Abstract
Herein, we have studied the analysis of fluorescence quenching for newly synthesized biologically active 3(2H)-pyridazinone derivative 5-(5-bromo-2-hydroxy-phenyl)-2-phenyl-2H-pyridazin-3-one [BHP] by various concentrations of aniline using UV-Visible spectroscopy, fluorescence spectroscopy and time-correlated single photon counting technique in five different solvents namely, methanol, ethanol, propan-2-ol, dimethylsulfoxide and ethyl acetate at room temperature. The fluorescence intensity of BHP molecule decrease with increasing in the aniline concentration and it is studied using the Stern-Volmer relation. The obtained Stern-Volmer plots were found to be linear in all the five solvents. The various parameters responsible for the fluorescence quenching such as quenching rate parameters (k q ), diffusion rate parameter (k d ) and the probability of quenching per encounter (p) were experimentally calculated in all five solvents. An activation energy of quenching (E a ) was calculated using the values of activation energy of diffusion (E d ) and p. It was found that the values of E a are greater than E d in all five solvents studied. Further, it is inferred that the fluorescence quenching reactions in BHP molecule are more significantly affected by activation energy processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 3(2H)-pyridazinones are the pyridazine derivatives which contain two adjacent nitrogen atoms at the one and two positions in a six-membered ring and a carbonyl group at the three position and they have different functionalities in their structure [1, 2]. A substantial number of pyridazinones derivatives are reported to possess antimicrobial, antitubercular, analgesic, anti-inflammatory, cyclooxygenase inhibitor, antidiabetic, antihypertensive, antiplatelet, anticancer, antifungal, antidepressant–anxiolytic, anticonvulsant, bronchodilatory (asthma), anti-allergic, antifeedant, inhibition of linolenic acid, activity for neurological disorders and many other properties [3]. Some of the major pyridazinone derivatives which have appeared in the market are indolidan, bemoradan, pimobendan, levosimendan as antihypertensive, minaprine as an antidepressant, emorfazone as anti-inflammatory and azanrinone as a cardiotonic [3].
Recently, spectroscopic and quantum chemical investigations on 3(2H)-pyridazinone derivatives such as levosimendan (IUPAC name: 2-[[4-[(4R)-4-methyl-6-oxo-4,5-dihydro-1H-pyridazin-3-yl]phenyl]hydrazinylidene]propanedinitrile) and bromopyrazone (IUPAC name: 1-phenyl-4-amino-5-bromopyridazon-(6)) compounds have been reported in the literature [4, 5]. In addition, the detailed structural, conformational, spectroscopic, electronic and nonlinear optical properties of the 3(2H)-pyridazinone derivatives namely flufenpyr (IUPAC name: {2-chloro- 4-fluoro-5-[5-methyl-6-oxo-4-(trifluoromethyl)-1(6H)-pyridazin-1-yl] phenoxy}acetic acid)) used in agriculture as a herbicide and amipizone (IUPAC name: 6-(p-(2- chloropropionylamino)phenyl)-5-methyl-4,5-dihydropyridazin-3- one)) designed to be antithrombotics and an inhibitor for platelet aggregations and the cardiovascular system) compounds estimated at the B3LYP (Becke’s three-parameter exact exchange functional (B3) combined with the gradient-corrected correlational functional of Lee, Yang and Parr (LYP)), B3PW91(Becke’s three parameters incorporating Perdew and Wang’s 1991 gradient-corrected exchange and correlation functions and includes 20% Hartree − Fock exchange) and HSEH1PBE (Heyd − Scuseria − Ernzerhof hybrid combined with Perdew, Burke and Ernzerhof’s exchange and correlation functions) levels of theory with the basis set 6-311G (d, p) [6]. Soliman et al. reported the molecular structure, spectroscopic properties, NLO (nonlinear optical), HOMO (highest occupied molecular orbital) – LUMO (lowest unoccupied molecular orbital) and NBO (natural bond orbital) analysis of 6-hydroxy-3(2H)-pyridazinone [7]. Recently, we have reported the photophysical properties particularly fluorescence quantum yield and ground and excited state dipole moments of 3(2H)-pyridazinone derivatives [8, 9]. However, there are no reports on the fluorescence quenching of these derivatives. So, we are the first to report the fluorescence quenching of said molecule.
