Abstract
In this work, we synthesized a series of CuCo2S4/g-C3N4 (CSC) nanocomposites by a facile solvothermal approach using D-penicillamine as sulphur source. The phase structure, morphology, chemical composition, and optical properties were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), high resolution transmission electron microscopy (HR-TEM), X-ray photoelectron spectroscopy (XPS), UV–vis diffuse reflectance spectroscopy (UV–vis DRS). The photocatalytic activities of the synthesized CSC nanocomposites were evaluated using degradation of methylene blue (MB) dye under visible-light irradiation. The light absorption capacity and photocatalytic activity of CuCo2S4 were enhanced by the successful incorporation with g-C3N4. The nanocomposite with 30% loading of g-C3N4 in CuCo2S4 exhibited maximum degradation efficiency compared to the pure CuCo2S4 towards the degradation of MB dye under the light irradiation. A possible mechanism for enhanced photocatalytic activity towards the pollutant degradation by the nanocomposites was proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
At present, the survival of the human society is threatened by various forms of organic pollutants and water scarcity. Water pollution due to rapid industrialization is one of the most severe problems associated with the depletion of fresh water resources. Improper discharges of dyes, pesticides, personal care products, pharmaceutical effluents, and etc. are the major sources of water pollutants [1,2,3,4]. Among the various wastewater treatment processes, advanced oxidation processes have been proven to be the efficient method for the degradation of pollutants in wastewater. In the past few decades, the semiconductors like TiO2 [5] and ZnO [6] have been extensively applied in photocatalytic processes, due to their unique properties like non-toxicity and photostability. Nonetheless, these materials possess insufficient separation efficiency for the photoinduced electron–hole pairs and poor visible-light absorption ability and so they were excluded from the practical applications. In order to utilize more visible-light illumination, which constitutes large part of the solar spectrum, materials with narrow band gaps are preferred [7,8,9]. Nowadays, extensive researches have been carried out in this field to design more efficient photocatalysts with substantial activity under the solar illumination.
Recently, various metal sulphides such as CuInS2 [10], Cu2ZnSnS4 [11], SnCoS4 [12] and CuSnS3 [13] have been extensively studied in the field of energy and environment. Among them, CuCo2S4, a binary metal sulphide photocatalyst has recently emerged in various energy storage applications, due to its unique electrical, magnetic, and catalytic properties [14]. To decrease high recombination rate of the photoexcited charge carriers in CuCo2S4, it should be combined with another appropriate semiconductors having matched band energies. Since 2009, it was found that graphitic carbon nitride (g-C3N4) is a metal-free semiconductor with high photocatalytic performance under visible light. Facile synthesis, good physicochemical stability, and narrow band gap of 2.7 eV are appealing properties of g-C3N4 to be a promising photocatalyst to address energy and environmental crises [15, 16]. Recently, many studies revealed that g-C3N4 incorporated semiconductors show good degradation efficiency than commercial ZnO and TiO2 photocatalysts. In this light, many heterojunction photocatalysts such as CaFe2O4/g-C3N4 [17], Co0.5Cd0.5S/g-C3N4 [18], CuCr2O4/g-C3N4 [19], and Ag2WO4/g-C3N4 [20] have been successfully applied in the photocatalytic processes.
In this work, we have prepared CuCo2S4/g-C3N4 nanocomposites by a simple solvothermal method using D-penicillamine as sulphur source. The fabricated photocatalysts was applied for photocatalytic degradation of methylene blue (MB) under visible-light illumination and the improved activity was obtained for the nanocomposite relative to the pure CuCo2S4. To the best of our knowledge, there is not any report about photocatalytic activity of CuCo2S4/g-C3N4 nanocomposites under visible light.
2 Experimental
2.1 Synthesis of g-C3N4
Ten grams of melamine was placed in a semi closed silica crucible and heated at 550 °C in a muffle furnace in air atmosphere for 4 h to obtain a pale yellow powder and finally washed with dil. HNO3 for further use [17].
