.82 to 0.79. The increased ZT for Rb2CuAsF6 and Rb2CuAsCl6 is justified by greater electrical conductivity and the Seebeck coefficient [77, 78]. Meanwhile, the decrease in ZT for Rb2CuAsBr6 can be attributed to the increase in heat losses at elevated temperatures [79]. Moreover, at room temperature, Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 revealed ZT values of 0.80, 0.79, and 0.78, respectively. Our research anticipates the discovery of these new materials with improved thermoelectric performance, making them promising candidates for innovative thermoelectric systems.
4 Conclusion
This work utilized density functional theory (DFT) to evaluate the structural, thermodynamic, elastic, electro-optic, and electron transport characteristics of Rb2CuAsX6 (X = F, Cl, Br) perovskites. The substitution of halide ions reveals the variation in mechanical, thermodynamic, optical, and thermoelectric response of studied compounds. The investigation of the elastic parameters and formation energy has confirmed that the examined perovskites are cubic in structure, stable, and ductile. The thermodynamic characteristics revealed the stability at elevated temperatures and suitability for industrial applications. The anisotropy and mechanical strength decrease in the order: Rb2CuAsF6 > Rb2CuAsCl6 > Rb2CuAsBr6. The band structure computations identified Rb2CuAsF6, Rb2CuAsCl6, and Rb2CuAsBr6 are p-type semiconductors with indirect band gaps of 1.25 eV, 1.10, and 0.83 eV, respectively. The values of Eg decrease during the replacement of F with Cl and Br. The optical characteristics demonstrated strong optical absorption and minimal energy loss in the visible and ultraviolet spectrum which highlights their suitability for solar energy conversion. Additionally, thermoelectric characteristics including higher power factor and figure of merit (ZT) values of 0.8, 0.79, 0.78, respectively, at room temperature were determined. Comparatively, Rb2CuAsF6 demonstrates superior absorption and ZT values than Rb2CuAsCl6 and Rb2CuAsBr6. Our research anticipates these perovskite combinations exhibit superior photoelectric and heat conversion efficiency, rendering them potential prospects for advanced solar cells and thermoelectric devices.
Data Availability
The corresponding author will provide the data generated during the study upon a reasonable request.
References
N.S. Lewis, D.G. Nocera, Powering the planet: chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. 103(43), 15729–15735 (2006)
M. Amin, H.H. Shah, A.G. Fareed, W.U. Khan, E. Chung, A. Zia, Z.U.R. Farooqi, C. Lee, Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 47(77), 33112–33134 (2022)
A. Shah, P. Torres, R. Tscharner, N. Wyrsch, H. Keppner, Photovoltaic technology: the case for thin-film solar cells. Science 285(5428), 692–698 (1999)
K. Maeda, K. Domen, Photocatalytic water splitting: recent progress and future challenges. J. Phys. Chem. Lett. 1(18), 2655–2661 (2010)
D. Beretta, N. Neophytou, J.M. Hodges, M.G. Kanatzidis, D. Narducci, M. Martin-Gonzalez, M. Beekman, B. Balke, G. Cerretti, W. Tremel, A. Zevalkink, Thermoelectrics: from history, a window to the future. Mater. Sci. Eng. 138, 100501 (2019)
H. Zhou, Q. Chen, G. Li, S. Luo, T.B. Song, H.S. Duan, Z. Hong, J. You, Y. Liu, Y. Yang, Interface engineering of highly efficient perovskite solar cells. Science 345(6196), 542–546 (2014)
J. Burschka, N. Pellet, S.J. Moon, R. Humphry-Baker, P. Gao, M.K. Nazeeruddin, M. Grätzel, Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499(7458), 316–319 (2013)
W.J. Yin, B. Weng, J. Ge, Q. Sun, Z. Li, Y. Yan, Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 12(2), 442–462 (2019)