The phenomenon of fluorescence quenching competes with the spontaneous emission and causes the reduction in the fluorescence intensity and lifetime of the fluorescence molecules [10]. Generally, it is a process in which the electronic excitation energy of an excited molecule is transferred to a quencher molecule via several mechanisms such as diffusion, charge transfer and energy transfer etc., it leads to the non-fluorescent emission of quencher molecule. Under steady state illumination, the rate of formation of an excited molecule M* is equal to its rate of deactivation and the concentration of excited molecule [M*] remains constant.
In the absence of any bimolecular step, the concentration of M* is given below
where
- Ra :
-
Rate of formation of the activated molecule.
- k f :
-
Rate constant for fluorescence.
- ∑k i :
-
Sum of the rate constants for all the unimolecular deactivation steps such as internal conversion (k IC ) and intersystem crossing (k ISC ) which originate from this state.
If another molecule Q is added to the solution which quenches the fluorescence intensity of molecule M by bimolecular quenching step, then:
Then the concentration of fluorophore (excited molecule) [M*] in the presence of the quencher is given below
If [M*] 0 and [M*] are the fluorophore concentrations in the absence and presence of quencher, the respective quantum yields F 0 and F are given below
then the ratio of two quantum yields gives:
Equation (7) is known as the Stern- Volmer [S-V] equation.
where
- ᅟ:
-
\( {\tau}_0=\frac{1}{\left({k}_f+\sum {k}_i\right)} \)
- K sv :
-
=k q τ 0
F 0 and F are the fluorescence intensities without and with quencher respectively.
K SV is the S-V constant and [Q] is the quencher concentration. The S-V constant K SV can be estimated from the slope of the linear plot of F 0 /F versus [Q]. k q is the S-V quenching rate parameter and τ 0 is the fluorescence lifetime of the solute in the absence of quencher.
The fluorescence quenching of organic molecules in solvents by various quenchers like carbon tetrachloride [11, 12], aniline [13,14,15], oxygen [16], TiO2 nanoparticles [17, 18] and silver nanoparticles [19] have been a subject of continued investigators for last two decades. The aim of above-said investigations is to know the whether the fluorescence quenching is dynamic or static in nature and these results of interactions between biologically active molecules with biosystems and nanoparticles may shine in the area of biological applications. In general, the fluorescence quenching phenomenon is very useful in the field of physical science, chemical science and medical science [20, 21].
In view of the biological importance of 3(2H)-pyridazinone derivatives and fluorescence quenching process, we have studied the fluorescence quenching of newly synthesized biologically active 3(2H)-pyridazinone derivative 5-(5-bromo-2-hydroxy-phenyl)-2-phenyl-2H-pyridazin-3-one [BHP] by the various concentration of aniline in five different solvents. Further, we have estimated various fluorescence quenching rate parameters responsible for fluorescence quenching using the Stern-Volmer relation.
Experimental
Materials
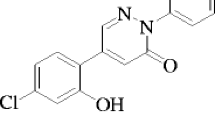
The biologically active 3(2H)-pyridazinone derivative 5-(5-bromo-2-hydroxy-phenyl)-2-phenyl-2H-pyridazin-3-one [BHP] molecule is synthesized according to reference [22], the completion of the reaction, purity of the molecule was checked by TLC (Thin-layer chromatography). Further, purity as 100% was confirmed by recording the spectrum using Agilent-single Quartz LC-MS (Liquid chromatography–mass spectrometry). The elemental analysis was carried out using Heraus CHN rapid analyzer. The melting point was determined by using a Shital melting-point apparatus. Functional groups were analyzed with Nicolet-5700 Fourier transform infrared (FTIR) spectrophotometer. Structural confirmation of molecule was analyzed with 1H and 13C NMR spectra were recorded on Bruker 400-MHz spectrometer using CDCl3 as a solvent and tetramethylsilane (TMS) as an internal standard and were discussed in reference [22]. The molecular structure of BHP is shown in Fig. 1. Solvents methanol, ethanol, propan-2-ol, dimethylsulfoxide (DMSO) and ethyl acetate (Solvents are chosen based on the high solubility of BHP molecule) were of spectroscopic grade and they were obtained from S.D. Fine Chemicals Ltd., India. The quencher aniline of ACS grade (>99.5% assay) was double distilled and tested for its purity before use. Solutions were prepared to keep the concentration of solute BHP fixed 1 × 10−5 M and varying quencher concentrations (0.00 μM, 0.06 μM, 0.13 μM, 0.20 μM, 0.26 μM and 0.33 μM) in all five different solvents.
