Abstract
A new thiophenyl pyrazoline probe for Cu2+ in aqueous solution was synthesized and characterized by IR, NMR, HRMS and X-ray analysis. The probe displays remarkably high selectivity and sensitivity for Cu2+ with a detection limit of 1.919 × 10−7 M in aqueous solution (EtOH:HEPES = 1:1, v/v, 0.02 M, pH = 7.2). In addition, the probe is further successfully used to image Cu2+ in living cells and the probe possesses good reversibility.

A new thiophenyl pyrazoline probe can be used to image Cu2+ in living cells with good reversibility
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper ion is one of essential heavy metal ions in human body and plays an important role in various physiologic processes [1, 2]. But disruption of copper homeostasis can result in a variety of diseases such as Menkes [3], Wilson’s diseases [4], familial amyotropic lateral sclerosis [5], Alzheimer’s disease [6], and prion diseases [7]. Moreover, long-term exposure to high levels of Cu2+ can induce liver and kidney damage [8]. Therefore, a sensitive method for detecting copper in biological samples is necessary and indispensable. A number of methods have been developed for the detection of copper, including atomic absorption spectrometry [9, 10], inductively coupled plasma atomic emission spectrometry [11, 12], voltammetry [13], electrochemical method [14, 15] and fluorescent chemosensors [16, 17]. However, most of these methods need relatively high cost apparatus and cannot be used in biological applications because they entail the destruction of the sample. Consequently, fluorescent chemosensors have attracted researchers’ attention due to their high sensitivity, selectivity and easy operational use [18, 19].
Up to now, a lot of fluorescent sensors for monitoring Cu2+ based on calcein [20], rhodamine derivatives [21], Schiff base [22, 23], quinoline [24], fluorescein [25], coumarin [26, 27], indole [28] and naphthalendiimide [29] have been reported. However, major shortcomings of them are inferior selectivity [23], reversibility [22–25] or water solubility [26, 27]. Therefore, the development of water soluble and reversible fluorescent probes for monitoring Cu2+ in living cells is more appealing.
In recent years, pyrazoline derivatives have gained much attention due to their outstanding properties, such as high fluorescence quantum yields and excellent stability [30–34]. As an continuation of our work on the development of fluorescent probe for monitoring metal ions [33–37], herein we report a new pyrazoline-based fluorescent probe 3 (Scheme 1) for Cu2+ recognition. This probe with high sensitivity and selectivity for monitoring Cu2+ in aqueous solution is suitable for imaging Cu2+ in living cells.
Experimental Details
Apparatus
Thin-layer chromatography (TLC) was conducted on silica gel 60 F254 plates (Merck KGaA). 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 300 (300 MHz and 75 MHz) spectrometer, using DMSO as solvent, and tetramethylsilane (TMS) as internal standard. Melting points were determined on an XD-4 digital micro melting point apparatus. IR spectra were recorded with an IR spectrophotometer VERTEX 70 FT-IR (Bruker Optics). HRMS spectra were recorded on a QTOF6510 spectrograph (Agilent). UV–vis spectra were recorded on a U-4100 (Hitachi). Fluorescent measurements were recorded on a Perkin–Elmer LS-55 luminescence spectrophotometer. All pH measurements were made with a Model PHS-3C pH meter (Shanghai, China) and operated at room temperature about 298 K.
Reagents
Deionized water was used throughout the experiment. All the reagents were purchased from commercial suppliers and used without further purification. The salts used in stock aqueous solutions of metal ions were NaNO3, Fe(NO3)3 9H2O, AgNO3, KNO3, Co(NO3)2 6H2O, Mg(NO3)2 6H2O, Ca(NO3)2 4H2O, Al(NO3)3 9H2O, Ba(NO3)2, Cr(NO3)3 9H2O, Ni(NO3)2 6H2O, Cd(NO3)2 4H2O, Pb(NO3)2, Cu(NO3)2 3H2O, Zn(NO3)2 6H2O and HgCl2.
