Abstract
Previously unknown 3,4-diamino-6-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles were synthesized by reaction of 4-amino-6-aryl-2-bromopyridine-3,5-dicarbonitriles with hydrazine hydrate. Study of the optical properties of the synthesized compounds revealed their fluorescence in solution with the emission maximum located in the range of λ 484–548 nm and quantum yield of 0.9–3.9%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pyrazolo[3,4-b]pyridine ring system is an important structural fragment of many biologically active compounds, including those exhibiting antiproliferative [1], antimicrobial [2, 3], anticancer [4–6], and antiparasitic activities [7], as well as inhibitory activity against acetylcholinesterase [8], casein kinase 1 (CK1), checkpoint kinase 1 (CHK1) [9], Aurora A kinase [10], and fibroblast growth factor (FGFR) [11]. Optical properties of pyrazolo[3,4-b]pyridine derivatives are no less interesting. In this regard, the synthesis of efficient fluorophores [12–14] and chemosensors based thereon [15, 16] was reported.
The most widely used method for the synthesis of 3-amino-1H-pyrazolo[3,4-b]pyridines is based on nucleophilic substitution of halogens [17] or, more rarely, oxygen- [3, 5, 18–19] or sulfur-containing fragments [20–22]. Herein we report the synthesis of new 3,4-diamino-6-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles 1 and their optical properties.
RESULTS AND DISCUSSION
We previously found that the bromine atom in 4-amino-6-aryl-2-bromopyridine-3,5-dicarbonitriles 2 [23, 24] can be readily replaced by an amino group in reactions with primary and secondary amines to produce the corresponding 2-[(di)alkylamino]pyridines [25]. In continuation of these studies, compounds 2 were reacted with hydrazine hydrate. According to published data [26–28], replacement of the halogen atom in 2 by hydrazine leads to the formation of 2-hydrazinylpyridine-3,5-dicarbonitriles which then undergo intramolecular heterocyclization to 3-amino-1H-pyrazolo[3,4-b]pyridines. As expected, the reaction of 2 with hydrazine hydrate also afforded 3,4-diamino-6-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles 1 in 83–92% yield (Scheme 1). It should be noted that, despite all attempts, we failed to isolate intermediate 2-hydrazinylpyridines.
The 1H NMR spectra of 1a–1f showed signals from protons of the aryl substituent. The two amino and one NH groups gave rise to broadened singlets or exchanged with water. In the IR spectra of 1a–1f, stretching vibrations of the conjugated cyano groups were observed at 2206–2218 cm–1, and N–H stretching bands were observed in the region 3161–3466 cm–1. In the mass spectra of 1a–1f, the base peak was that of the molecular ion.
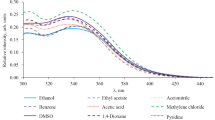
Compounds 1a–1f were isolated as yellow to orange crystalline solids poorly soluble in organic solvents (except for DMSO). Using compound 1b as an example, we found that the position of the electronic absorption maximum almost does not depend on the solvent nature. Moreover, in many cases we failed to determine its exact position because of overlap by the solvent band. The fluorescence maximum of 1a–1f ranges from λ 452 to 514 nm, and it shifts to longer wavelengths as the solvent polarity increases (Table 1). Depending on the substituent in the benzene ring, the fluorescence maximum of 1a–1f in DMSO appears in the range λ 484–548 nm (Table 2). Electron-donating groups generally increase the fluorescence intensity and shift its maximum to shorter wavelengths (Fig. 1). Compounds 1a–1f in the solid state showed almost no fluorescence.
EXPERIMENTAL
The IR spectra were recorded on an FSM-1202 FTIR spectrometer (Russia) from samples dispersed in mineral oil. The 1H NMR spectra were recorded on a Bruker DRX-500 spectrometer (USA) using DMSO-d6 as solvent and tetramethylsilane as internal standard. The mass spectra (electron impact, 70 eV) were run on a Finnigan MAT INCOS-50 mass spectrometer (USA). Elemental analysis was performed with a Vario Micro cube CHN analyzer (Germany). The fluorescence spectra were measured on an Agilent Cary Eclipse spectrofluorometer (USA). The melting points were determined with an OptiMelt MPA100 automated melting point apparatus (USA). The progress of reactions and the purity of the isolated compounds were monitored by TLC on Sorbfil PTSKh-AF-A-UF plates using ethyl acetate as eluent; visualization was done under UV light, by treatment with iodine vapor, and by thermal decomposition. Compounds 2a–2f were synthesized as described in [23]. Hydrazine hydrate (100%) was commercial product (Germany).