2.2 Synthesis of CuCo2S4/g-C3N4 Nanocomposites (CSC)
The CSC nanocomposites were synthesized by simple solvothermal method. Stochiometric amount of synthesized g-C3N4 was dispersed in 40 ml of ethylene glycol through ultrasonication process for 30 min by a digital ultrasonic cleaner (bath type, India), with power of 150W and frequency of 40 kHz. Then 1 mmol of Cu(NO3)2·3H2O, 2 mmol of Co(NO3)2·6H2O and 4 mmol of D-penicillamine were added into above suspension with continuous stirring for 30 min under room temperature. Finally the mixture was transferred into a Teflon lined autoclave and heated at 200 °C for 24 h and cooled naturally to obtain the black precipitate. Then, the precipitate was washed three times with deionized water and dried in vacuum at 80 °C overnight. The g-C3N4 loading percentages were 10, 20, and 30% and they denoted as CSC10, CSC20, and CSC30 nanocomposites, respectively. The pure CuCo2S4 was also prepared under same condition without the addition of g-C3N4.
2.3 Characterization
The phase structure and purity were analysed using X-ray diffraction (XRD). The XRD patterns were recorded using X-ray diffractometer (Rigaku) with Cu Kα radiation (λ = 1.5418 Å) at a scan rate of 2 °C/min. Morphology of the samples was observed with a field emission scanning electron microscopy, Zeiss EVO18 electron microscope and a transmission electron microscopy, JEOL JEM 2100 microscope. The chemical composition of the samples investigated by X-ray photoelectron spectroscopy (XPS) equipped with X-ray source with energy of 1489.7 eV, using Scienta omicron GMBH instrument. UV–vis diffuse reflectance spectra (UV–vis DRS) were analysed using Jasco V-750 diffuse reflectance spectrophotometer. The change in dye concentration during the degradation process was analysed by a Shimadzu UV-1700 spectrophotometer.
2.4 Photocatalytic Studies
The photocatalytic studies were carried out in a typical visible-light photo reactor under room temperature using 300 W halogen lamp. In a typical process, 50 mg of the photocatalyst was suspended in 100 mL of 10 mg L−1 of MB solution and dispersed by ultrasonication. After that, the solution was stirred under dark condition for 20 min to attain the adsorption–desorption equilibrium between the dye solution and the photocatalyst. Then the light source was turned on to study the photodegradation reaction. In different reaction courses, 2 mL of the suspension was withdrawn and the suspension was centrifuged at 5000 rpm to obtain the clear solution and the absorbances were analysed using the spectrophotometer.
3 Results and Discussion
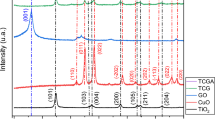
The powder XRD patterns of CuCo2S4 and CSC nanocomposites are illustrated in Fig. 1a, b. The characteristic peak of pure g-C3N4 at 27.5° attributes to the (002) plane (Fig. 1a). For the CuCo2S4 powder, the diffraction peaks at 26.6°, 31.3°, 38.0°, 47.0°, 49.9°, and 54.8° are corresponding to the (022), (113), (004), (224), (115), and (044) planes of cubic CuCo2S4, respectively (JCPDS Card no. 42-1450) [21]. There is no other impurity peak corresponding to D-penicillamine, which ascertains the phase purity of the synthesized samples. Furthermore, the peaks of both g-C3N4 and CuCo2S4 co-existed in the CSC nanocomposites, which confirm that g-C3N4 was successfully incorporated into CuCo2S4 matrix (Fig. 1b).
The FT-IR spectra of g-C3N4, CuCo2S4, and CSC nanocomposites are illustrated in Fig. 2. The broad band around 3100–3300 cm−1 corresponds to the N–H stretching vibration. In addition, the peak at 812 cm−1 corresponds to the breathing mode of s-triazine, whereas the peaks in the region of 1240–1650 cm−1 are due to the stretching vibrations of aromatic C–N heterocycles (Fig. 2a) [22,23,24,25]. For the pure CuCo2S4, the peak at 623 cm−1 is ascribed to the vibration of Cu-S and the peak at 1243 cm−1 is related to the Co-S bond [26]. In Fig. 2c, all the characteristic peaks of g-C3N4 and CuCo2S4 are observed, which further confirms successful incorporation of g-C3N4 and CuCo2S4 counterparts.
Morphology of the fabricated samples was investigated by FESEM analysis. Figure 3a, b shows the FESEM image of pure CuCo2S4, which confirms that the sample is composed of spongy sphere like morphology. Figure 3c shows that the bulk g-C3N4 has composed of unique sheet like morphology. Figure 3d displays anchoring of CuCo2S4 particles on the surface of g-C3N4 sheets, which prevents further agglomeration of CuCo2S4 particles. This is highly beneficial for the superior charge separation in the CuCo2S4 particles. Thus FESEM and HRTEM studies are in good agreement with XRD and FT-IR analysis. The SAED pattern of the CSC30 nanocomposite in Fig. 3f indicates the polycrystalline nature of CuCo2S4 with interplanar distance 0.279 nm, which perfectly matched with (004) diffraction patterns of CuCo2S4.