Spectroscopic Measurements
In all the spectroscopic measurements quartz cuvettes were used. For the absorption spectra we have used two sides transparent, then for fluorescence spectra and fluorescence lifetimes, we have used four sides transparent cuvette. These cuvettes are having 1 cm width and 5 cm height.
-
Absorption spectra: Absorption spectra were recorded using UV-visible spectrophotometer [Model: Hitachi U-3310 at USIC, K U Dharwad, India] in the range of 200 nm – 800 nm with bandwidth 1.0 nm and deuterium lamp used as a light source.
-
Fluorescence spectra: Fluorescence spectra were recorded in absence and presence of the quencher using fluorescence spectrophotometer [Model: Hitachi F-7000 at USIC, K U Dharwad, India] in the range of 300 nm – 600 nm with bandwidth 1 nm, voltage 400 V and Xenon lamp used as a light source.
-
Fluorescence lifetimes: Fluorescence lifetimes were recorded in the absence of the quencher using time-correlated single photon counting technique (TCSPC) [Model: ISS 90021 at USIC, K U Dharwad, India] in the bi-exponential fitting having chi-square value nearly unity using laser diodes as a source.
All these spectroscopic measurements were carried out at room temperature [300 K]. The experimental values are reproducible within 5% of the experimental error.
Results and Discussion
Fluorescence spectra of BHP molecule along with their corresponding excitation and emission wavelengths in methanol, ethanol, propan-2-ol, DMSO and ethyl acetate solvents for various quencher concentrations (aniline) were recorded and are shown in Fig. 2. Initially, the fluorescence intensity F 0 was measured without the quencher and then the fluorescence intensity F was measured for various concentrations of aniline. The experimentally measured values of fluorescence intensity for BHP molecule in five different solvents for various concentrations of aniline and are shown in Table 1. Using the experimentally measured values of F and F 0 , S–V plots were plotted and are shown in Fig. 3. From Fig. 3 it is found that S-V plots are linear with intercept nearly unity. An observed linear S-V plot indicates that the fluorescence quenching in said molecule is purely dynamic in nature. Using the least square fit method the obtained values of slope, intercept and correlation coefficient of S-V plots were shown in Table 2. The slope value of S-V plot gives the S-V constant (K SV ). Further, the fluorescence lifetime decay curve without quencher (τ 0 ) for BHP molecule in methanol, ethanol, propan-2-ol, DMSO and ethyl acetate solvents were recorded and shown in Fig. 4. The corresponding values of fluorescence lifetime are shown in Table 3. The quenching rate parameter k q is measured using the experimentally measured values of K SV and τ 0 and is given by an Eq. (8) and these measured values are shown in Table 3.
In order to study the diffusion process of fluorescence quenching mechanism in BHP molecule, we have estimated the diffusion coefficient of solute D S and the quencher D Q can be estimated using the Stoke’s Einstein Eq. (9) [23].
where
- K :
-
Boltzmann constant,
- T :
-
Temperature
- η :
-
Viscosity of the solvent
- a :
-
The Stoke’s Einstein number a = 6 for solute and a = 3 for quencher [24].