Synthesis of 2-(5-Phenyl-3-Thiophen-2-yl-4,5-Dihydro-Pyrazol-1-yl)-Pyridine (3)
The synthetic route of proposed compound 3 is shown in Scheme 1. Starting materials chalcone (1) and 2-hydrazinylpyridine (2) were prepared according to literature [38, 39]. A mixture of chalcone (1) (0.419 g, 2 mmol), 2-hydrazinylpyridine (2) (0.258 g, 2.4 mmol), NaOH (0.250 g, 6 mmol) and ethanol (25 ml) was stirred at reflux for 1 h. After cooling, water (100 mL) was added to the mixture. The mixture was filtered and the crude product was crystallized from ethanol to give compound 3 as white crystals, Yield: 45.2 %; mp: 164–165 °C; IR (KBr, cm−1): 3084.8, 2917.6, 1588.6, 1472.6, 1430.1, 1127.9, 766.0, 698.8; 1H NMR (300 MHz, DMSO): δ 3.14 (dd, 1H, J = 5.1, 17.4 Hz, 4-Htrans), 3.91 (dd, 1H, J = 12.3, 17.4 Hz, 4-Hcis), 5.76 (dd, 1H, J = 5.1, 12.3 Hz, 5-H of pyrazoline), 6.67 (t, 1H, J = 6 Hz, pyridine-H), 7.11 (dd, 1H, J = 3.6, 5.1 Hz, pyridine-H), 7.17–7.22 (m, 3H, Ar-H), 7.27 (s, 1H, Ar-H), 7.29–7.33 (m, 3H, Hz, thiophene-H), 7.96 (d, 1H, J = 3.7 Hz, pyridine-H); 13C NMR (75 MHz, DMSO): 155.33, 147.97, 146.63, 143.70, 137.86, 135.76, 129.03, 128.81, 127.34, 126.03, 114.86, 108.85, 60.87, 43.40. HRMS: calcd for [M + H]+ C18H16N3S: 306.1065; found: 306.1076.
A single crystal of 3 was obtained from ethanol solution and was characterized using X-ray crystallography (Fig.1).
Cell Culture and Imaging
Hela cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10 % calf bovine serum (HyClone) at 37 °C in humidified air and 5 % CO2. For fluorescence imaging, the cells (5 × 104 mL-1) were seeded into 24-well plates, and experiments to assay Cu2+ uptake were performed in the same media supplemented with 1 μM of CuCl2 for 0.5 h. The cells were washed twice with PBS buffer before the staining experiments, and incubated with 1 μM of probe 3 for 1 h in the incubator. After washing twice with PBS, the cells were imaged under a Phase Contrast Microscope (Nikon, Japan).
Results and Discussion
Absorption Properties
The absorption spectra of probe 3 in aqueous solution (EtOH:HEPES = 1:1, v/v, 0.02 M, pH = 7.2) were investigated. As shown in Fig. 2, in the absence of Cu2+ ion, probe 3 showed an absorption maximum at 356 nm with ε 2.1 × 104 M-1 cm−1 (Fig. S1, Fig. S2). Upon the addition of Cu2+, the absorption peak at 356 nm decreased with a red shift of 10 nm. Because of the binding of the probe and Cu2+, conjugate electrons of the complex delocalized more than free probe, resulting in a red shift of maximum absorption. It is obvious that none of other cations produced such a decrease in their absorption spectra.
Selectivity Studies
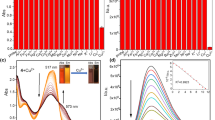
The fluorescence spectra of probe 3 with various metal ions in aqueous solution (EtOH:HEPES = 1:1, v/v, 0.02 M, pH = 7.2) were conducted to examine the selectivity. As shown in Fig. 3, the fluorescence spectra of probe 3 showed a strong fluorescence emission at 452 nm and the addition of 5 equiv. of Cu2+ induced a significant decrease in fluorescence intensity. However, the addition of other metal ions, including Al3+, Fe3+, Co2+, Ni2+, Ba2+, Ca2+, Cd2+, Cr3+, K+, Mg2+, Na+, Ag+, Hg2+, Zn2+ did not induce obvious fluorescence change of the probe, which indicates the selectivity of the probe to Cu2+. The fact was also confirmed by color change from stronger blue fluorescence in absence of Cu2+ to almost no-fluorescence in presence of Cu2+ under the irradiation at 365 nm. For other metal ions, no changes were observed under the same condition. Therefore, it also proved the selectivity of the probe to Cu2+ (Fig. 3 inset).
