Abstract
Fluorescence excitation-emission matrix spectroscopy (EEMs) has become a very popular technique in characterization of aquatic dissolved organic matter (DOM) coupled with a parallel factor (PARAFAC) model, denoted as (EEMs-PARAFAC). This research addresses the poorly researched relationship correlation between dissolved ions and fluorescence in a natural water environment. The relationship between the EEMs-PARAFAC components and ionic composition was studied in freshwater lakes, rivers, and seawater from locations in China. The natural water environment is different from a simulated environment having a fixed ionic composition. We used electrical conductivity (EC) to reflect the ionic strength as an indicator to evaluate the relationship in a series of water bodies. Results show that the EC generally had a positive correlation with DOM in natural water environment, but no correlation was found with water from the highly saline Yellow Sea. The Chaohu Lake samples contained one component having a significant negative correlation with EC, i.e., r > 0.6, p < 0.05, while other surface waters contained components having both positive and negative correlations (r > 0.5, p < 0.05). The negative correlation with EC also highlighted that humic acid-like components and protein-like materials (c1-c3) were positively correlated with DOM, while the protein-like component (c4) was negatively correlated with DOM. The EC equation proposed provided a good fit with the EC values of surface waters. The use of EC would be a useful and rapid method for analyzing the variation in the fluorescence component and its effect on water quality. This study highlights the need to account for variation in EC when assessing EEMs-PARAFAC of natural waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dissolved organic matter (DOM) is an important component of aquatic environment which comes from the decomposition of organisms and from the synthetic activities of microorganisms [1, 2]. It can be fractionated into variables such as humic acid (HA) and fulvic acid (FA) molecules [3, 4] and plays a significant role in the function of water ecosystem. For example, it is provides a mechanism for the migration of a variety of substances (such as potentially toxic elements, nanoparticles, ions, nutrient elements), causing nonpoint source pollution [5,6,7], and can also be transformed into thousands of carcinogenic byproducts during water disinfection [8, 9]. Many characterization techniques have been developed [10,11,12] to investigate these behaviors, such as the Fourier-transform ion cyclotron resonance (FT-ICR) mass spectroscopy [13], mass spectrometry (MS) [14], homo and hetero-correlated multidimensional nuclear magnetic resonance (NMR) spectroscopy [10], X-ray photoelectron energy spectroscopy (XPS) [15], UV-Vis spectroscopy (UV) [11] and excitation-emission matrix spectroscopy (EEMs) [16] . These methods have greatly improved the ability to recognize the characteristics of DOM. However, it is still a challenge to analyze the complexity and heterogeneity of DOM and how to detect it quickly. Among them, UV and EEMs spectroscopies are the simplest operational methods since they have no complex steps in the pretreatment of samples. UV is an important reference index for water quality analysis, such as the absorption value at 254 nm (UV254). Unlike the UV254, EEMs can trace the origin of DOM which is difficult for UV, and identify the components and relative concentrations of organic matter qualitatively and quantitatively [12, 17]. EEMs has established a correlation with a variety of water quality indicators [12, 18], such as chemical oxygen demand (COD), dissolved organic carbon (DOC), and biochemical oxygen demand (BOD). Little is known of correlation potential of electrical conductivity (EC) and fluorescence.
The influence of the water matrix is rarely considered during EEMs analysis. Natural waters include varying quantities of dissolved cations (e.g., Mg2+, Ca2+, Na+, K+) and anions (e.g., Cl−, SO42−, NO3−, HCO3−) which can interact with the natural organic matter (NOM), resulting in the potential to create an abundance of inorganic or metal organic salts [19,20,21,22]. Among them, calcium and bicarbonate are of great importance in the total dissolved solids in many fresh surface waters [23]. The optical fluorescence properties of DOM are predominantly from conjugated double bonds and aromatic rings under visible and ultraviolet light radiation [24]. Both metal and nonmetallic ions can affect fluorescence activity including quenching and signal enhancement [25, 26]. For example, Ca2+ and Na+ both important components of natural waters have a significant influence by reducing the HA’s fluorescence through individual or combined interactions with other ions [12]. The variation in ionic composition and known influence on fluorescence intensity should be of concern in applying the technique to monitor water quality. It is feasible that by establishing a good relationship between the ionic composition of water and the fluorescence of DOM as well as other related parameters a simple method for water quality monitoring can be developed. However, in the surface water environment, the variability of ionic composition and the multiple potential interactions has not been evaluated comprehensively in relation to its influence on the fluorescence of DOM.
