Abstract
Legumes are notorious for coevolutionary arms races where chemical defenses are employed to ward off herbivores—particularly insect seed predators. Locoweeds are legumes containing the toxic alkaloid swainsonine which can poison livestock, but its role as a deterrent for insects is unknown. Swainsonine is produced by the fungal endophyte Alternaria section Undifilum, and the chemical composition of the toxin has been well characterized. Despite this knowledge, the ecological roles and evolutionary drivers of swainsonine toxins in locoweeds remain uncertain. Here, we quantify swainsonine concentrations and herbivory levels in the hyper-diverse locoweed Astragalus lentiginosus to evaluate its role as an evolved chemical defense. We found that A. lentiginosus shows considerable variation in swainsonine concentrations according to variety, in particular showing presence/absence variation at both population and local geographic scales. Surprisingly, herbivory levels from presumed generalist insects emerging from fruits showed no correlation with swainsonine concentrations. Conversely, seed and fruit herbivory levels linked to specialist Acanthoscelides seed beetles increased with concentrations of swainsonine—suggesting a possible coevolutionary arms race. Our results highlight that variation in endophyte-produced toxin systems may not follow classical expectations for geographic variation and ecological roles of plant chemicals. We discuss the implications of these results on plant-endophytic toxin systems and coevolutionary dynamics more broadly, highlighting a considerable need for more research in these systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antagonistic coevolution between plants and phytophagous insects that consume them has long been hypothesized to be a primary driver of Earth’s plant and insect diversity (Ehrlich and Raven 1964). This model is characterized by an arms race between plant chemical defenses and insect resistance, punctuated by episodes of plant range expansion and insect specialization, promoting ecogeographic reproductive isolation and speciation in both groups (Maron et al. 2019). This coevolutionary relationship is particularly prevalent among legumes (Fabaceae), where pre-dispersal seed predation by insects is common, and chemical defenses are particularly abundant (Janzen 1971)—likely contributing to Fabaceae ranking as the third most speciose plant family (7% of all plants, Christenhusz and Byng 2016; Judd et al. 1999). This hypothesis, frequently termed “escape and radiate” (Thompson 1994), has been a topic of extensive research in evolutionary biology for decades, yet the proximal mechanisms for how insect herbivory mediates diversification remain unclear (Marquis et al. 2016; Maron et al. 2019). Furthermore, while plant defenses are well characterized in many groups, their relationship with herbivory is typically assumed and not well characterized, limiting a more mechanistic understanding of plant-insect coevolutionary dynamics (Moore et al. 2014).
The diversity of legumes in both species and chemical defenses is embodied by the genus Astragalus, the single most speciose genus of plants with over 3,000 described species, including approximately 400 in Western North America alone (Maassoumi 2020; Folk et al. 2024). Astragalus are notorious for their diversity of toxic compounds presumed to be plant defenses, including several nitrotoxins, selenium hyperaccumulation, and an indolizidine alkaloid swainsonine (Williams and Barneby 1977; Emerick and DeMarco 1990; Cook et al. 2009; Liu et al. 2017). Many Astragalus species are commonly referred to as “Locoweeds”—a group of toxic plants known to poison livestock with a syndrome referred to as “locoism” (Cook et al. 2009; Kingsbury 1964). These toxic plants cause negative economic impacts reaching several million dollars per year due to death, reproductive complications, and weight loss of livestock (Wu et al. 2016; Zhao et al. 2013; James et al. 1992, Ralphs et al. 2000). Of the many Astragalus toxins, the toxin responsible for locoism is swainsonine, with cases of livestock locoism documented across the globe (Molyneux and James 1982; Molyneux et al. 1994; Wu et al. 2016). Interestingly, swainsonine concentration has been documented to vary greatly among species and within Astragalus species (Ralphs et al. 2008, Gardner et al. 2001, 2004; Cook et al. 2009, 2011). For example, mean swainsonine concentrations have been documented to vary by as much 100x between species (A. amphioxys < 0.001% and A. mollissimus > 0.1%, Ralphs et al. 2008), and in a recent screen of A. lentigininosus, swainsonine was detected at some level in 31 taxonomic varieties—while being totally absent from 8 others (Cook et al. 2016b). However, previous screens for swainsonine have only included limited numbers of samples per taxon and did not rigorously examine population and geographic variation. The stark variation among taxa is intriguing and requires spatially explicit sampling within taxa to better disentangle the ecological forces that shape this variation, particularly at the sub-specific level.