Fluorescence spectra of probe 3 (10 μM) with addition of Al3+, Fe3+, Co2+, Ni2+, Ba2+, Ca2+, Cd2+, Cr3+, K+, Mg2+, Na+, Ag+, Hg2+, Zn2+, Cu2+ in aqueous solution (EtOH:HEPES 1:1, v/v, 0.02 M, pH = 7.2) with an excitation at 356 nm and emission at 452 nm. Inset: the color change of probe 3 (10 μM) in aqueous solution (EtOH:HEPES 1:1, v/v, 0.02 M, pH = 7.2) solution with 5 equiv. Cu2+
Anion effect on the selectivity of the probe for Cu2+ was also carried out. The results showed that the fluorescence intensity of 3-Cu2+ did not change in the cases of CuSO4, CuCl2, Cu(NO3)2 and Cu(OAc)2 (Fig. S3).
Tolerance Over Other Metal Ions
In order to test the practical application of the probe for Cu2+ ion, the interference of other common foreign ions on the fluorescence intensity of probe 3 was also studied. As shown in Fig. 4, only Cr3+ and Hg2+ slightly disturbed the fluorescence intensity of 3-Cu2+ and the initial fluorescence intensity of 3-Cu2+ did not change significantly with other metal ions such as Al3+, Fe3+, Co2+, Ni2+, Ba2+, Ca2+, Cd2+, K+, Mg2+, Na+, Ag+ and Zn2+. The results indicated that probe 3 had a high selectivity for Cu2+ in the presence of other related species.
Fluorescence spectra of probe 3 (10 μM) with addition of Al3+, Fe3+, Co2+, Ni2+, Ag+, Hg2+, Zn2+, Ba2+, Cd2+, Cr3+ (5 equiv.) and Mg2+, Ca2+ (100 equiv.) and K+, Na+ (200 equiv.) in aqueous solution (EtOH:HEPES = 1:1, v/v, 0.02 M, pH = 7.2) containing 5 equiv. of Cu2+ with an excitation at 356 nm and emission at 452 nm
Cu2+-Titration
As shown in Fig. 5, fluorescence titration experiments clearly showed fluorescence “turn-off”. At the beginning, with the addition of Cu2+ at a concentration lower than 1.0 equiv. of probe 3, a significant decrease in the fluorescence intensity was observed and the fluorescence of probe 3 was almost completely quenched with only 2 equiv. of Cu2+ ions. As a further increase in Cu2+ concentration, the fluorescence intensity did not change (Fig. 5, inset). As shown in Fig. S5, the detection limit of probe 3 for the determination of Cu2+ was estimated to be 1.919× 10–7 M (R = 0.995). Therefore, the data demonstrate that compound 3 can be used as an excellent “turn-off” probe for detection of Cu2+ ion.
Binding of Probe 3 with Cu2+
To investigate the coordination information between probe 3 and Cu2+, the stoichiometry between probe 3 and Cu2+ in the complex system was determined by the changes in the absorption response of probe 3 with varying concentrations of Cu2+. In Fig. 6, there is an inflection point when the molar fraction was 0.5, indicating a 1:1 stoichiometry of the Cu2+ to probe 3 in the complex. The association constant (Ka) of Cu2+ with probe 3 in HEPES buffered solution at pH 7.20 was found to be 6.796× 104 M−1 (Fig. S4). In addition, the Cu2+-titration was also supposed a 1:1 Cu2+ complex formation. A proposed complex binding was presented in Scheme 2. Cu2+ was bound to one nitrogen atom from pyridine, one nitrogen atom from pyrazoline and one sulfur atom from thiophene. The model was confirmed by a theory calculation. Based on first-principles Density Functional Theory (DFT), the calculations were performed using the CASTEP code [40]. The exchange and correlation interactions were modeled using the generalized gradient approximation (GGA) with the PW91 functional [41]. In the geometrical optimization, all forces on atoms were converged to 0.05 eV∕Å, the maximum ionic displacement was within 0.002 Å and the total stress tensor was reduced to the order of 0.1 GPa (Fig. 7).