The EC of the solution is characterized by the ionic contribution of the electrolyte dissolved in water, which is directly proportional to the salinity that is an important index of natural water. Salinity is usually calculated by the primary cations (Mg2+, Ca2+, Na+, K+) and anions (SO42−, Cl−, CO32−, HCO3−). The total content of main ion components in natural water accounts for almost all salt content. Therefore, the EC is often proportional to the concentration of ionic species in water but depends on the water characteristics [27]. The molar conductance of main ions in natural water decreases in the order of K + > Ca2 + > Mg2 + > Na + and SO42− > Cl− > CO32− > HCO3−, indicating that different ionic composition can lead to the change of EC [27] which led to changes in the fluorescence signal for DOM [12]. However, the effect of ionic composition in a complex surface water environment has limited study even though this feature has marked effects on a number of aspects of water treatment. Little is known about the effect of ionic composition on optical properties of DOM. For a reverse osmosis membrane, the EC test is an important parameter to evaluate performance of the membrane and membrane fouling is closely related to the fluorescent components of DOM [12]. If the relationship between EC and DOM does exist, it is of great benefit to the understanding of membrane fouling behaviors. Also, EC has relationship with other water quality parameters can be explored through the bridging role of DOM fluorescence properties. For example, specific DOM species are closely related to the generation of disinfection by-products during water chlorination [28, 29]. A simple scheme as shown in Fig. 1 express the significance of the relationship.
In recent years, the coupling of EEMs and parallel factor (PARAFAC) has led to the EEMs-PARAFAC model, which has become a popular technique for analyzing the DOM of surface water and in water treatment processes [12, 30, 31]. In this study, the correlation between EC and the EEMs-PARAFAC model fractioned aquatic DOM of the Xiangjiang River was analyzed, including not only the NOM filtered by 0.45 μm membranes, but also those components filtered by the 30 kDa and 10 kDa ultrafiltration membranes. This was further assessed in different water environments from locations across China.
Materials and Methods
Sampling Sites
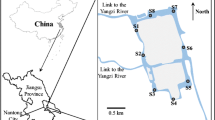
Our sampling sites were fixed along Xiangjiang River, located in Xiangtan City, Hunan, China. Analyses of water quality parameters were collected from November 2018 to March 2019. The Xiangjiang River is the largest River in the Hunan Province and an important drinking water source, flowing through the city’s center with its direction fixed from south to north. Figure 2 shows eight distributed sites labeled as numbers from 1 to 8. Site 1 was the drainage outlet of the sewage treatment plant in Xiangtan City, Site 2 drained Xiangtan City’s industrial areas. Sites 3 and 4 were located at the junction points of the Xiangjiang River with its Lianshui and Juanshui tributaries, respectively. Site 5 was fixed on the Xiangjiang River’s upstream area. Sites 6 and 7 were located in Xiangtan City’s central area. Site 8 was located downstream. Analyses of water quality parameters were collected from November 2018 to March 2019.
Water Collection
Water samples were collected during a five-month period from eight sampling sites located along the Xiangjiang River in Xiangtan City, China. Glass bottles with TFE-lined screw caps were used for water sampling. Prior to use, the bottles were soaked in 1 M HCl solution overnight and then rinsed with an ultrapure water. The samples were transported to laboratory for treatment within 24 h. Background water matrix measurements included UV254, EC, dissolved oxygen (DO), pH value, temperature and dissolved organic carbon (DOC). The EC values at measured temperatures were incorporated into the test values at 25 °C. The UV254 was measured on a TU-1901 UV-visible spectrophotometer (Purkinje General Instrument Co., Ltd., Beijing, China). Water quality parameters were analyzed after the samples were filtered through a membrane (pore size = 0.45 μm, 30 kDa and 10 kDa). pH and EC were measured using an Ohaus Starter ST 2100 pH meter and an Ohaus Starter ST10C-C conductivity meter, respectively. The DO concentration was determined using a Huanyu JPB-607A portable dissolved oxygen meter (Shanghai Precision and Scientific Instrument Co., Ltd., China). In other aquatic environments, only pH, temperature, and EC values were tested. The DOC was measured using an Elementar Vario MICRO Cube (Elementar Analysensysteme GmbH, Germany), respectively.