3,4-Diamino-6-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (1a). A mixture of 0.299 g (1 mmol) of 4-amino-2-bromo-6-phenylpyridine-3,5-dicarbonitrile (2a) and 0.15 g (3 mmol) of hydrazine hydrate in 5 mL of 1,4-dioxane was refluxed for 4 h. After completion of the reaction (TLC), the mixture was diluted with 30 mL of distilled water, and the precipitate was filtered off, washed with small portions of distilled water, and recrystallized from 1,4-dioxane. Yield 0.228 g (91%), mp 291–292°C (decomp.). IR spectrum, ν, cm–1: 3454, 3358, 3177 (NH, NH2), 2206 (CN), 1654 (C=C). 1H NMR spectrum (DMSO-d6), δ, ppm: 5.72 s (2H, NH2), 7.27 s (2H, NH2), 7.48–7.51 m (3H, C6H5), 7.71–7.74 m (2H, C6H5), 12.20 s (1H, NH). Mass spectrum: m/z 250 (Irel 100%). Found, %: C 62.45; H 4.09; N 33.46. C13H10N6. Calculated, %: C 62.39; H 4.03; N 33.58. M 250.27.
Compounds 1b–1f were synthesized in a similar way.
3,4-Diamino-6-(4-methylphenyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (1b). Yield 0.219 g (83%), mp 306–307°C (decomp.). IR spectrum, ν, cm–1: 3467, 3395 (NH, NH2), 2218 (C≡N), 1696 (C=C). 1H NMR spectrum (DMSO-d6), δ, ppm: 2.39 s (3H, CH3), 7.38 d (2H, C6H4, J = 7.7 Hz), 7.62 d (2H, C6H4, J = 7.7 Hz), 8.78 br.s (3H, NH, NH2). 13C NMR spectrum (DMSO-d6), δC, ppm: 21.51, 81.85, 89.97, 116.15, 129.53, 129.57, 142.14, 146.87, 148.41, 155.41, 155.51, 159.70. Mass spectrum: m/z 264 (Irel 100%). Found, %: C 63.52; H 4.63; N 31.85. C14H12N6. Calculated, %: C 63.62; H 4.58; N 31.80. M 264.29.
3,4-Diamino-6-(3,4-dimethoxyphenyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (1c). Yield 0.276 g (89%), mp 246–247°C (decomp.). IR spectrum, ν, cm–1: 3439, 3379, 3300 (NH, NH2), 2208 (C≡N), 1662 (C=C). 1H NMR spectrum (DMSO-d6), δ, ppm: 3.82 s (3H, OCH3), 3.83 s (3H, OCH3), 5.70 s (2H, NH2), 7.07 d (1H, C6H3, J = 8.3 Hz), 7.22 s (2H, NH2), 7.32–7.38 m (2H, C6H3), 12.17 br.s (1H, NH). 13C NMR spectrum (DMSO-d6), δC, ppm: 56.14, 56.20, 80.72, 91.48, 111.63, 112.91, 119.35, 122.16, 131.95, 148.74, 148.80, 150.44, 153.98, 154.15, 161.25. Mass spectrum: m/z 310 (Irel 100%). Found, %: C 58.18; H 4.61; N 27.00. C15H14N6O2. Calculated, %: C 58.06; H 4.55; N 27.08. M 310.32.
3,4-Diamino-6-(2-chlorophenyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (1d). Yield 0.262 g (92%), mp 308–309°C (decomp.). IR spectrum, ν, cm–1: 3448, 3177 (NH, NH2), 2218 (C≡N), 1687 (C=C). 1H NMR spectrum (DMSO-d6), δ, ppm: 7.57 t (1H, C6H4, J = 7.6 Hz), 7.64 t (1H, C6H4, J = 7.4 Hz), 7.68–7.72 m (2H, C6H4), 8.80 br.s (5H, NH, NH2). 13C NMR spectrum (DMSO-d6), δC, ppm: 66.82, 83.90, 90.49, 115.15, 128.06, 130.21, 131.34, 131.91, 132.97, 146.95, 148.70, 154.93, 157.80. Mass spectrum: m/z 286/284 (Irel 33/100%). Found, %: C 54.73; H 3.25; N 29.60. C13H9ClN6. Calculated, %: C 54.84; H 3.19; N 29.52. M 284.71.