Reversibility and Effect of pH
The response of probe 3 to Cu2+ was confirmed to be reversible by the EDTA. As shown in Fig. 8, upon addition of 1 equiv. EDTA to the mixture of probe 3 (10 μM) and Cu2+ (10 μM) in aqueous solution (EtOH:HEPES = 1:1, v/v, 0.02 M, pH = 7.2), the fluorescent intensity was almost completely recovered, indicating that the EDTA replaced the receptor 3 to chelate Cu2+. Thus, the experimental observations suggested that probe 3 should be a reversible for Cu2+. On the other hand, the effect of pH on the fluorescence response of probe 3 to Cu2+ was investigated. As shown in Fig. S5, in absence of Cu2+, nearly no substantial change in fluorescence intensity of probe 3 was observed in the pH range from 5.5 to 9.0, but the fluorescence intensity of 3-Cu2+ has different responsive behaviors in different pH ranges. With increasing pH value from 5.5 to 7, the fluorescence intensity of 3-Cu2+ decreases because the deprotonation of probe 3 increases the conjugation between probe 3 and Cu2+. The significant increase of the fluorescence intensity with the increasing pH value from 7.5 to 9.0 should be attributed to the displacing of Cu2+ from the complex, leading to the recovery of the fluorescence of probe 3. Thus, the coordination between probe 3 and Cu2+ is stable in the pH range of 7.0–7.5, indicating that the probe is promising for biological applications.
Imaging of Intracellular Cu2+
The ability of probes to sensitively and selectively detect analyte in living cells is significant for biological application. Considering that higher level of Cu2+ in tumors takes a possible key role in promoting angiogenesis, we carried out assay in Hela cells.
From Fig. 9, we can clearly observe significant confocal imaging changes of probe 3 (1 μM) in the medium upon addition of Cu2+ (1 equiv.) for 1 h at 37 °C. Hela cells incubated with probe 3 initially display a strong fluorescent image, but the fluorescence image completely quenched in the presence of Cu2+. When EDTA (0.5 mM) was added to the medium containing probe 3 (1 μM) and Cu2+ (1 μM), fluorescent image was returned because EDTA chelated strongly Cu2+ to lead to free probe 3.
Fluorescence microscope images of living Hela cells. a: Fluorescence image of probe 3 (1 μM); d: Fluorescence image of probe 3 (1 μM) in the presence of Cu2+ (1 μM); g: Fluorescence image of probe 3 (1 μM) in the presence of Cu2+ (1 μM) and EDTA (0.5 mM); b, e and h: Bright-field; c, f and i: Overlay image
Conclusions
In summary, a new pyrazoline-based probe 3 was developed. The probe can monitor Cu2+ with high sensitivity and selectivity over other competitive metal ions in aqueous solution (EtOH:HEPES = 1:1, v/v, 0.02 M, pH = 7.2). The binding ratio of probe 3 and Cu2+ was determined to be 1:1, which was confirmed by the Job’s plot and the Cu2+-titration results. The binding constant (Ka) for 3-Cu (II) was calculated to be 6.796× 104 M−1 and the detection limit of probe 3 for Cu2+ was 1.919× 10−7 M. Additionally, the probe could serve as a reversible fluorescent probe to image Cu2+ in living cells.