It was long thought that swainsonine was a secondary plant metabolite of locoweeds, however it is now understood to be produced by fungal endophytes (Cook et al. 2014; Braun et al. 2003; Pryor et al. 2009). In Astragalus, Alternaria section Undifilum endosymbionts are responsible for the synthesis of swainsonine and the associated toxicity (Baucom et al. 2012; Oldrup et al. 2010; Lu et al. 2016). Alternaria section Undifilum are vertically transmitted by way of hyphae present in the seed coat and removal of the seed coat in Oxytropis plants (a locoweed genus sister to Astragalus) leads to no detection in swainsonine in progeny plants (Oldrup et al. 2010; Grum et al. 2012). This endophyte as well as swainsonine, has been found in all parts of the plant and the endophyte does not appear to grow outside of living plants as mycelia (Cook et al. 2009, 2011). The high variation in swainsonine concentration observed in Astragalus and Oxytropis has been linked to the amount of the fungal endophyte present within a given plant (Cook et al. 2009, 2011). A common garden experiment further demonstrated that endophyte genotype is responsible for determining swainsonine toxin concentrations (Cook et al. 2013).
Despite the economic impacts and considerable investigation over the last decade, the ecological role of the swainsonine toxin for locoweed plants remains unclear. The widespread distribution of swainsonine in Astragalus taxa points to an ecologically important role for this toxin—presumably as a defense against herbivory. However, empirical study of the effects of swainsonine concentrations on herbivory are lacking or absent in the literature. Swainsonine does not appear to deter grazing by large ungulate mammals, locoism can take weeks of consuming the same plants to develop (Cook et al. 2014; Pfister et al. 2003). Thus, the interaction between Astragalus and swainsonine producing fungal symbionts is unlikely to have evolved as a defense against herbivory by mammals. Furthermore, clipping of locoweeds to simulate herbivory does not lead to an increase in swainsonine production, indicating swainsonine is unlikely to be an induced defense response (Ralphs et al. 2002; Cook et al. 2016a). Plant toxins are often associated with coevolutionary defenses against insect herbivores, especially pre-dispersal seed predators of legumes (Janzen 1971). A variety of insects have been documented to consume Astragalus seeds, including lepidopterans, hemipterans, and seed chalcid hymenopterans. By far the most significant seed predators are larvae of specialist seed beetles and weevils which can have profound impacts on plant fitness by consuming more than 75% of seeds (Green and Palmbald 1975; Combs et al. 2011, 2013). Of note is a monophyletic group of seed beetle species within the genus Acanthoseclides (Chrysomelidae: Bruchinae) that feed nearly exclusively on Astragalus seeds (Johnson 1970; Kingsolver 2004). Importantly, swainsonine concentrations appear to be highest in the seeds and pods of Astragalus oxyphysus and other locoweeds (Molyneux et al. 1991). Therefore, we hypothesize that swainsonine serves as a defensive compound against beetle seed predation, which accounts for profound fitness losses in Astragalus populations and represents a strong ecological selective pressure. Studies across many other plant-toxin systems have found largely clinal variation between prevalence of plant defenses and intensity of herbivory and represent the primary evidence for herbivores as the selective agent driving plant defense evolution (reviewed Agrawal et al. 2012; Züst et al. 2012). We therefore also predict to find a correlation between swainsonine concentration and intensity of seed and/or fruit herbivory as this would represent a coevolutionary hotspot with both organisms under intense selection (Thompson 2005; Brodie et al. 2002).