Q.A. Akkerman, M. Gandini, F. Di Stasio, P. Rastogi, F. Palazon, G. Bertoni, J.M. Ball, M. Prato, A. Petrozza, L. Manna, Strongly emissive perovskite nanocrystal inks for high-voltage solar cells. Nat. Energy 2(2), 1–7 (2016)
G.E. Eperon, G.M. Paternò, R.J. Sutton, A. Zampetti, A.A. Haghighirad, F. Cacialli, H.J. Snaith, Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 3(39), 19688–19695 (2015)
T. Krishnamoorthy, H. Ding, C. Yan, W.L. Leong, T. Baikie, Z. Zhang, M. Sherburne, S. Li, M. Asta, N. Mathews, S.G. Mhaisalkar, Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 3(47), 23829–23832 (2015)
P. Ramasamy, D.H. Lim, B. Kim, S.H. Lee, M.S. Lee, J.S. Lee, All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 52(10), 2067–2070 (2016)
W. Zhang, G.E. Eperon, H.J. Snaith, Metal halide perovskites for energy applications. Nat. Energy 1(6), 1–8 (2016)
X. Wu, High-efficiency polycrystalline CdTe thin-film solar cells. Solar Energy 77(6), 803–814 (2004)
B. Albiss, M. Al-Widyan, Numerical simulation, preparation, and evaluation of Cu (In, Ga) Se2 (CIGS) thin-film solar cells. ChemEngineering 7(5), 87 (2023)
M.M.A. Moon, M.F. Rahman, J. Hossain, A.B.M. Ismail, Comparative study of the second generation a-Si: H, CdTe, and CIGS thin-film solar cells. Adv. Mater. Res. 1154, 102–111 (2019)
K. Assiouan, A. Marjaoui, J.E. Khamkhami, M. Zanouni, H. Ziani, A. Bouchrit, A. Achahbar, Theoretical investigation of Rb2AuBiX6 (X = Br, cl, F) double perovskite for thermoelectric and optoelectronic applications. J. Phys. Chem. Solids 188, 111890 (2024)
T. Leijtens, G.E. Eperon, S. Pathak, A. Abate, M.M. Lee, H.J. Snaith, Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 4(1), 2885 (2013)
A.H. Slavney, R.W. Smaha, I.C. Smith, A. Jaffe, D. Umeyama, H.I. Karunadasa, Chemical approaches to addressing the instability and toxicity of lead–halide perovskite absorbers. Inorg. Chem. 56(1), 46–55 (2017)
A. Babayigit, A. Ethirajan, M. Muller, B. Conings, Toxicity of organometal halide perovskite solar cells. Nat. Mater. 15(3), 247–251 (2016)
M.R. Jani, M.T. Islam, S.M. Al Amin, M.S.U. Sami, K.M. Shorowordi, M.I. Hossain, S. Chowdhury, S.S. Nishat, S. Ahmed, Exploring solar cell performance of inorganic Cs2TiBr6 halide double perovskite: a numerical study. Superlatt. Microstruct. 146, 106652 (2020)
L.Z. Lei, Z.F. Shi, Y. Li, Z.Z. Ma, F. Zhang, T.T. Xu, Y.T. Tian, D. Wu, X.J. Li, G.T. Du, High-efficiency and air-stable photodetectors based on lead-free double perovskite Cs2AgBiBr6 thin films. J. Mater. Chem. C 6(30), 7982–7988 (2018)
M.A. Khan, H.A. Alburaih, N.A. Noor, A. Dahshan, Comprehensive investigation of opto-electronic and transport properties of Cs2ScAgX6 (X = Cl, Br, I) for solar cells and thermoelectric applications. Solar Energy 225, 122–128 (2021)
E.T. McClure, M.R. Ball, W. Windl, P.M. Woodward, Cs2AgBiX6 (X = Br, Cl): new visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 28(5), 1348–1354 (2016)
Y. Xu, T. Gong, J.N. Munday, The generalized Shockley–Queisser limit for nanostructured solar cells. Sci. Rep. 5(1), 13536 (2015)
A. Ayyaz, G. Murtaza, A. Ahmed, S.M. Ramay, A. Usman, G. Farid, M. Naeem, Comparative DFT-based investigation of physical properties of Cs2MBiBr6 (M = Ag, Cu, and Au) Perovskites: sustainable materials for renewable energy. Comput. Condens. Matter. 38, e00885 (2024)
A. Ayyaz, G. Murtaza, M. Umer, A. Usman, H.H. Raza, Structural, elastic, optoelectronic, and transport properties of Na-based halide double perovskites Na2CuMX6 (M = Sb, Bi, and X = Cl, Br) as renewable energy materials: a DFT insight. J. Mater. Res. 38(20), 4609–4624 (2023)
A. Ayyaz, G. Murtaza, M. Naeem, A. Usman, S.M. Ramay, M. Irfan, H. Irfan, DFT exploration of elastic, optoelectronic, and thermoelectric properties of stable and eco-friendly double perovskites Cs2YAuX6 (X = Cl, Br) for green energy applications. J. Phys. Chem. Solids 188, 111936 (2024)