Excitation-Emission Matrix Spectroscopy (EEMs) Measurement and Analysis
EEMs of DOM of 0.45 μm, 30 kDa and 10 kDa membrane filtered water samples were tested using an FL-4600 fluorescence spectrophotometer (Hitachi High Technologies, Tokyo, Japan). A molecular weight cut-off of 30 kDa, 10 kDa, and 3 kDa for ultrafiltration membranes was conducted using a 300-ml MSC 300 ultrafiltration cup (Shanghai Mosu Science Equipment Co., Ltd., China) under a nitrogen pressure of 0.1 MPa with a magnetic stirring speed of 100 r/min. After water samples permeated the membrane, the filtrates were collected for quality measurements using an ultraviolet light at 254 nm (UV254) to measure the intensity of attenuated light to obtain the absorbance per path length (abs/cm) and a dissolved organic carbon (DOC) analysis method. The excitation wavelengths and emission wavelengths were fixed from 200 nm to 400 nm in 2 nm steps, and from 200 to 500 nm in 3 nm steps, respectively. The fluorescence intensity of an ultra-pure water as a blank sample was subtracted. The Rayleigh and Raman scatter were removed following the reported procedure as found in [32]. A preliminary experiment ensured it did not cause the internal filtration due to the DOC variation among samples using dilution. The corrected EEMs data was fractioned into different components using the PARAFAC model [33]. The split-half analysis was used to identify the best number of the factors. The maximum fluorescence intensity (Fmax) of each derived component was used to evaluate quantitative and qualitative differences between samples. Their correlation with EC was evaluated using correlation coefficient (r) with p value. The EEMs-PARAFAC model analysis was performed on MATLAB using the N-way toolbox.
Results and Discussion
Water Characteristics
Water characteristics included UV254, EC, DO, pH, temperature and DOC with average values of 0.06 cm−1, 480 μS/cm, 10.03 mg/L, 7.45, 13.43 °C and 4.22 mg/L, respectively. The average 100 times ratio of UV254 to DOC as an SUVA was 1.4 (cm−1.L/mg). The results of DOM EEMs of upstream river site 8 and DOM EEMs of downstream site 5 are as follows: Both the upstream and downstream river water had fluorescence characteristics (see Fig. 3a-b), primarily including typical fluorophores of humic acid-like material, protein-like material and fulvic acid-like material. These components are often found in aquatic environments such as wastewater [34], river water [35] and drinking water [36]. Each category can be divided some other subclasses. According to fluorescence region integration (FRI) developed by Chen et al. [37], these EEMs regions were divided into five parts: I) aromatic protein-like materials, II) aromatic protein-like materials, III) fulvic acid-like materials, IV) soluble microbial metabolites, and V) humic substance-like material. The average intensity ratios of the above fluorescence fractions on upstream DOM to downstream DOM were 1.05 for I, 1.36 for II, 0.86 for III, 1.77 for IV and 1.17 for V. These fraction ratios showed higher values except for III. The aromatic protein-like materials and soluble microbial metabolites showed higher ratio values. As shown in Fig. 3c, the EC variations of all samples for each time measurement were relatively stable with the downstream site 1 and site 8 having higher EC values. Upstream EC values were relatively lower. In addition, at the intersection of water sources site 3 and site 4, higher EC values were recorded.
Results of three dimension excitation-emission matrix spectroscopy of DOM: (a) DOM of upstream river site 8, (b) DOM of downstream site 5, and (c) EC distribution of all sample sites after measuring five times during 2018–2019 year. The Roman numeral colors represent five categories of EEM data: I) aromatic protein-like materials, II) aromatic protein-like materials, III) fulvic acid-like materials, IV) soluble microbial metabolites, and V) humic substance-like materials. Signatures of four EEMs-PARAFAC model components a-d, denoted as c1, c2, c3 and c4, respectively. These components were identified in 40 filtered waters samples using a 0.45 μm pore size membrane. Contour plots show the spectral shapes of excitation and emission-derived components (d-g). Excitation (Ex.) is shown as a dashed line, and emissions (Em.) is shown as a solid line representing loadings (h-k)
EEMs-PARAFAC-based assessment of DOM in natural and engineered systems has been a high priority and a potential assessment method has been investigated [38]. Figure 3d-g shows four EEMs-PARAFAC-based independent components (denoted as c1, c2, c3 and c4), respectively. Figure 3h-k show their spectral loadings, corresponding to c1-c4 components. It shows the excitation (denoted as Ex) and emission (denoted as Em) loadings for each component, which were obtained from EEMs-PARAFAC models on random halves of the data array. The c1 exhibits an excitation maximum at a wavelength of 266 nm and a secondary excitation peak at 338 nm with a maximum emission at 446 nm, which has been classified as a high-molecular weight hydrophobic humic acid-like fluorophore [39]. The c2 shows two excitation maxima at 244 and 304 nm with a single emission maximum at 386 nm, which has been identified as an intermediate molecular weight fulvic acid-like fluorophore [39]. The c2 is marine and terrestrial humic material resulting from possible microbial reprocessing, while c1 is a terrestrially-derived component [40]. The c3 had two main excitation maxima at 232 nm and 274 nm, with an emission peak at 338 nm. They all resemble protein-like structures similar to high-excitation wavelength tryptophan acid-like materials [40]. The c4 had the stronger peaks at lower excitation wavelengths of 222 nm, with characteristic emission wavelengths (nm) ranging between 300 nm and 450 nm, which typically represent low excitation wavelength tryptophan acid-like substances [41].