The composition of the prepared the CSC30 nanocomposite was analysed by the EDS elemental technique. Figure 4a–g clearly indicates the presence of only C, N, Cu, Co, and S elements. The signals for C and N elements originated from g-C3N4 and the peaks for Cu, Co, and S elements are from CuCo2S4 counterparts of the nanocomposite.
To further confirm the composition and chemical states of the CSC30 nanocomposite, XPS analysis was carried out.
The survey scan displays the presence of main elements including Cu, Co, S, C, and N, which further confirms the results of EDX analysis (Fig. 5a). The high resolution XPS spectrum of Cu 2p shows broad peaks at 932.7 and 952.7 eV correspond to Cu p3/2 and Cu 2p1/2, which indicates the oxidation state of Cu is +2 (Fig. 5b). The spectrum of Co 2p (Fig. 5c) shows two peaks at 778 and 793.2 eV correspond to Co 2p3/2 and Co 2p1/2, which confirms the existence of both Co2+ and Co3+ oxidation states of Co. In the S 2p spectrum (Fig. 5d), the peak at 162 eV indicates the existence of sulphur in S2− oxidation state [21]. In C 1 s spectrum (Fig. 5e), peak at 284.5 eV corresponds to C–C bond and the second peak at 287 eV belongs to sp3 hybridised carbon in C–N group of g-C3N4. The N 1 s spectrum (Fig. 5f) is deconvoluted into three different peaks. The peak observed at 397.9 eV corresponds to the sp2 hybridised aromatic N atom (C–N=C) and the peak at 399.7 eV belongs to the N atom that involved in the bridging of three carbon atom N–(C)3. Finally the peak observed at 402.3 eV corresponds to the amino C–N–H group [27]. Thus, the elemental composition and chemical states in the CSC30 nanocomposite are confirmed by XPS analysis.
UV–vis DRS technique was used to analyse the optical properties of the prepared samples. The band gap energy of the prepared samples was evaluated using the following equation [28].
where, hv is the photoenergy, α is absorption coefficient, B is proportionality constant, n is related to the transition type and v is the light frequency. As observed in Fig. 6a, the g-C3N4 shows strong absorption with an absorption edge nearly at 460 nm and the CuCo2S4 powder shows substantial absorption in the whole range of visible light. Figure 6b illustrates band gap calculations for the fabricated samples. It is evident that band gaps of the CuCo2S4, g-C3N4, CSC10, CSC20, and CSC30 are 1.4, 2.74, 1.2, 1.06, and 1.01 eV, respectively. The low band gaps of the CSC nanocomposites indicates that these photocatalysts could have remarkable photocatalytic performance under visible light.
The transient photocurrent studies of the CuCo2S4 and CSC30 nanocomposite are shown in Fig. 7. Both of the CuCo2S4 and CSC30 nanocomposite show fast photochemical current responses under visible light.
The CSC30 nanocomposite exhibits the strongest photo-electrochemical activity, whose photocurrent density is higher than that of the pure CuCo2S4. This is mainly due to the proper loading of g-C3N4 on the surface of the CuCo2S4, leading to an effective heterojunction structure which acts as good conducting pathway for electron transportation in the photocatalytic process.
3.1 Photocatalytic Activity
The photocatalytic degradation process was studied by examining the absorbance changes of MB under visible-light irradiation. Figure 8a illustrates temporal UV–vis spectra for MB during the degradation reaction over the CSC30 nanocomposite. As can be seen, the pollutant is nearly degraded after the light irradiation for 180 min. Figure 8b shows the C/C0 of MB as a function of irradiation time over the CuCo2S4 and CSC nanocomposites where C is the concentration with respect to time (t) and C0 is the initial concentration of MB. Figure 8b shows that in the absence of any photocatalyst, there is no degradation of MB dye. It is clear that photocatalytic activity of the nanocomposites are obviously higher than that of the pure CuCo2S4. The degradation efficiency was nearly 90, 88, 80, and 65% for the CSC30, CSC20, CSC10, and CuCo2S4, respectively. Figure 8c shows that the degradation efficiency increases with an increase g-C3N4 loading. This is due to easier transport of the charge carriers created by the effective incorporation of g-C3N4, as confirmed by transient photocurrent studies. The important factor that determines the commercialization of a photocatalyst is the stability and reusability. Hence, recycling experiments were carried out to check stability of the photocatalyst. Three cycles of degradation studies were carried out using over the CSC30 nanocomposite as photocatalyst under the same conditions. The photocatalytic degradation ability of the CSC30 nanocomposite was slightly decreased in the third degradation cycle and it retained nearly 83% of its activity as shown in the (Fig. 8d). Thus, the CSC30 nanocomposite shows good photocatalytic stability and reusability for the photocatalytic applications.