Since the radii of the solute molecule have a larger size than the solvent molecule. R = R S + R Q is the sum of the radii of solute and quencher are estimated using the method as suggested by Edward [25] and these values are shown at the bottom of Table 4. The diffusion coefficients D S for solute and D Q for quencher were calculated using Eq. (9) with help of literature values of viscosity [13] of the respective solvents and the estimated values of radii and these values are shown in Table 4. In this calculation, the viscosity of the solute and the quencher are taken as equal to the viscosity of the medium, because their concentrations are very small. Thus, using values of D and R the values of diffusion rate parameter k d was estimated using an Eq. (10) and these values are shown in Table 5.
where N ′ is the Avogadro’s number per millimole.
Further, using the values of k q and k d the probability of quenching per encounter p was estimated using an Eq. (11)
The calculated values of p in all five solvents are shown in Table 5. From Table 5, it is observed that the probability of quenching per encounter is less than unity and this fact may infer that the fluorescence quenching process in BHP molecule is governed by activation energy other than the material diffusion. In solution, the value of activation energy for diffusion E d and activation energy for quencher E a are needed in order to study the role of an activation process. Using the values gas constant R and calculated values of p and E d , E a is determined according to Eq. (12).
According to Eq. (12), the probability of quenching per encounter p is related to the activation energy. The calculated values of E a are shown in Table 5. From Table 5, it is observed that values of E a are greater than the E d in all five solvents studied. It indicates that in bimolecular fluorescence quenching reaction, the influences of activation energy for quencher process is more than the activation energy for diffusion.
Conclusion
From the present discussions of fluorescence quenching of BHP molecule by various concentrations of aniline in five different solvents namely methanol, ethanol, propan-2-ol, DMSO and ethyl acetate. Herein, we noted that;
-
The said molecule undergoes fluorescence quenching by aniline in all five different solvents at room temperature.
-
S-V plot is linear in all five different solvents.
-
The values of quenching rate parameter (k q ) are greater than diffusion rate parameter (k d ) in all five different solvents.
-
The value of probability of quenching per encounter is less than unity in all five different solvents.
-
The value of activation energy for quenching process is larger than the activation energy for diffusion in all five different solvents.
In view of these facts, it may be inferred that the fluorescence quenching of BHP molecule by the various concentrations of aniline in five different solvents is not solely due to diffusion but there is also a contribution of activation energy.
References
Alvarez-Builla J, Vaquero JJ, Barleuenga J (2011) Modern heterocyclic chemistry. Wiley-VCH, UK
Asif M (2012) Some recent approaches of biologically active substituted Pyridazine and Phthalazine drugs. Curr Med Chem 19:2984
Dubey S, Bhosle PA (2015) Pyridazinone:an important element of pharmacophore possessing broad spectrum of activity. Med Chem Res 24:3579
Bahceli S, Gokce H (2014) Study on spectroscopic and quantum chemical calculation of levosimendan. Indian Pure Appl Phys 52:224
Gokce H, Bahceli S (2014) Spectroscopic and quantum chemical studies on bromopyrazone. Spectrochim Acta A 133:741
Avcı D, Bahceli S, Tamer O, Atalay Y (2015) Comparative study of DFT/B3LYP B3PW91 and HSEH1PBE methods applied to molecular structures and spectroscopic and electronic properties of flufenpyr and amipizone. Can J Chem 93:1147
Soliman SM, Albering J, Abu-Youssef MA (2015) Molecular structure, spectroscopic properties, NLO, HOMO-LUMO and NBO analyses of 6-hydroxy-3(2H)-Pyridazinone. Spectrochim Acta A 136:1086
