Abstract
New highly fluorescent 2-imino-2H-pyrano[3,2-c]pyridin-5(6H)-onesderivatives were synthesized using a simple route. The present molecules were prepared by two methods with good yield. The structures were characterized by NMR1H, 13 C, and elemental analysis. Also, the effect of solvent and concentration on the fluorescence properties were demonstrated. However, the high fluorescence intensity in the range of 70,000–75,000 a. u. was obtained with a concentration equal to 10− 6 M of prepared molecules. The intensity was influenced also by the molecule structure and solvent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrano[3,2-c]pyridines are functionalized oxygen and nitrogen containing heterocyclic consisting of pyrane and pyridone units (Fig. 1). Generally, pyranes compounds have been considered as biologically active molecules, and valuable scaffolds founded in many natural products including carbohydrates, antibiotics, and pheromones [1]. Also, it can be used as ligands for metal complexes, electroluminescent devices,and medicinal chemistry [2, 3] because of their potential fluorescent properties [4]. However, pyrane [5] and 2-pyridone rings were used as fluorophores and presented a high fluorescent properties [6].Also, it formed a new fluorescent dyes [7].
Recently, the N-Alkylated 2-pyridone is one of the most important heterocyclic compounds which have lately attracted considerable interest owing to their wide range of applications in various fields [8, 9] as fluorescence, and pharmacology [10]. Multi-compound reaction is one of the most methods to produce heterocyclic derivatives [11,12,13]. However, the synthesis of pyrano[3,2-c]pyridones have been reported. In 2010,Xuesen Fan et al. [14] reported the synthesis of novel pyrano[3,2-c]pyridines via a multicomponent reaction of 5-formyl-2’-deoxyuridine and 4-hydroxylpyridones and malononitrile using [bmim]BF4 as an ionic liquid at 80 °C. Then, Anatoliy et al. [15] reported two stepwise versions of this process involving the synthesis of the intermediate Knoevenagel products and their subsequent reactions with 4-hydroxy-6- methyl-2(1H)-pyridone derivative in refluxing methanol in the presence of NEt3.In 2016,Michail studied another domino reaction for synthesized Spiro[indoline-3,4’-pyrano[3,2-c]pyridine]-2,5‘(6’H)-diones, by condensing of one pot isatin, 4-hydroxy-6-methylpyridin-2(1H)-one, and malononitrile in the presence sodium acetate as a catalyst under refluxing ethanol for 15 min [16]. Fluorescencebased on heterocyclicmolecules gives solution of several chemistry problems, such as metal ion detection, cancer imaging, cyanure elimination [17].Therefore, the development of fluorescent properties of molecules adds a considerable value for environmental and life science. Several researchers reported the synthesis of fluorescent molecules such as coumarins [18], imidazole [19], pyrazole [20, 21], chromene [22], pyrimidin-one [23] and bis-3-cyano-2-pyridones [24], this later presented a high fluorescent intensity and can be used as ion indicator.
As a part of our aim at the development of new simple and efficient procedures for the synthesis of some important heterocyclic systems and their application [25], herein, we report a novel approach to synthesize various pyrano[3,2-c]pyridones derivatives. Also, to our knowledge, the fluorescence properties of substituted pyrano[3,2-c]pyridones derivatives was studied for the first time till now.
Results and Discussions
Synthesis of Heterocyclic Molecules
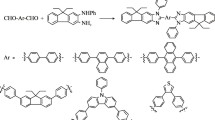
Initially, we were interested tosynthesize2-imino-2H-pyrano[3,2-c]pyridin-5(6H)-ones derivatives alkylated on nitrogen. Theretrosynthetic pathway for their synthesis is shown in Scheme 1. Two methods has been investigated for the 2-imino-2H-pyrano[3,2-c] pyridin-5(6H)-ones synthesis.
For the method (a): The first step is based on the formation of pyrane ring from the reaction of intermediate 1 with malononitrile or ethylcyanoacete in methanol in presence of piperidine as basic catalyst at reflux for 4hresulting in the formation of 2imino-2H-pyrane as starting material. The intermediate 2-((diméthylamino)methylene)-3-oxobutanoate d’éthyle was synthesized according to the previous literature procedure [26]. The second step involved the use of enaminopyranes (3a-b) as key intermediates. These latter were prepared by the reaction of 2-iminopyranes (2a-b) derivatives with amount of dimethylacetal dimethyl-formamide (DMFDMA). The mixture was stirred at room temperature for 48 h without solvent, this leads to a precipitate corresponding to 3a-b. Thus, new enaminone esters (3a-b) are prepared in moderate yields (59–67%).The enaminones formed are then condensed with various primary amines (Phenethylamine, Benzylamine, Cyclohexylamine, Aniline, Sec-butylamine, propylamine, Buthylamine, Hexylamine) as nucleophilic agents at 90 °C during 3 h, under solvent freeconditions to give the iminopyranopyridone ring(4a-h). The synthetic method was shown in Scheme 2 respectively.