Correlation Coefficient of Components and EC
We tested the EC correlation with components of the Xiangjiang River. Figure 4 shows the plots of EC vs. fluorophores filtered by a 0.45 μm pore size membrane (p < 0.05). It showed that the r values between EC and components (c1, c2, c3 and c4) were 0.54, 0.76, 0.67 and − 0.65, respectively. The positive correlation occurred between EC and the components c1, c2 and c3, as well as the negative correlation between c4 and EC. The r value between EC and c2 was the best, followed by c3, and finally c4 and c1. Overall, two kinds of correlation characteristics show that both positive correlations and negative correlations can co-exist in the water environment.
Results of plots of EC vs. EEMs-PARAFAC model components of dissolved organic matter samples. The results showed that three components were positive correlation with EC and one component was negative correlation with EC. Statistical results showed that the p-values for correlation of Fmax with EC values are lower than 0.05 for all plots
Correlation Result of Other Membrane Fractioned DOM Components and EC
The EC values of 0.45 μm, 30 kDa, and 10 kDa filtered water samples and raw water samples are almost the same. The standard errors were lower than 0.013 μS/cm. This shows that it is difficult for ultrafiltration membranes with 30 kDa and 10 kDa to retain ions, and the total ions of raw water influenced the whole filtered samples with different-size membranes ranging from a 0.45 μm membrane to a 10 kDa membrane. EEMs of water samples filtered by different membranes show similar shapes. Quantitative similarities for molecular weight fractionations were analyzed using uncorrected matrix correlation (UMC) as shown in Table 1. Uncorrected matrix correlation evaluates the degree of similarity between two-component fluorescent mixtures through the sum of the eigenvalues of the m x n matrices using the following equation [42, 43]:
where A and B are the matrices under comparison. ‖A‖ is [(ATA)]1/2, and ‖B‖ is [trace(BTB)]1/2.
The UMC value ranged between 0 and 1 and was used to measure the degree of spectral overlapping between two components. The 0 and 1 values for matrices indicate null and complete spectral overlapping, respectively. Between the fractionations of 0.45 μm and 30 kDa membrane filtered samples, it showed that the UMC values of c1, c2, c3 and c4, were 0.9977, 0.9955, 0.9938 and 0.9749, respectively. Between the fractionations of 0.45 μm and 10 kDa membrane filtered samples, the UMC values of c1, c2, c3 and c4 were 0.9960, 0.9940, 0.9927 and 0.9642, respectively. Between the fractionations of 0.45 μm and 10 kDa membrane filtered samples, UMC values of c1, c2, c3 and c4, were 0.9994, 0.9986, 0.9951 and 0.9794, respectively. All of the results showed that all components have a relatively complete spectral overlapping, which were comparable among all the filtered water samples.
Different ultrafiltration samples produced different correlation results between EC and the DOM fluorophores, as shown in Fig. 5. This variation in correlation results showed that the r values between EC and components c2 and c3 of the 30 kDa fractioned DOM were expressed as r = 0.68 for c2 and 0.45 for c3. These r values were smaller than those r values between EC and 0.45 μm filtered c2 and c3; then, the r = 0.76 for c2 and 0.67 for c3. The 10 kDa fractioned DOM had r values where r = 0.75 for c2 and 0.71 for c3, suggesting that EC had a higher influence on those organic matter of 0.45 μm–30 kDa, and less influence on 10 kDa fractioned DOM, rather than that between 10 kDa and 30 kDa fractioned DOM. The correlation between EC and c1 was slightly different from that between c2 and c3. The r between EC and the 10 kDa fractioned fluorophores was smaller than that above the 10 kDa fractioned DOM; thus, the EC‘s influence on that organic matter above 10 kDa was significant. The negative correlation between EC and c4 continued throughout the molecular weight distribution, with values ranging from −0.52 to −0.66. Also, EC was highly correlated with components below 0.45 μm to 30 kDa and less than 10 kDa. These results showed that the membrane filtered DOM fluorescence with the ionic variations were different. These results also showed the ultrafiltration DOM samples to be varied with the ions, and their correlation variations agree with those occurring in 0.45 μm membrane filtered samples, but their correlation degree was different. Those results better explained the ionic bonding to DOM, possibly resulting in fluorescent emission.