References
Robinson NJ, Winge DR (2010) Copper metallochaperones. Annu Rev Biochem 79:537–562
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108:1517–1549
Waggoner DJ, Bartnikas TB, Gitlin JD (1999) The role of copper in neurodegenerative disease. Neurobiol Dis 6:221–230
Camakaris J, Voskoboinik I, Mercer JF (1999) Breakthroughs and views molecular mechanisms of copper homeostasis. Biochem Biophys Res Commun 261:225–232
Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27:723–749
Hung YH, Bush AI, Cherny RA (2010) Copper in the brain and Alzheimer’s disease. J Biol Inorg Chem 15:61–76
Brown DR, Kozlowski H (2004) Biological inorganic and bioinorganic chemistry of neurodegeneration based on prion and Alzheimer diseases. Dalton Trans 33:1907–1917
Finkel T, Serrano M, Blasco MA (2007) The common biology of cancer and ageing. Nature 448:767–774
Gonzales APS, Firmino MA, Nomura CS, Rocha FRP, Oliveira PV, Gaubeur I (2009) Peat as a natural solid-phase for copper preconcentration and determination in a multicommuted flow system coupled to flame atomic absorption spectrometry. Anal Chim Acta 636:198–204
Budziak D, Silva EL, Campos SD, Carasek E (2003) Application of Nb2O5–SiO2 in pre-concentration and determination of copper and cadmium by flow system with flame atomic absorption spectrometry. Microchim Acta 141:169–174
Liu Y, Liang P, Guo L (2005) Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta 68:25–30
Guo Y, Din BJ, Liu YW, Changc XJ, Meng SM, Liu JH (2004) Preconcentration and determination of trace elements with 2-aminoacetylthiophenol functionalized Amberlite XAD-2 by inductively coupled plasma–atomic emission spectrometry. Talanta 62:207–213
Ensafi AA, Khayamian T, Benvidi A (2006) Simultaneous determination of copper, lead and cadmium by cathodic adsorptive stripping voltammetry using artificial neural network. Anal Chim Acta 561:225–232
Cheng WL, Sue JW, Chen WC, Chang JL, Zen JM (2010) Activated nickel platform for electrochemical sensing of phosphate. Anal Chem 82:1157–1161
Li C, Wang L, Deng L, Yu H, Huo J, Ma L, Wang J (2009) Electrochemical assessment of the interaction of dihydrogen phosphate with a novel ferrocenyl receptor. J Phys Chem B 113:15141–15144
Lai SJ, Chang XJ, Fu C (2009) Cadmium sulfide quantum dots modified by chitosan as fluorescence probe for copper (II) ion determination. Microchim Acta 165:39–44
Luo Y, Li Y, Lv BQ, Zhou ZD, Xiao D, Choi MMF (2009) A new luminol derivative as a fluorescent probe for trace analysis of copper (II). Microchim Acta 164:411–417
Pan M, Lin XM, Li GB, Su CY (2011) Progress in the study of metal–organic materials applying naphthalene diimide (NDI) ligands. Coord Chem Rev 255:1921–1936
Guerchais V, Fillaut JL (2011) Sensory luminescent iridium (III) and platinum (II) complexes for cation recognition. Coord Chem Rev 255:2448–2457
Liu M, Zhao HM, Quan X, Chen S, Yu HT (2010) Distance-independent quenching of quantum dots by nanoscale-graphene in self-assembled sandwich immunoassay. Chem Commun 46:1144–1146
Kumar M, Kumar N, Bhalla V, Sharma PR, Kaur T (2012) Highly selective fluorescence turn-on chemodosimeter based on rhodamine for nanomolar detection of copper ions. Org Lett 14:406–409
Aksuner N, Henden E, Yilmaz I, Cukurovali A (2009) A highly sensitive and selective fluorescent sensor for the determination of copper (II) based on a schiff base. Dye Pigment 83:211–217
Wen ZC, Yang R, He H, Jiang YB (2006) A highly selective charge transfer fluoroionophore for Cu2+. Chem Commun 42:106–108