Secondly,the2-imino-2H-pyrano[3,2-c]pyridin-5(6H)-ones derivativeswere also synthesized by method (b) which consisting the multicomponent reaction between synthesized2H-iminopyranes (2a-b),triethylorthoformate, and primary amine in the presenceof acetic anhydride at 80 °C for 5 h (Scheme 3). Various primary aliphatic, aromatic, and cyclic amines were used to investigating the versatility of this methodology and synthesizing new structures. The resultsof afforded products prepared by method (a) and (b) were showed in Fig. 2.
Figure 2 shows that the 2-iminopyranes intermediates (2a-3b) were synthesized with moderate yields (40–59%). For the synthesis of 2-imino-2H-pyrano[3,2-c]pyridin-5(6H)-ones by the method (a) give high yield (59–85%) compared with the method (b).The mechanism proposed for the preparation of substituted 2-imino-2H-pyrano[3,2-c]pyridin-5(6H)-ones (4a-h) was described in Scheme 4. First, intermediate I was obtained by a nucleophile addition reaction between primary amines and double bond of enamino-2-imino-2H-pyran (3a-b). Then, an intermolecular cyclization reaction between thedouble bond of amino group and ester groupin intermediate I to obtain intermediate II. Finally, thesubstituted 2-imino-2H-pyrano[3,2-c]pyridin-5(6H)-ones (4a-h) were obtained by an aromatization step with the elimination of ethanol.
Fluorescence Properties of 2-imino-2H-Pyrano[3,2-c]pyridin-5(6H)-ones Derivatives
The photophysicalproperties of fluorescent molecules were recorded to identify the highly, more intense and sensitive prepared molecules. However, the effect of concentration, and solvent on the intensity was studied for six derivatives:4a, 4b, 4c, 4e, 4f, and 4h at room temperature.First, four solutions were prepared withdifferent concentrations of each heterocyclic molecule in methanol:10− 4, 10− 5, 10− 6, and 10− 7 M with specific excitation wavelengths λex for each compound (Fig. 3).The fluorescence spectra of all products showed good fluorescence intensity at theirwavelength excitation in methanol except in higher concentration (10− 4, 10− 5), where the intensity decreases. With higher concentration, all spectra demonstrate a red shift of bands to higher wavelength with a curios decreasing of fluorescence intensity (Table 1). The results closed that molecular aggregation and the fluorescence quenching occur when the concentration increases [27].
Comparing the fluorescence intensity of compounds in methanol (10− 6 M), they are classified in order of decrease as follows: 4c, 4a, 4f, 4b, 4e, and 4h (Table 1). It is known in the literature that the cyclic substituents and the presence of electron donor groups (-OH, = NH) increase the fluorescence intensity, which verifies the principle of fluorescence based on the delocalization of π electrons, the reason why that high intensity were recorded [28].The compound 4cgave the highest intensity due to the presence of the benzyl ring (aromatic ring and H acid of -CH2), and it decreases for the compound 4f (2 H acid - CH2-CH2) to 4h which contains a linear aliphatic chain (R = Hexyl).
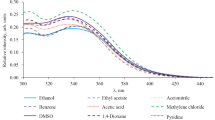
Secondly, the solvent effect on the fluorescence was studied using the concentration 10− 6 M for 4a-c, 4e-f, and 4h molecules. Figure 4 shows the solvent effect on the fluorescence of 4a, 4b and 4c compounds. For the compound 4a, the best fluorescence intensity was recorded with methanol, acetonitrile, and H2O and slowly lowers with dichloromethane. While the 4b compound presentsa high intensity in methanol and chloroformwith a shift of bands to lower wavelength in the lastone and it presentsmoderatefluorescence intensity with other solvent. For the compound 4c, the fluorescence intensity in chloroform and dichloromethane was slowly lower then methanol. And it demonstrates a very lower intensity with H2O and acetonitrile.The difference between the three compounds 4a, 4b, and 4c is the number of methylene fragment between azote and the phenyl ring. So, decreasing the methylene group between the phenylic and other cyclic fragment leads to the decrease of fluorescence intensity in polar and apolar solvents.
In the case of 4e, 4f, and 4h (Fig. 5), high intensity was afforded with different solvents. The 4h compound presents a high intensity with polar solvent such as methanol, dichloromethane, acetonitrile, ethyl acetate, and chloroform.This result confirms also that the number of methylene fragment influenced the fluorescence intensity.In addition, the presence of the acid protons on R substituent of the pyrano[3,2-c]pyridine ring has a positive effect.