a Temporal UV–vis spectra of MB during the degradation reaction over the CSC30 nanocomposite. b Photocatalytic degradation of MB over the CuCo2S4 and CSC nanocomposites. c Plots for LnC/C0 versus irradiation time for different photocatalysts. d Stability test of the CSC30 nanocomposite towards MB degradation upon three cycles
3.2 Photocatalytic Mechanism
In photocatalytic processes, photocatalytic performance is mainly dependent to the increase in production of electron–hole pairs and suppression of them from fast recombination. Using the relation proposed by Butler and Ginley [29, 30], the conduction band (CB) and valence band (VB) potentials of the semiconductors presented in the CSC nanocomposites were calculated.
where E VB is the VB potential and E CB is the CB potential, Ee is the energy of free electrons on the hydrogen scale, E g is band gap of the semiconductor, and χ is the absolute electronegativity, which calculated by the following equation [31].
where a, b, and c are the number of atoms in the semiconductor. For g-C3N4, E g is 2.74 eV and χ is 4.67 eV. The calculated E CB and E VB are − 1.22 and 1.48 eV, respectively. For the CuCo2S4, E g is 1.4 eV, χ is 5.32 eV, E CB and E VB were calculated to be 0.13 and 1.53 eV, respectively. Under visible-light irradiation, CuCo2S4 and g-C3N4 absorb visible light to produce the charge carriers. The CB edge potential of g-C3N4 is quite higher than that of CuCo2S4. As a consequence, the excited electrons transfer from the CB of g-C3N4 to that of CuCo2S4 and the holes generated in the VB of CuCo2S4 migrate to the VB of g-C3N4. The schematic diagram for the proposed mechanism is illustrated in Fig. 9. Based on this mechanism, electrons are successfully separated from the holes, which leads to the increased photocatalytic activity for the CSC nanocomposites [32, 33].
4 Conclusions
In this work, we synthesized novel biomolecule-assisted method for preparation of the CSC nanocomposites using D-penicillamine by simple solvothermal method and their photocatalytic activities were investigated towards MB degradation under visible light. Different characterization techniques confirmed formation of heterojunction between CuCo2S4 and g-C3N4, which was effective in separation of the photoinduced charge carriers. It was found that photocatalytic performance of the nanocomposites are higher than the pure CuCo2S4 powder. Finally, a realistic mechanism was proposed for the enhanced photocatalytic activity using potential edge energies of the semiconductors presented in the nanocomposite.