Results of plots of EC vs. EEMs-PARAFAC model components of DOM filtered samples where (a) 30 kDa pore size membrane, and (b) 10 kDa pore size membrane. In this figure, three components were found positive correlation with EC and one component was negative correlation with EC. Statistical results showed that the p-values for correlation of Fmax with EC values were lower than 0.05 for all plots
A Survey in Other Water Environments
Based on the identified knowledge from investigation of Xiangjiang River, a survey was conducted to see if the knowledge could also be used to other aquatic environments. Surveys of other aquatic environments were based on water quality from the Yellow Sea in Dalian City in the Liaoning Province, Chaohu Lake in Hefei, Anhui Province, the Yangtse River in Nanjing’s Jiangsu Province, and the Zhujiang River in the Guangzhou Province, where 0.45 μm membranes were used to filter water samples and were analyzed using EC and EEMs. The samples providing data were collected between Oct. 2019 and Nov. 2019. The Yellow Sea sampling point was located in Dalian City in eastern China and included 12 sites and 42 samples supporting the data used in this study. We measured the EC values of seawater, which is much higher than that of other surface water. Chaohu Lake provided 18 samples tested in eight sites. It is located in Hefei City, Anhui Province, China, with an area of 2063 km2. It is the fifth largest freshwater lake in China. Eutrophication in the Chaohu lake has been very serious for decades with an overabundance of algae. The Yangtse River is the longest river in Asia. From this river, we obtained 42 samples from 42 sites with data located in Nanjing, downstream of the Yangtse River. The Zhujiang River (also known as the Pearl River) is the second largest river in China. We obtained samples from its 46 sampling sites to generate the data needed for this study. The sampling sites for the Zhujiang (Pearl) River were located in Guangzhou City, China.
In this study, we conducted a short-term 2-month water sample collection to observe whether the correlation characteristics between EC and the components of the Xiangjiang water samples collected in our long-term (November 2018 to March 2019) study as presented in this paper are also suitable for other waters. There are eight kinds of ions distributed widely in typical China natural water environment, including Ca2+ (mg/L), Mg2+ (mg/L), Na+ (mg/L), K+ (mg/L), HCO3−, (mg/L), SO42− (mg/L), Cl− (mg/L) and NO3− (mg/L). The characteristics of a natural water environment showed that the ionic composition not only depends on the different variations of different regions, but also on the water environmental characteristics. Therefore, we observed the correlation characteristics of DOM’s fluorescence with EC in other typical water environments, including the highly saline Yellow Sea in northern China (test temperature around 20 °C, pH around 7.57), the algae-polluted Chaohu lake in central China (test temperature around 19.2 °C, pH around 7.75), the Zhujiang River in southern China (test temperature around 20.6 °C, pH around 7.34), and the eastern Yangtse River (test temperature around 20.7 °C, pH around 7.84). The experimental data was collected from October to November 2019 during a short period (around two weeks for each test). The sampling locations in China‘s map and the results of EEMs-PARAFAC model components of DOM from different water environments are shown in Fig. 6.
Sampling locations in China’s province map and the EEMs-PARAFAC components of DOM in these water environments. In the plots of EEMs and components, x-axes stand for the emission wavelength (Em.) and excitation wavelength (Ex.), respectively, while y- axes stand for excitation wavelength (Ex.) and emission wavelength (Em.), respectively
The Yellow Sea water with its high salinity contained two components, and the correlation coefficient of EC with the components was small (r < 0.2) (see Figs. 6 and 7a): The r values of c1, c2, and EC were r = 0.1, p > 0.05, and r = 0.15, p > 0.05, respectively. The correlation was not significant in the Yellow Sea except that c1 and c2 in the Yellow Sea samples represented protein-like components, whereas the components in other waters were similar to those in the Xiangjiang River samples. The Chaohu Lake water was characterized by severe eutrophication and contained four components (see Fig. 6). As shown in Fig. 7b, the r values of c1, c2, c3, c4 as correlated by EC were r = −0.43, p < 0.05, r = 0.04, p > 0.05, r = −0.35, p < 0.05, and r = − 0.71, p < 0.05, respectively. Among them, the negative correlation of EC with c4 was the most significant, but with other components the correlation was not remarkable (|r| < 0.5). The EC values of the Zhujiang River and Yangtze River were lower than those of the Yellow Sea and Chaohu Lake, and the correlation of EC with components was also significantly different from the Fmax of EEMs-PARAFAC components shown in Figs. 6 and 7c-d. There were four components identified in the Zhujiang River. The correlation coefficients of c1, c2, c3, and c4 with EC were r = 0.64, p < 0.05, r = 0.73, p < 0.05, r = 0.76, p < 0.05 and r = −0.40, p < 0.05, respectively. There were also four components identified in Yangtze River. The correlation coefficients of c1, c2, c3, and c4 with EC were r = 0.51, p < 0.05, r = 0.78, p < 0.05, r = 0.34, p < 0.05 and r = −0.44, p < 0.05, respectively. The correlation was dependent on water characteristics. For example, the correlation coefficients of EC with c4 in the Zhujiang River and Yangtze River were lower than those in Chaohu Lake and Xiangjiang River, while other components in the Chaohu lake water did not exhibit a correlation with EC, which was the opposite of what happened in other waters. The monitoring period of other waters was shorter than that of the Xiangjiang River, and the dimensions of EC were not large, which may affect the analysis of correlation characteristics. The composition of ions and DOM in seawater and surface water is different, and the pollution degree of surface water is different, which may lead to different effects of ions on optical properties of DOM. However, in any case, similar trends can be found that coincide with the Xiangjiang River, which showed that the negative correlation of EC with c4 existed in all of the tested surface waters, and a positive correlation with other components was related to the surface water characteristics.