3,4-Diamino-6-(3,4-dichlorophenyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (1e). Yield 0.287 g (90%), mp 322–323°C (decomp.). IR spectrum, ν, cm–1: 3466, 3335, 3161 (NH, NH2), 2216 (C≡N), 1657 (C=C). 1H NMR spectrum (DMSO-d6), δ, ppm: 5.74 br.s (2H, NH2), 7.36 s (2H, NH2), 7.73 d.d (1H, C6H3, J = 8.3, 2.1 Hz), 7.77 d (1H, C6H3, J = 8.4 Hz), 7.95 d (1H, C6H3, J = 2.1 Hz), 12.27 br.s (1H, NH). 13C NMR spectrum (DMSO-d6), δC, ppm: 80.73, 91.62, 118.54, 129.34, 130.86, 131.00, 131.39, 132.55, 139.81, 148.72, 153.50, 153.91, 158.80. Mass spectrum, m/z (Irel, %): 320 (65), 318 (18), 316 (100). Found, %: C 48.80; H 2.60; N 26.39. C13H8Cl2N6. Calculated, %: C 48.92; H 2.53; N 26.33. M 319.15.
3,4-Diamino-6-(3-nitrophenyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (1f). Yield 0.338 g (85%), mp 243–244°C (decomp.). IR spectrum, ν, cm–1: 3460, 3343, 3185 (NH, NH2), 2210 (C≡N), 1672 (C=C). 1H NMR spectrum (DMSO-d6), δ, ppm: 7.84 t (1H, C6H4, J = 8.1 Hz), 8.22 d.t (1H, C6H4, J = 7.8, 1.4 Hz), 8.39 d.d (1H, C6H4, J = 8.1, 2.4 Hz), 8.56–8.61 m (1H, C6H4). Mass spectrum: m/z 295 (Irel 100%). Found, %: C 52.77; H 3.00; N 33.32. C13H9N7O2. Calculated, %: C 52.88; H 3.07; N 33.21. M 397.44.
CONCLUSIONS
2,4-Diamino-6-arylpyridine-3,5-dicarbonitriles have been synthesized, and their electronic absorption and luminescence spectra have been recorded. The synthesized compounds show fluorescence in solution with an emission maximum located at λ 484 to 548 nm and do not fluoresce in the solid state.
REFERENCES
Ye, Q., Cao, J., Zhou, X., Lv, D., He, Q., Yang, B., and Hu, Y., Bioorg. Med. Chem., 2009, vol. 17, p. 4763. https://doi.org/10.1016/j.bmc.2009.04.043
Witherington, J., Bordas, V., Garland, S.L., Hickey, D.M.B., Ife, R.J., Liddle, J., Saunders, M., Smith, D.G., and Ward, R.W., Bioorg. Med. Chem. Lett., 2003, vol. 13, p. 1577. https://doi.org/10.1016/S0960-894X(03)00134-3
Nagender, P., Malla Reddy, G., Naresh Kumar, R., Poornachandra, Y., Ganesh Kumar, C., and Narsaiah, B., Bioorg. Med. Chem. Lett., 2014, vol. 24, p. 2905. https://doi.org/10.1016/j.bmcl.2014.04.084
Mohamed, M.S., Awad, Y.E.E.-D., El-Hallouty, S.M., and El-Araby, M., Open J. Med. Chem., 2012, vol. 2, p. 78. https://doi.org/10.4236/ojmc.2012.23010
Ravi Kumar, N., Poornachandra, Y., Krishna Swaroop, D., Jitender Dev, G., Ganesh Kumar, C., and Narsaiah, B., Bioorg. Med. Chem. Lett., 2016, vol. 26, p. 5203. https://doi.org/10.1016/j.bmcl.2016.09.062
Hao, S.-Y., Qi, Z.-Y., Wang, S., Wang, X.-R., and Chen, S.-W., Bioorg. Med. Chem., 2021, vol. 31, article ID 115985. https://doi.org/10.1016/j.bmc.2020.115985
Ribeiro, J.L.S., Soares, J.C.A.V., Portapilla, G.B., Providello, M.V., Lima, C.H.S., Muri, E.M.F., de Albuquerque, S., and Dias, L.R.S., Bioorg. Med. Chem., 2021, vol. 29, article ID 115855. https://doi.org/10.1016/j.bmc.2020.115855
Umar, T., Shalini, S., Raza, M.K., Gusain, S., Kumar, J., Seth, P., Tiwari, M., and Hoda, N., Eur. J. Med. Chem., 2019, vol. 175, p. 2. https://doi.org/10.1016/j.ejmech.2019.04.038
Huart, A.-S., Saxty, B., Merritt, A., Nekulova, M., Lewis, S., Huang, Y., Vojtesek, B., Kettleborough, C., and Hupp, T.R., Bioorg. Med. Chem. Lett., 2013, vol. 23, p. 5578. https://doi.org/10.1016/j.bmcl.2013.08.046
Shi, J., Xu, G., Zhu, W., Ye, H., Yang, S., Luo, Y., Han, J., Yang, J., Li, R., Wei, Y., and Chen, L., Bioorg. Med. Chem. Lett., 2010, vol. 20, p. 4273. https://doi.org/10.1016/j.bmcl.2010.04.083