Desai VR, Hungund SM, Sidarai AH, Basanagouda M, Kadadermath JS (2017) Spectroscopic study for a novel pyridazin-3(2H)-one derivatives. LAM academic publishing, Germany
Desai VR, Hungund SM, Basanagouda M, Kadadermath JS, Sidarai AH (2016) Solvent effects on the electronic and fluorescence spectra of HNP: estimation of ground and excited state dipole moments. J Fluoresc 26:1391
Lackowicz JR (1983) Principles of fluorescence spectroscopy. Plenum Press, New York
Kadadevarmath JS, Malimath GH, Melavanki RM, Patil NR (2014) Static and dynamic model fluorescence quenching of laser dye by carbon tetrachloride in binary mixtures. Spectrochim Acta a 117:630
Patil NR, Melavanki RM, Kapatkar SB, Chandrashekhar K, Patil HD, Umapathy S (2011) Fluorescence quenching of biologically active carboxamide by aniline and carbon tetrachloride in different solvents using Stern–Volmer plots. Spectrochim Acta A 79:1985
Desai VR, Hungund SM, Basanagouda M, Kadadermath JS, Thipperudrappa J, Sidarai AH (2017) Spectroscopic studies on newly synthesized 5-(2-hydroxy-5-methoxy-phenyl)_2-phenyl-2-H-pyridazin-3-one molecule. J Mol Liq 225:613
Sidarai AH, Desai VR, Hunagund SM, Basanagouda M, Kadadevarmath JS (2016) Fluorescence quenching of DMB by aniline in benzene–acetonitrile mixture. Int Lett Chem Phys Astron 65:32
Sidarai AH, Desai VR, Hunagund SM, Basanagouda M, Kadadevarmath JS (2016) Effect of solvent polarity on the fluorescence quenching of TMC molecule by aniline in benzene-acetonitrile mixtures. Can J Phys 94:1125
Olmsted J (1974) Oxygen quenching of fluorescence of organic dye molecules. J Chem Phys Let 26:33
Raghavendra UP, Basanagouda M, Sidrai AH, Thipperudrappa J (2016) Spectroscopic investigations on the interaction of biologically active 4-aryloxymethyl coumarins with TiO2 nanoparticles. J Mol Liq 222:601
Desai VR, Hungund SM, Pujar MS, Basanagouda M, Kadadermath JS, Sidarai AH (2016) Specroscopic interactions of titanium dioxide nanopaticles with pharmacologically active 3(2H)-pyridazinone derivative. J Mol Liq 233:166
Deepa HR, Suresh Kumar HM, Basanagouda M, Thipperudrappa J (2014) Influence of silver nanoparticles on absorption and fluorescence properties of laser dyes. Can J Phys 92:163
Keizer J (1987) Diffusion effects on rapid bimolecular chemical reactions. Chem rev 87:167
Eftink MR, Ghiron CA (1981) Fluorescence quenching studies with proteins. Anal Biochem 114:199
Basanagouda M, Kulkarni MV (2011) Novel one-pot synthesis for 2,5-Diaryl and 5-aryl- pyridazin-3(2H)-ones. Synth Commun 41:2569
Einstein A (1956) Investigations on the theory of Brownian movement. Dover, New York
Kadadevarmath JS, Giraddi TP, Malimath GH, Chikkur GC (1996) Electronic excitation energy quenching of an organic liquid scintillator by carbon tetrachloride in different solvents. J Radiat Measure 26:117
Edward JT (1970) Molecular volumes and the stokes–Einstein equation. J Chem Edu 47:261
Acknowledgements
Authors (Vani and Shirajahammad) acknowledge the financial support under UGC-UPE fellowship (KU/SCH/UGCUPE/2014-15/916 and KU/SCH/UGC-UPE/2014-15/915) from Karnatak University Dharwad, India. Authors are thankful to Prof. N.M. Badiger director and Smt. C.R. Bharati technical staff of University Scientific and Instruments Center (USIC) Karnatak University Dharwad, India for providing UV-Visible spectrophotometer, fluorescence spectrophotometer and TSCPC instruments facilities. Authors are immensely grateful to Dr. Shivaram N. Patil (NAVKIS P.U Academy, Dept. of Physics, Mysore, Karnataka, India) for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desai, V.R., Hunagund, S.M., Basanagouda, M. et al. Analysis of Fluorescence Quenching for Newly Synthesized Biologically Active 3(2H)-pyridazinone Derivative by Aniline. J Fluoresc 27, 1839–1846 (2017). https://doi.org/10.1007/s10895-017-2121-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2121-3