References
X. Zhang, S. Li, S. Hu, J. Chen, W. Jiang, J. Zhang, L. Ji, ,L. Cai, Y. Wang, W. Song, J. Liu, Mater. Lett. 185, 50 (2016)
Y. Zhang, G. Zhu, M. Hojamberdiev, J. Gao, J. Hao, J. Zhou, P. Liu, Appl. Surf. Sci. 371, 231 (2016)
Q. Zhang, B. Xu, S. Yuan, M. Zhang, T. Ohno, Catal. Today 284, 27 (2017)
J. Chen, W. Mei, Q. Huang, N. Chen, C. Lu, H. Zhu, J. Chen, W. Hou, J. Alloy. Compd. 688, 225 (2016)
D. Maruthamani, D. Divakar, M. Kumaravel, J. Ind. Eng. Chem. 30, 33 (2015)
A. Senthilraja, B. Subash, B. Krishnakumar, D. Rajamanickam, M. Swaminathan, M. Shanthi, Mat. Sci. Semicon. Proc. 22, 83 (2014)
T. Li, X. Hu, C. Liu, C. Tang, X. Wang, S. Luo, J. Mol. Catal. A-Chem. 425, 124 (2016)
S. Yang, C. Chen, L. Liu, L. Zhu, X. Xu, Mater. Res. Bull. 92, 29 (2017)
C. Yu, K. Wang, P. Yang, S. Yang, C. Lu, Y. Song, S. Dong, J. Sun, J. Sun, Appl. Surf. Sci. 420, 233 (2017)
H. Fakhri, A.R. Mahjoub, A.H. Cheshme Khavar, Appl. Surf. Sci. 318, 65 (2014)
T.Y. Ho, L.Y. Chen, Energy Procedia 61, 2050 (2014)
J. Ye, T. Chen, Q. Chen, W. Chen, Z. Yu, S. Xu, J. Mater. Chem. A 4, 13194 (2016)
S. Vadivel, D. Maruthamani, B. Paul, S.S. Dhar, A. Habibi-Yangjeh, S. Balachandran, B. Saravanakumar, A. Selvakumar, K. Selvam, RSC Adv. 6, 74177 (2016)
R.H. Fath, S.J. Hoseini, New J. Chem. 41, 3392 (2017)
M. Mousavi, A. Habibi-Yangjeh, S.R. Pouran, J. Mater. Sci.: Mater. Electron. (2017). doi.https://doi.org/10.1007/s10854-017-8166-x
Q. Liu, Y. Guo, Z. Chen, Z. Zhang, X. Fang, Appl. Catal. B-Environ. 183, 231 (2016)
S. Vadivel, D. Maruthamani, A. Habibi-Yangjeh, B. Paul, S.S. Dhar, K. Selvam, J. Colloid Interf. Sci. 480, 126 (2016)
X. Fang, J. Song, H. Shi, S. Kang, Y. Li, G. Sun, L. Cui, Int. J. Hydrogen Energ. 42, 5741 (2017)
A. Akhundi, A. Habibi-Yangjeh, J. Colloid Interf. Sci. 504, 697 (2017)
K. Vignesh, M. Kang, Mater. Sci. Eng. B- Adv. 199, 30 (2015)
J. Tang, Y. Ge, J.F. Shen, M. Ye, Chem. Commun. 52, 1509 (2016)
G. Zhang, J. Zhang, M. Zhang, X. Wang, J. Mater. Chem. 22, 8083 (2012)
J. Sun, Y. Yuan, L. Qiu, X. Jiang, A. Xie, Y. Shen, J. Zhu, Dalton Trans. 41, 6756 (2012)
G. Liao, S. Chen, X. Quan, H. Yu, H. Zhao, J. Mater. Chem. 22, 2721 (2012)
M. Lu, Z. Pei, S. Weng, W. Feng, Z. Fang, Z. Zheng, M. Huang, P. Liu, Phys. Chem. Chem. Phys. 16, 21280 (2014)
J. Shen, J. Tang, P. Dong, Z. Zhang, J. Ji, R. Baines, M. Ye, RSC Adv. 6, 13456 (2016)
S. Tonda, S. Kumar, Y. Gawli, M. Bhardwaj, S. Ogale, Int. J. Hydrogen Energ. 42, 5971 (2017)
M. Mousavi, A. Habibi-Yangjeh, M. Abitorabi, J. Colloid Interf. Sci. 480, 218 (2016)
M.A. Butler, D.S. Ginley, J. Electrochem. Soc. 125, 228 (1978)
A. Habibi-Yangjeh, M. Shekofteh-Gohari, Sep. Purif. Technol. 184, 334 (2017)
W. Zhang, X. Xiao, L. Zheng, C. Wan, Appl. Surf. Sci. 358, 468 (2015)
J. Fu, Y. Tian, B. Chang, F. Xi, X. Dong, J. Mater. Chem. 22, 21159 (2012)
T. Xian, H. Yang, L.J. Di, J.F. Dai, J. Alloy. Compd. 622, 1098 (2015)
Acknowledgements
This work was financially supported by DST- Science and Engineering Research Board (SERB) India, under “Early Career Research Award Scheme” (ECR/2016/001535/CS) to Dr. S. Vadivel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hariganesh, S., Vadivel, S., Maruthamani, D. et al. Facile Solvothermal Synthesis of Novel CuCo2S4/g-C3N4 Nanocomposites for Visible-Light Photocatalytic Applications. J Inorg Organomet Polym 28, 1276–1285 (2018). https://doi.org/10.1007/s10904-018-0828-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-0828-5