Liu ZP, Zhang CL, Wang XQ, He WW, Guo ZJ (2012) Design and synthesis of a ratiometric fluorescent chemosensor for Cu(II) with a fluorophore hybridization approach. Org Lett 14:4378–4381
Chen XT, Tong AJ (2012) Modification of silica nanoparticles with fluorescein hydrozide for Cu(II) sensing. Dye Pigment 95:776–783
Olimpo GB, Natalia M, Leidi CF, Jose Carlos NF, Vioctor V, Frank HQ, Marco TN, Bruce KC (2012) Design and synthesis of a new coumarin-based “turn-on” fluorescent probe selective for Cu2+. Tetrahedron Lett 53:5280–5283
Ahamed BN, Ghosh P (2011) Selective colorimetric and fluorometric sensing of Cu (II) by iminocoumarin derivative in aqueous buffer. Dalton Trans 40:6411–6419
Kaur P, Kaur S, Singh K, Sharma PR, Kaur T (2011) Indole-based chemosensor for Hg2+ and Cu2+ ions: Applications in molecular switches and live cell imaging. Dalton Trans 40:10818–10821
Li QQ, Peng M, Li NN, Qin JG, Li Z (2012) New colorimetric chemosensor bearing naphthalendiimide unit with large blue-shift absorption for naked eyes detection of Cu2+ ions. Sensor Actuat B 173:580–584
Sarkar A, Bhattacharya SC (2012) Selective fluorescence resonance energy transfer from serum albumins to a bio-active 3-pyrazolyl-2-pyrazoline derivative: A spectroscopic analysis. J Lumin 132:2612–2618
Wang ML, Zhang JX, Liu JZ, Xub CX, Ju HX (2002) Intramolecular energy and charge transfer in 5-(9-anthryl)-3-(4-nitrophenyl)-1-phenyl-2-pyrazoline. J Lumin 99:79–83
Li MM, Huang SY, Ye H, Ge F, Miao JY, Zhao BX (2013) A new pyrazoline-based fluorescent probe for Cu2+ in live cells. J Fluoresc 23:799–806
Zhang Z, Wang FW, Wang SQ, Ge F, Zhao BX, Miao JY (2012) A highly sensitive fluorescent probe based on simple pyrazoline for Zn2+ in living neuron cells. Org Biomol Chem 10:8640–8644
Gong ZL, Ge F, Zhao BX (2011) Novel pyrazoline-based selective fluorescent sensor for Zn2+ in aqueous media. Sensor Actuat B 159:48–153
Liu WY, Li HY, Lv HS, Zhao BX, Miao JY (2012) A rhodamine chromene-based turn-on fluorescence probe for selectively imaging Cu2+ in living cell. Spectrochim Acta A 95:658–663
Liu WY, Li HY, Zhao BX, Miao JY (2012) A new fluorescent and colorimetric probe for Cu2+ in live cells. Analyst 137:3466–3469
Liu WY, Li HY, Zhao BX, Miao JY (2011) Synthesis, crystal structure and living cell imaging of a Cu2+-specific molecular probe. Org Biomol Chem 9:4802–4805
Gresser R, Hartmann H, Wrackmeyer M, Leo K, Riede M (2011) Synthesis of thiophene-substituted aza-BODIPYs and their optical and electrochemical properties. Tetrahedron 67:7148–7155
Alhaider AA, Abdelkader MA, Lien EJ (1985) Design, synthesis and pharmacological activities of 2-substituted 4-phenylquinolines as potential antidepressant drugs. J Med Chem 28:1394–1398
Segall MD, Lindan PJD, Probert MJ, Pickard CJ, Hasnip PJ, Clark SJ, Payne MC (2002) First-principles simulation: ideas, illustrations and the CASTEP code. J Phys: Condens Matte 14:2717–2744
Mattsson AE, Armiento R, Schultz PA, Mattsson TR (2006) Nonequivalence of the generalized gradient approximations PBE and PW91. Phys Rev B 73:195123–195130
Acknowledgments
This study was supported by 973 Program (2010CB933504) and National Natural Science Foundation of China (90813022 and 20972088).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Meng-Meng Li and Wen-Bo Zhao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 310 kb)
Rights and permissions
About this article
Cite this article
Li, MM., Zhao, WB., Zhang, TT. et al. A New Thiophenyl Pyrazoline Fluorescent Probe for Cu2+ in Aqueous Solution and Imaging in Live Cell. J Fluoresc 23, 1263–1269 (2013). https://doi.org/10.1007/s10895-013-1259-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1259-x