Equation of EC as Function of Component
The water samples for the EEMs collections of DOM were always pre-treated with a simple filtration method using a 0.45 μm membrane or other membrane if needed. Based on the test results in in this study, the 0.45 μm pore size membrane or ultrafiltration membrane pore size (of 30 kDa and 10 kDa), it is difficult to reduce the EC values; thus it has a wide range of influence on the three kinds of molecular weight. The EC effect showed a negative correlation of EC with three DOM components c1, c2, and c3, and the positive correlation with one DOM component c4, which were found existing in surface water environments. Ions coexist in different forms in the water environment. We assumed that all the ions that determine the EC in a natural water environment have an effect on the fluorescence properties of DOM, including those that cause quenching and fluorescence enhancement. It can play an interactive role of ion quenching and enhancement thereby leading to a decrease or an increase in the level of fluorescence because even though some ions have no effect on fluorescence, the amount of ions are proportional to the binding of unaffected DOM. These ions are correlated with all of the corrected component values by a factor. Because there was a certain linear correlation between components and EC, the following proposed equation presents the EC values correlated with all components as:
where b is the EC-related environmental factor constant (μs/cm); I is the number of components; ki is the ith corrected factor corresponding to the corrected component (AU), ci, and EC is the electric conductivity (μs/cm). The interaction between DOM and ions can also be affected by other factors, which is why the b value was added, which represents the influence of other environmental characteristics. The greater the absolute value of b, the higher the influence of other factors. If b is equal to zero, it implies that all components can express the EC value adequately.
Because the equation used MATLAB (Version R2010B) to fit the experimental data, the equations and plots of the predicted measured vs. predicted values are given in Table 2. The results showed that the fittings had high r values, as high as 0.86 for 0.45 μm filtered membranes and 0.75 for 30 kDa filtered membranes to test samples from the Xiangjiang River, 0.84 for 10 kDa filtered membranes in Xiangjiang River, 0.77 for Chaohu Lake, 0.79 for Zhujiang River, and 0.81 for the Yangtse River. Compared with other surface water, it showed the highest b value of 359.8507 in the seriously polluted Chaohu lake, implying a significant influence of other factors in the accuracy of the EC equation using components as independent variables. In short-term monitoring of the latter surface water, the dispersion of the EC range was not wide, but it was still in good agreement with the equation. Combined with the good fit of the Eq. 2, the result demonstrated that EC is dependent on DOM’s fluorescence components, which supported our assumption.
Conclusions
This study discussed the relationship between the EC values and aquatic dissolved organic matter’s fluorescence components. The correlation plays significant roles: It can reveal the interaction characteristics of strength of ions and DOM; it can provide added knowledge to support the use of EEMs in the evaluation of variations in DOM. The results led to the following conclusions: Even though no correlation could be found between highly saline seawater’s EC and its two protein components, an EC correlation was found with the components of Xiangjiang River showing that EC is positively correlated with c1, c2, and c3, and negatively correlated with c4. The evaluation of EEMs on the DOM of an ultrafiltration system may be affected by the ions. 30 kDa and 10 kDa filters cannot be used to filter the ions of raw water samples, so the EC effect on DOM throughout the whole molecular weight of DOM showed that they were better correlated well with c2–c4 of a 10 kDa filtered water sample (r > 0.6) and with c2 and c4 using 30 kDa filtered water samples (r > 0.5). The surface water environment is different from the sea water with high salinity. The correlation between EC and DOM in the surface is universal, and they have different characteristics; thus, the negative correlation between EC and c4 existed in all of investigated surface waters, whereas the positive correlation between other three components was dependent on their water characteristics. An assumed EC equation is the function of components that fit well with surface waters with a correlation coefficient, r, greater than 0.75, making it a potential monitoring tool to indicate the variations of fluorescent components showing water quality. In addition, it also advocates not using EEMs-PARAFAC to investigate component variations without considering the EC values. These findings were successfully tested in this study and can accurately and rapidly evaluate the variations of aquatic DOM, which can help researchers better understand its monitoring value in water and water remediation and can lead to convenient management of water and water remediation.