D.Y. Hu, X.H. Zhao, T.Y. Tang, L.M. Lu, L. Li, L.K. Gao, Y.L. Tang, Exploring the structural, electronic and optical properties of vacancy-ordered double perovskites Cs2TlAsX6 (X = I, Br, Cl) based on first-principles. Phys. Lett. A 427, 127917 (2022)
D.Y. Hu, X.H. Zhao, T.Y. Tang, L. Li, Y.L. Tang, Insights on structural, elastic, electronic and optical properties of double-perovskite halides Rb2CuBiX6 (X = Br, Cl). J. Phys. Chem. Solids 167, 110791 (2022)
G. Nazir, Q. Mahmood, M. Hassan, Tuning of band gap by anions (Cl, Br, I) of double perovskites Rb2AgAsX6 (Cl, Br, I) for solar cells and thermoelectric applications. Phys. Scripta 98(2), 025811 (2023)
S. Charef, A. Assali, A. Boukortt, Optoelectronic and thermoelectric properties of novel double halide perovskites Na2AgAsX6 (X = Cl, Br) for efficient green solar cells. Mater. Today Commun. 38, 108065 (2024)
P. Blaha, K. Schwarz, G.K. Madsen, D. Kvasnicka, J. Luitz, wien2k. An augmented plane wave+ local orbitals program for calculating crystal properties, 60(1) (2001)
Z. Wu, R.E. Cohen, More accurate generalized gradient approximation for solids. Phys. Rev. B 73(23), 235116 (2006)
G. Rehman, M. Shafiq, Saifullah, R. Ahmad, S. Jalali-Asadabadi, M. Maqbool, I. Khan, H. Rahnamaye-Aliabad, I. Ahmad, Electronic band structures of the highly desirable III–V semiconductors: TB-mBJ DFT studies. J. Electron. Mater. 45, 3314–3323 (2016)
A. Ayyaz, G. Murtaza, M. Shafiq, M.Q. Shah, N. Sfina, S. Ali, Exploring structural, thermodynamic, elastic, electro-optic, and thermoelectric characteristics of double perovskites Rb2XInBr6 (X = Na, K) for photovoltaic applications: a DFT approach. Solar Energy 265, 112131 (2023)
V. Lucarini, J.J. Saarinen, K.E. Peiponen, E.M. Vartiainen, Kramers–Kronig Relations in Optical Materials Research (Springer, Cham, 2005)
G.K. Madsen, D.J. Singh, BoltzTraP. A code for calculating band-structure dependent quantities. Comput. Phys. Commun. 175(1), 67–71 (2006)
A. Otero-de-la-Roza, D. Abbasi-Pérez, V. Luaña, Gibbs A new version of the quasiharmonic model code. II. Models for solid-state thermodynamics, features and implementation. Comput. Phys. Commun. 182(10), 2232–2248 (2011)
J. Tao, J.P. Perdew, H. Tang, C. Shahi, Origin of the size-dependence of the equilibrium van der Waals binding between nanostructures. J. Chem. Phys. (2018). https://doi.org/10.1063/1.5018572
G.S. Painter, Improved correlation corrections to the local-spin-density approximation. Phys. Rev. B 24(8), 4264 (1981)
V.G. Tyuterev, N. Vast, Murnaghan’s equation of state for the electronic ground state energy. Comput. Mater. Sci. 38(2), 350–353 (2006)
W. Travis, E.N.K. Glover, H. Bronstein, D.O. Scanlon, R.G. Palgrave, On the application of the tolerance factor to inorganic and hybrid halide perovskites: a revised system. Chem. Sci. 7(7), 4548–4556 (2016)
A.E. Fedorovskiy, N.A. Drigo, M.K. Nazeeruddin, The role of Goldschmidt’s tolerance factor in the formation of A2BX6 double halide perovskites and its optimal range. Small Methods 4(5), 1900426 (2020)
M.A. Ali, A.A. Alothman, M. Mushab, A. Khan, M. Faizan, DFT insight into structural, electronic, optical and thermoelectric properties of eco-friendly double perovskites Rb2GeSnX6 (X = Cl, Br) for green energy generation. J. Inorg. Organometall. Polym. Mater. 33(11), 3402–3412 (2023)
R. Ullah, M.A. Ali, A. Khan, R.A. Alshgari, M.S.S. Mushab, A. Samad, Effect of cation exchange on structural, electronic, magnetic and transport properties of Ba2MReO6 (M = In, Gd). J. Magn. Magn. Mater. 546, 168816 (2022)
M.A. Blanco, A.M. Pendás, E. Francisco, J.M. Recio, R. Franco, Thermodynamical properties of solids from microscopic theory: applications to MgF2 and Al2O3. J. Mol. Struct. 368, 245–255 (1996)