Zhao, B., Li, Y., Xu, P., Dai, Y., Luo, C., Sun, Y., Ai, J., Geng, M., and Duan, W., ACS Med. Chem. Lett., 2016, vol. 7, p. 629. https://doi.org/10.1021/acsmedchemlett.6b00066
Chen, J., Liu, W., Ma, J., Xu, H., Wu, J., Tang, X., Fan, Z., and Wang, P., J. Org. Chem., 2012, vol. 77, p. 3475. https://doi.org/10.1021/jo3002722
Deore, R., Dingore, K., and Jachak, M., J. Fluoresc., 2015, vol. 25, p. 1549. https://doi.org/10.1007/s10895-015-1674-2
Kendre, D.B., Toche, R.B., and Jachak, M.N., Tetrahedron, 2007, vol. 63, p. 11000. https://doi.org/10.1016/j.tet.2007.08.052
García, M., Romero, I., and Portilla, J., ACS Omega, 2019, vol. 4, p. 6757. https://doi.org/10.1021/acsomega.9b00226
Orrego-Hernández, J., Lizarazo, C., Cobo, J., and Portilla, J., RSC Adv., 2019, vol. 9, p. 27318. https://doi.org/10.1039/C9RA04682H
Sirakanyan, S.N., Ghazaryan, S.G., Hakobyan, E.K., and Hovakimyan, A.A., Russ. J. Org. Chem., 2020, vol. 56, p. 840. https://doi.org/10.1134/S1070428020050176
Sirakanyan, S.N., Hakobyan, E.K., and Hovakimyan, A.A., Russ. J. Org. Chem., 2018, vol. 54, p. 929. https://doi.org/10.1134/S1070428018060167
Hamza, E.Kh., Hamdy, N.A., Zarie, E.S., Fakhr, I.M.I., Elwahy, A.H.M., and Awad, H.M., J. Heterocycl. Chem., 2019, vol. 57, p. 182. https://doi.org/10.1002/jhet.3764
Sanad, S.M.H., Hawass, M.A.E., Ahmed, A.A.M., and Elneairy, M.A.A., Synth. Commun., 2018, vol. 48, p. 1847. https://doi.org/10.1080/00397911.2018.1468911
Sanad, S.M.H., Abdel-Fattah, A.M., Attaby, F.A., and Elneairy, M.A.A., J. Heterocycl. Chem., 2018, vol. 56, p. 651. https://doi.org/10.1002/jhet.3444
Abdel Fattah, A.M., Elneairy, M.A.A., and Gad-Elkareem, M.A.M., Phosphorus, Sulfur Silicon Relat. Elem., 2007, vol. 182, p. 1351. https://doi.org/10.1080/10426500601160991
Bardasov, I.N., Alekseeva, A.U., and Ershov, O.V., Tetrahedron Lett., 2018, vol. 59, p. 1398. https://doi.org/10.1016/j.tetlet.2018.02.069
Bardasov, I.N., Mihailov, D.L., Alekseeva, A.U., Ershov, O.V., and Nasakin, O.E., Tetrahedron Lett., 2013, vol. 54, p. 21. https://doi.org/10.1016/j.tetlet.2012.10.015
Ershov, O.V., Mikhailov, D.L., Bardasov, I.N., Ievlev, M.Yu., and Belikov, M.Yu., Russ. J. Org. Chem., 2017, vol. 53, p. 886. https://doi.org/10.1134/S1070428017060124
Ershov, O.V., Ievlev, M.Yu., Belikov, M.Yu., and Maksimova, V.N., Russ. J. Org. Chem., 2018, vol. 54, p. 873. https://doi.org/10.1134/S1070428018060088
Tranfić, M., Halambek, J., Cetina, M., and Jukić, M., J. Mol. Struct., 2011, vol. 1001, p. 145. https://doi.org/10.1016/j.molstruc.2011.06.033
El-Sayed, A.A., Amr, A.E., El-Ziaty, A.K., and Elsayed, E.A., Molecules, 2019, vol. 24, article no. 1965. https://doi.org/10.3390/molecules24101965
ACKNOWLEDGMENTS
A part of this study was performed in the framework of state assignment for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of fundamental research.
Funding
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (project no. 0849-2020-0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2022, Vol. 58, No. 7, pp. 754–759 https://doi.org/10.31857/S0514749222070084.
Rights and permissions
About this article
Cite this article
Al-Shuaeeb, R.A.A., Alekseeva, A.Y., Yashchenko, N.N. et al. Synthesis and Optical Properties of 3,4-Diamino-6-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles. Russ J Org Chem 58, 997–1001 (2022). https://doi.org/10.1134/S1070428022070089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022070089