References
Summers RS, Roberts PV (1988) Activated carbon adsorption of humic substances: I. Heterodisperse mixtures and desorption. J Colloid Interface Sci 122:367–381
Hyung H, Kim J-H (2008) Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: effect of NOM characteristics and water quality parameters. Environ Sci Technol 42:4416–4421
Klaus U, Pfeifer T, Spiteller M (2000) APCI-MS/MS: a powerful tool for the analysis of bound residues resulting from the interaction of pesticides with DOM and humic substances. Environ Sci Technol 34:3514–3520
He P-j, Xue J-f, Shao L-m, Li G-j, Lee D-J (2006) Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill. Water Res 40:1465–1473
Ravichandran M (2004) Interactions between mercury and dissolved organic matter––a review. Chemosphere 55:319–331
Kalbitz K, Wennrich R (1998) Mobilization of heavy metals and arsenic in polluted wetland soils and its dependence on dissolved organic matter. Sci Total Environ 209:27–39
Reuter J, Perdue E (1977) Importance of heavy metal-organic matter interactions in natural waters. Geochim Cosmochim Ac 41:325–334
Zhu G, Yin J, Zhang P, Wang X, Fan G, Hua B, Ren B, Zheng H, Deng B (2014) DOM removal by flocculation process: fluorescence excitation–emission matrix spectroscopy (EEMs) characterization. Desalination 346:38–45
Bian Y, Wang C, Zhu G, Ren B, Zhang P, Hursthouse AS (2018) Occurrence and control of N-nitrosodimethylamine in water engineering systems. Environ Eng Res 24:1–16
Nebbioso A, Piccolo A (2013) Molecular characterization of dissolved organic matter (DOM): a critical review. Anal Bioanal Chem 405:109–124
Li P, Hur J (2017) Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: a review. Crit Rev Environ Sci Technol 47:131–154
Zhu G, Bian Y, Hursthouse AS, Wan P, Szymanska K, Ma J, Wang X, Zhao Z (2017) Application of 3-D fluorescence: characterization of natural organic matter in natural water and water purification systems. J Fluoresc 27:2069–2094
Kujawinski EB, Behn MD (2006) Automated analysis of electrospray ionization Fourier transform ion cyclotron resonance mass spectra of natural organic matter. Anal Chem 78:4363–4373
Zhang H, Zhang Y, Shi Q, Ren S, Yu J, Ji F, Luo W, Yang M (2012) Characterization of low molecular weight dissolved natural organic matter along the treatment trait of a waterworks using Fourier transform ion cyclotron resonance mass spectrometry. Water Res 46:5197–5204
Zhu G, Bian Y, Hursthouse AS, Xu S, Xiong N, Wan P (2020) The role of magnetic MOFs nanoparticles in enhanced iron coagulation of aquatic dissolved organic matter. Chemosphere:125921
Zhu G, Fang H, Xiao Y, Hursthouse AS (2020) The application of fluorescence spectroscopy for the investigation of dye degradation by chemical oxidation. J Fluoresc 30:1271–1279
Song K, Shang Y, Wen Z, Jacinthe P-A, Liu G, Lyu L, Fang C (2019) Characterization of CDOM in saline and freshwater lakes across China using spectroscopic analysis. Water Res 150:403–417
Christian E, Batista JR, Gerrity D (2017) Use of COD, TOC, and fluorescence spectroscopy to estimate BOD in wastewater. Water Environ Res 89:168–177
Sargsyan S (2016) A method for determining and exploring the distribution of organic matters and hardness salts in natural waters. Appl Water Sci 7:1–6
Davies CW (1938) 56. The extent of dissociation of salts in water. Part VI. Some calcium salts of organic acids. J Chem Soc 10:277–281
Apelblat A (1993) Solubilities of organic salts of magnesium, calcium, and iron in water. J Chem Thermodyn 25:1443–1445
Kloster N, Brigante M, Zanini G, Avena M (2013) Aggregation kinetics of humic acid: effects of Ca2+ concentration, Functions of Natural Organic Matter in Changing Environment, Springer
Howard CS (1933) Determination of Total dissolved solids in water analysis. Ind Eng Chem Anal Ed 5:4–6
Provenzano MR, D'Orazio V, Jerzykiewicz M, Senesi N (2004) Fluorescence behaviour of Zn and Ni complexes of humic acids from different sources. Chemosphere 55:885–892