E. Francisco, J.M. Recio, M.A. Blanco, A.M. Pendás, A. Costales, Quantum-mechanical study of thermodynamic and bonding properties of MgF2. J. Phys. Chem. A 102(9), 1595–1601 (1998)
S.A. Dar, V. Srivastava, U.K. Sakalle, V. Parey, Ferromagnetic phase stability, magnetic, electronic, elasto-mechanical and thermodynamic properties of BaCmO3 perovskite oxide. J. Electron. Mater. 47, 3809–3816 (2018)
S.A. Mir, S. Yousuf, D.C. Gupta, First principle study of mechanical stability, magneto-electronic and thermodynamic properties of double perovskites: A2MgWO6 (A = Ca, Sr). Mater. Sci. Eng. B 250, 114434 (2019)
S.A. Dar, V. Srivastava, S.N. Tripathi, U.K. Sakalle, A complete DFT description on structural, electronic, elastic, mechanical and thermodynamic properties of some intermetallic AuX2 (X = Al, Ga, In) compounds. Eur. Phys. J. Plus 133(12), 541 (2018)
J. Luo, A. Yang, Z. Xie, First-principles study on the direct bandgap double perovskite series Cs2LiInX6 (X = F, Cl, and Br). ACS Omega 6(48), 32408–32416 (2021)
M.S. Islam, R. Ahmed, M. Mahamudujjaman, R.S. Islam, S.H. Naqib, A comparative study of the structural, elastic, thermophysical, and optoelectronic properties of CaZn2X2 (X = N, P, As) semiconductors via ab-initio approach. Results Phys. 44, 106214 (2023)
Z.J. Wu, E.J. Zhao, H.P. Xiang, X.F. Hao, X.J. Liu, J. Meng, Crystal structures and elastic properties of superhard Ir N2 and Ir N3 from first principles. Phys. Rev. B 76(5), 054115 (2007)
J.C. Tan, B. Civalleri, C.C. Lin, L. Valenzano, R. Galvelis, P.F. Chen, T.D. Bennett, C. Mellot-Draznieks, C.M. Zicovich-Wilson, A.K. Cheetham, Exceptionally low shear modulus in a prototypical imidazole-based metal-organic framework. Phys. Rev. Lett. 108(9), 095502 (2012)
F. Mouhat, F.X. Coudert, Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 90(22), 224104 (2014)
G. Feng, X. Jiang, W. Wei, P. Gong, L. Kang, Z. Li, Y. Li, X. Li, X. Wu, Z. Lin, W. Li, High pressure behaviour and elastic properties of a dense inorganic–organic framework. Dalton Trans. 45(10), 4303–4308 (2016)
S. Kojima, Poisson’s ratio of glasses, ceramics, and crystals. Materials 17(2), 300 (2024)
Q. Long, X. Nie, S.L. Shang, J. Wang, Y. Du, Z. Jin, Z.K. Liu, C15NbCr2 laves phase with mechanical properties beyond Pugh’s criterion. Comput. Mater. Sci. 121, 167–173 (2016)
C.M. Zener, S. Siegel, Elasticity and anelasticity of metals. J. Phys. Chem. 53(9), 1468–1468 (1949)
Hajjir Titrian, Ugur Aydin, Martin Friák, Duancheng Ma, Dierk Raabe, Jörg. Neugebauer, Self-consistent scale-bridging approach to compute the elasticity of multi-phase polycrystalline materials. MRS Online Proc. Library 1524, 301–307 (2013)
M. Friák, W.A. Counts, D. Ma, B. Sander, D. Holec, D. Raabe, J. Neugebauer, Theory-guided materials design of multi-phase Ti–Nb alloys with bone-matching elastic properties. Materials 5, 1853–1872 (2012)
L.-F. Zhu, M. Friák, L. Lymperakis, H. Titrian, U. Aydin, A.M. Janus, H.-O. Fabritius et al., Ab initio study of single-crystalline and polycrystalline elastic properties of Mg-substituted calcite crystals. J. Mech. Behav. Biomed. Mater. 20, 296–304 (2013)
T. Ou, Q. Zhuang, H. Yan, S. Feng, P. Li, X. Ma, Lead-free halide double perovskites Rb2InSbX6 (X = F, Cl, Br, I): a first-principles study of structural and optoelectrical properties. Chem. Phys. 573, 112015 (2023)
A. Boutramine, S. Al-Qaisi, S. Samah, N. Iram, T.A. Alrebdi, S. Bouzgarrou, A.S. Verma, S. Belhachi, R. Sharma, Optoelectronic and thermoelectric properties of new lead-free K2NaSbZ6 (Z = Br, I) halide double-perovskites for clean energy applications: a DFT study. Opt. Q. Electron. 56(3), 417 (2024)
M. Hilal, B. Rashid, S.H. Khan, A. Khan, Investigation of electro-optical properties of InSb under the influence of spin-orbit interaction at room temperature. Mater. Chem. Phys. 184, 41–48 (2016)