The result shows that the solvation power increases the fluorescence intensity; moreover, the use of polar solvents such as methanol confirms the solvation parameter as well as the possibility of intermolecular hydrogen bonds formation with the free doubles of oxygen and nitrogen atoms of pyridine-2-one group (Fig. 6).
Conclusion
In the present study, the heterocyclic 2-imino-2H-pyrano[3,2-c]pyridin-5(6H)-ones derivatives were prepared successfully with two methods affording high yield (40–85%) [25]. The method (a) gives products using two steps, and the method (b) involves the one pot synthesis of the derivatives. This one gives moderate yields (58–69%). Thesynthetic derivatives presented a high fluorescent intensity in methanol with a concentration of 10− 6 M. The fluorescence intensity increases according to the structure of each prepared derivative. The study of solvent effect on the fluorescence intensity of 4a, 4b, 4c, 4e, 4f, and 4h demonstrates that the intensity is related to the structure and cyclic aromatic rings where the cyclic aromatic derivatives presented a high fluorescent intensity than the aliphatic one. In addition, with polar solvent, the cyclic aromatic compounds presented excellent fluorescence intensity in the range of 60,000 and 70,000 (a.u.).Our results demonstrates that 2-imino-2H-pyrano[3,2-c]pyridin-5(6H)-ones derivatives can be used as indictor for a next study.
Data Availability
All of the material is owned by the authors and/or no permissions are required and it is aviable.
References
Shahi M, Foroughifar N, Mobinikhaledi A (2015) Synthesis and antimicrobial activity of some tetrahydro quinolone diones and pyrano [2, 3-d] pyrimidine derivatives. Iran J Pharm Res 14(3):757
Pinna GA, Loriga G, Murineddu G et al (2001) Synthesis and anti-HIV-1 activity of new delavirdine analogues carrying arylpyrrole moieties. Chem Pharm Bull 49(11):1406–1411
Magesh CJ, Makesh SV, Perumal PT (2004) Highly diastereoselective inverse electron demand (IED) diels–alder reaction mediated by chiral salen–AlCl complex: the first, target-oriented synthesis of pyranoquinolines as potential antibacterial agents. Bioorg Med Chem Lett 14(9):2035–2040
Gaginella TS, Walsh RE (1992) Sulfasalazine Dig Dis Sci 37(6):801–812
Wang B-B, Wang Y, Wu W-N et al (2020) A near-infrared colorimetric and fluorescent dual-channel probe for cyanide detection based on dicyanomethylene-4H-pyran. Inorg Chem Commun 122:108245
Kasprzyk W, Koper F, Flis A et al (2021) Fluorescence assay for the determination of glutathione based on a ring-fused 2-pyridone derivative in dietary supplements. Analyst 146(6):1897–1906
Porobić SJ, Božić B, Dramićanin MD et al (2020) Absorption and fluorescence spectral properties of azo dyes based on 3-amido-6-hydroxy-4-methyl-2-pyridone: Solvent and substituent effects. Dyes Pigm 175:108139
Fujita Y, Oguri H, Oikawa H (2005) Biosynthetic studies on the antibiotics PF1140: a novel pathway for a 2-pyridone framework. Tetrahedron Lett 46(35):5885–5888
Wilson JE, Garvey J, Taveras KM (2019) Synthesis of isoindolinones: Intramolecular [4 + 2] cycloaddition/retro [4 + 2] of pyridone propiolamides. Tetrahedron Lett 60(41):151105
Kumar NV, Rajendran S (2004) A one-pot synthesis of 4-methylpyrano [3, 2-C] quinolin-2, 5 [6H]-diones. Heterocycl Commun 10(4–5):289–294
Majidi Arlan F, Poursattar Marjani A, Javahershenas R et al (2021) Recent developments in the synthesis of polysubstituted pyridines via multicomponent reactions using nanocatalysts. New J Chem 45(28):12328–12345
Kafi-Ahmadi L, Poursattar Marjani A, Nozad E (2021) Ultrasonic-assisted preparation of Co3O4 and Eu-doped Co3O4 nanocatalysts and their application for solvent-free synthesis of 2-amino-4H-benzochromenes under microwave irradiation. Appl Organomet Chem 35(8):e6271
Kafi-Ahmadi L, Khademinia S, Poursattar Marjani A et al (2022) Fabrication of 5-aryl-1H-tetrazoles derivatives by solid-state synthesized MgFe2O4 and MgFe2ZnxO4 + δ heterogeneous nanocatalysts. Res Chem Intermed 48(7):2973–2986
Fan X, Feng D, Qu Y et al (2010) Practical and efficient synthesis of pyrano [3, 2-c] pyridone, pyrano [4, 3-b] pyran and their hybrids with nucleoside as potential antiviral and antileishmanial agents. Bioorg Med Chem Lett 20(3):809–813