Sunner J, Nishizawa K, Kebarle P (1981) Ion-solvent molecule interactions in the gas phase. The potassium ion and benzene. J Phys Chem 85:1814–1820
Stoughton R, Rollefson G (1939) The influence of ionic strength on the quenching of fluorescence in aqueous solutions. J Am Chem Soc 61:2634–2638
Lei Y (2004) Environmental chemistry of aquaculture water. China Agriculture Press, Beijing
Hua B, Veum K, Yang J, Jones J, Deng B (2010) Parallel factor analysis of fluorescence EEM spectra to identify THM precursors in lake waters. Environ Monit Assess 161:71–81
Hua B, Veum K, Koirala A, Jones J, Clevenger T, Deng B (2007) Fluorescence fingerprints to monitor total trihalomethanes and N-nitrosodimethylamine formation potentials in water. Environ Chem Lett 5:73–77
Yang L, Hur J, Zhuang W (2015) Occurrence and behaviors of fluorescence EEM-PARAFAC components in drinking water and wastewater treatment systems and their applications: a review. Environ Sci Pollut R 22:6500–6510
Zhou Z, Guo L, Shiller AM, Lohrenz SE, Asper VL, Osburn CL (2013) Characterization of oil components from the Deepwater Horizon oil spill in the Gulf of Mexico using fluorescence EEM and PARAFAC techniques. Mar Chem 148:10–21
Bahram M, Bro R, Stedmon C, Afkhami A (2006) Handling of Rayleigh and Raman scatter for PARAFAC modeling of fluorescence data using interpolation. J Chemom 20:99–105
Moradi S, Sawade E, Aryal R, Chow CW, van Leeuwen J, Drikas M, Cook D, Amal R (2018) Tracking changes in organic matter during nitrification using fluorescence excitation–emission matrix spectroscopy coupled with parallel factor analysis (FEEM/PARAFAC). J Environ Chem Eng 6:1522–1528
Nimptsch J, Woelfl S, Kronvang B, Giesecke R, González HE, Caputo L, Gelbrecht J, von Tuempling W, Graeber D (2014) Does filter type and pore size influence spectroscopic analysis of freshwater chromophoric DOM composition? Limnologica 48:57–64
Phong DD, Hur J (2015) Insight into photocatalytic degradation of dissolved organic matter in UVA/TiO2 systems revealed by fluorescence EEM-PARAFAC. Water Res 87:119–126
Hur J, Hwang SJ, Shin J-K (2008) Using synchronous fluorescence technique as a water quality monitoring tool for an urban river. Water Air Soil Pollut 191:231–243
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37:5701–5710
Sanchez NP, Skeriotis AT, Miller CM (2014) A PARAFAC-based long-term assessment of DOM in a multi-coagulant drinking water treatment scheme. Environ Sci Technol 48:1582–1591
Ishii SK, Boyer TH (2012) Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: a critical review. Environ Sci Technol 46:2006–2017
Piotr Kowalczuk MJD, Young H, Kahn AE, Cooper WJ, Gonsior M (2009) Characterization of dissolved organic matter fluorescence in the South Atlantic bight with use of PARAFAC model: interannual variability. Mar Chem 113:182–196
Zhu G, Wang C, Dong X (2017) Fluorescence excitation–emission matrix spectroscopy analysis of landfill leachate DOM in coagulation–flocculation process. Environ Technol 38:1489–1497
Sanchez NP (2013) Fluorescence based approach to drinking water treatment plant natural organic matter (NOM) characterization, treatment, and management. University of Akron, Akron
Burdick DS, Tu XM (1989) The wavelength component vectorgram: a tool for resolving two-component fluorescent mixtures. J Chemom 3:431–441
Acknowledgements
This work is financially supported by Natural Science Foundation of Hunan Province of China (Grant number 2018JJ2128), National Natural Science Foundation of China (Grant number 51408215), and the China Postdoctoral Science Foundation (Grant number 2017 M622578).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, G., Xiong, N., Wang, X. et al. Correlation Characteristics of Electrical Conductivity of Surface Waters with the Fluorescence Excitation-Emission Matrix Spectroscopy-Parallel Factor Components of Dissolved Organic Matter. J Fluoresc 30, 1383–1396 (2020). https://doi.org/10.1007/s10895-020-02628-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02628-6