M. Gajdoš, K. Hummer, G. Kresse, J. Furthmüller, F.J.P.R.B. Bechstedt, Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 73(4), 045112 (2006)
G. Grüner, Electrodynamics of Solids: Optical Properties of Electrons in Matter (Cambridge University Press, Cambridge, 2002)
D.R. Penn, Wave-number-dependent dielectric function of semiconductors. Phys. Rev. 128(5), 2093 (1962)
S. Menakh, B. Daoudi, A. Boukraa, K. Ferkous, First-principles calculations to investigate structural, elastic, electronic and optical properties of A2OsH6 for storage hydrogen and optoelectronic devices. Comput. Condens. Matter 31, e00684 (2022)
M.Q. Shah, M. Shafiq, A. Naeem, G. Murtaza, A. Ayyaz, A. Usman, S.M. Deen, M.A. El-Sheikh, Effect of position occupancy of different elements on the structural stability, optoelectronic, thermoelectric and elastic properties of Cs2CuAsX6 (X: Cl, Br, I) halide double perovskite: DFT analysis. Mater. Sci. Semicond. Process. 174, 108187 (2024)
S. Hu, Z. Ren, Metal halide perovskites as emerging thermoelectric materials. ACS Energy Lett. 6(11), 3882–3905 (2021)
S.A. Khandy, D.C. Gupta, Electronic structure, magnetism and thermoelectric properties of double perovskite Sr2HoNbO6. J. Magn. Magn. Mater. 458, 176–182 (2018)
N.A. Noor, Q. Mahmood, M. Rashid, B.U. Haq, A. Laref, The pressure-induced mechanical and optoelectronic behavior of cubic perovskite PbSnO3 via ab-initio investigations. Ceram. Int. 44(12), 13750–13756 (2018)
G.M. Mustafa, A. Slam, S. Saba, N.A. Noor, M.W. Iqbal, A. Dahshan, Optoelectronic and thermoelectric characteristics of halide based double perovskites K2YAgX6 (X = Br, I) for energy storage applications. Polyhedron 229, 116184 (2023)
R.A. Kishore, A. Nozariasbmarz, B. Poudel, S. Priya, High-performance thermoelectric generators for field deployments. ACS Appl. Mater. Interfaces 12(9), 10389–10401 (2020)
G.M. Mustafa, S. Saba, Q. Mahmood, N.A. Kattan, N. Sfina, T. Alshahrani, A. Mera, G.A. Mersal, M.A. Amin, Study of optoelectronic, thermoelectric, mechanical properties of double perovskites Cs2AgAsX6 (X = cl, br, I) for solar cells and energy harvesting. Opt. Q. Electron. 55(6), 527 (2023)
J.Y. Al-Humaidi, A. Ullah, N.U. Khan, J. Iqbal, S. Khan, A. Algahtani, V. Tirth, T. Al-Mughanam, M.S. Refat, A. Zaman, First-principle insight into the structural, electronic, elastic and optical properties of Cs-based double perovskites Cs2XCrCl6 (X = K, Na). RSC Adv. 13(30), 20966–20974 (2023)
A. Kaltzoglou, P. Falaras, Recent developments on hybrid perovskite materials for solar energy conversion and environmental protection. Current Opin. Chem. Eng. 33, 100708 (2021)
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number RGP2/358/44. This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R7), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
The funding source is not available.
Author information
Authors and Affiliations
Contributions
Ahmad Ayyaz: Conceptualization, Writing-original draft, Review & editing. Figures and Tables G. Murtaza: Formal analysis, Supervision. Youssef Bakkour: Validation, Visualization. Murefah mana Al-Anazy: Data curation, Investigation, Software.
Corresponding authors
Ethics declarations
Conflict of interest
The authors do not have any known conflict of interest in the publication of this work.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ayyaz, A., Murtaza, G., Bakkour, Y. et al. Impact of Halide Ion Occupancy on Thermodynamic, Mechanical, Electro-optic, and Electron Transport Characteristics of Rb2CuAsX6 (X = F, Cl, Br) Double Perovskites Using Density Functional Theory. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03079-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03079-3