Shestopalov AM, Larionova NA, Fedorov AE et al (2013) Synthesis of Isomeric Isothiazolo [4′, 3′: 4, 5]-and isothiazolo [4′, 5′: 4, 5] thieno [3, 2-b] pyrano [2, 3-d] pyridines by combination of domino reactions. ACS Comb Sci 15(10):541–545
Elinson MN, Ryzhkov FV, Vereshchagin AN et al (2016) Multicomponent assembling of isatins, malononitrile and 4-hydroxy-6-methylpyridin-2 (1H)-ones: one-pot efficient approach to privileged spiro [indoline-3, 4’-pyrano [3, 2-c] pyridine]-2, 5’(6’H)-dione scaffold. Mendeleev Commun 5(26):399–401
Popović Z, Liu W, Chauhan VP et al (2010) A nanoparticle size series for in vivo fluorescence imaging. Angew Chem 122(46):8831–8834
Nyuchev AV, Sharonova EA, Lenshina NA et al (2011) Synthesis of fluorescent coumarin triazolylglycosides. Tetrahedron Lett 52(32):4196–4199
Kafi-Ahmadi L, Khademinia S, Poursattar Marjani A et al (2022) Microwave-assisted preparation of polysubstituted imidazoles using Zingiber extract synthesized green Cr2O3 nanoparticles. Sci Rep 12(1):19942
Bikas S, Poursattar Marjani A, Bibak S et al (2023) Synthesis of new magnetic nanocatalyst Fe3O4@CPTMO-phenylalanine-Ni and its catalytic effect in the preparation of substituted pyrazoles. Sci Rep 13(1):2564
Pursuwani BH, Bhatt BS, Vaidya FU et al (2021) Fluorescence, DNA Interaction and cytotoxicity studies of 4, 5-Dihydro-1H-Pyrazol-1-Yl Moiety Based Os (IV) Compounds: synthesis, characterization and biological evaluation. J Fluoresc 31(2):349–362
Poursattar Marjani A, Asadzadeh F, Danandeh Asl A (2022) Fe3O4@Glycerol-Cu as a novel heterogeneous magnetic nanocatalyst for the green synthesis of 2-amino-4H-chromenes. Sci Rep 12(1):22173
Khashaei M, Kafi-Ahmadi L, Khademinia S et al (2022) A facile hydrothermal synthesis of high-efficient NiO nanocatalyst for preparation of 3,4-dihydropyrimidin-2(1H)-ones. Sci Rep 12(1):8585
Mehiaoui N, Kibou Z, Gallavardin T et al (2021) Novel bis-(3-cyano-2-pyridones) derivatives: synthesis and fluorescent properties. Res Chem Intermed 47(4):1331–1348
Yazid D, Tewfik A-D, Nawel M et al (2022) Ecofriendly synthesis of formamidine derivatives catalyzed by Al-MCM-41 Mesoporous materials. Lett Org Chem 19(5):347–352
Kibou Z, Cheikh N, Villemin D et al (2016) A rapid synthesis of highly functionalized 2-pyridones and 2-aminopyridines via a microwave-assisted multicomponent reaction. J Mater Environ Sci 7:3061–3067
Fukaminato T, Kawai T, Kobatake S et al (2003) Fluorescence of photochromic 1, 2-bis (3-methyl-2-thienyl) ethene. J Phys Chem B 107(33):8372–8377
Lermontova S, Grigor’ev I, Ladilina EY et al (2018) Porphyrazine structures with Electron-withdrawing substituents as the base for materials for Photonics and Biomedicine. Russ J Coord Chem 44(4):301–315
Acknowledgements
The authors would like to thank the General Directorate for Scientific Research and Technological Development (DGRST) for the financial support.
Funding
There is no funding for this researche.
Author information
Authors and Affiliations
Contributions
Nawel Mehiaoui: redaction, synthesis of molecules and experimentale study.
Ridha Hassaine: fluorescence ananlysis end interpretation.
Amina Berrichi: redaction and interpretation.
Zahira Kibou: synthesis of substrate molecules.
Noureddine Choukchou‑Braham analysis and interpretation.
Corresponding author
Ethics declarations
Competing Interests
The author declared that there is noconflict of interest.
Ethics Approval
This study was performed in our laboratory and approved by our group.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
I give my consent for the publication of identifiable details, which can includeMaterial, Figures which to be published in this Journal. I confirm that I have seen and been given the opportunity to read both the Material and the Article as attached to be published by this journal. I havediscussed this consent form authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehiaoui, N., Hassaine, R., Berrichi, A. et al. Synthesis of Highly Heterocyclic Fluorescent Molecules: 2-imino-2H-pyrano[3,2-c] Pyridin-5(6H)-ones Derivatives. J Fluoresc 33, 1995–2001 (2023). https://doi.org/10.1007/s10895-023-03212-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03212-4