Astragalus lentiginosus is the most taxon-rich plant species in North America (Knaus 2010) with 40 accepted taxonomic varieties (Plants of the World Online, Maassoumi 2020) and is distributed throughout arid and semiarid regions of western North America from southern Canada to northern Mexico. These varieties are distinguished by their morphological diversity, and many occupy specialized edaphic conditions that contribute to ecogeographic displacement (Knaus 2010; Knaus et al. 2005). Acanthoscelides beetles have been reared from 16 of 20 sampled A. lentiginosus varieties and can contribute to seed mortality up to 93% (Morse, unpublished data). This system is therefore ideal for investigating not only how swainsonine varies within and among populations in a geographic context, but also correlations between toxin concentration and seed predation.
Here, we employ a hierarchically structured sampling approach that is spatially explicit allowing us to examine the geographic scale of swainsonine variation within populations, between sites, and across taxonomic varieties, as well as examine associations between swainsonine level and insect herbivory. This extensive field survey includes nearly 500 field collected specimens spanning 18 Astragalus lentiginosus varieties collected from 59 sites in the American southwest. We evaluated three questions investigating swainsonine variation in Astragalus lentiginosus: (1) What is the scale of ecogeographic variation in swainsonine and insect herbivory of Astragalus among sites and taxonomic varieties? (2) Does geographic variation in swainsonine, a toxin produced by an endophytic association, differ from clinal patterns typically observed in plant endogenous chemical defenses? (3) Does the relationship between swainsonine and herbivory differ between Astragalus seed specialists and other insect herbivores? We answer these questions and discuss their implications for broader topics like the evolutionary ecology of locoweeds, defense strategies for herbivory in legumes, and patterns of variation in toxin-producing endophytes.
Methods
Collection of Astragalus lentiginosus Plant Material and Environmental Data
A field survey was conducted in 2019 to collect A. lentiginosus specimens from across the southwest portion of their range. Plant populations were identified by searching for recent geolocalized herbarium specimens. Specimens were collected in the field from 59 field sites across California, Nevada, Arizona, and Utah between May and July. The sampled populations were identified to species and variety using online georeferenced herbarium records (e.g. Jepson’s eflora; Intermountain Biota). At each site, ripe fruits were collected from up to 20 total plants, resulting in 487 individual plants used for swainsonine and herbivory data collection. A subset of ripe fruits from each site were placed in mason jars sealed with double wrapped cheesecloth to rear developing insect larvae from fruits.
GPS coordinates and elevation were taken for each plant specimen using a Garmin eTrex 20 (Garmin, Olathe, Kansas). For each collection site, we used the Worldclim 2 database to extract bioclimatic data from source location data at 30 arcsecond resolution (Fick and Hijmans 2017). Here we utilize BIO1, BIO12 and BIO15 for this study as they have been shown to be the most reliable predictors from species distribution modeling for A. lentiginosus varieties (Grillo et al. unpublished).
Swainsonine Quantification
Swainsonine detection and concentration was measured in 487 Astragalus lentiginosus specimens by using a modification of a previously published procedure (Gardner and Cook, 2011). A measured quantity of seeds was placed in a 2 mL screw-cap microcentrifuge tube. The ground material was extracted in 1.5 mL of 2% acetic acid for 18 h with agitation. After extraction, the samples were centrifuged, and a measured volume of extract (0.5 mL) was added to 0.5 mL of 20 mM ammonium acetate in a 1 mL auto-sampler vial. Samples were analyzed by HPLC-MS (Thermo Scientific LCQ Advantage) to quantitate swainsonine as previously described (Gardner et al. 2001). In brief, swainsonine was measured from a reconstructed ion chromatogram (m/z = 156) and quantitation based on an external calibration standard using a standard curve of an authenticated swainsonine standard. The detection limit of swainsonine was 0.01 mg/g of dry weight using this extraction procedure. The resulting swainsonine concentration of the injected sample was converted to microgram swainsonine per milligram dry weight (ug/mg) of the mass of the plant material extracted, and this concentration was used for all downstream analyses.
Herbivory Quantification
Herbivory was measured in two distinct ways for all 487 specimens used for swainsonine concentration. Fruit herbivory was measured in a maximum of 30 randomly-selected fruits per plant (mean 24.4 fruits, 4–30 fruits used), and of these fruits, each was scored for presence or absence of insect exit holes. When exit holes were present, hole size was measured and categorized as either small (< 1 mm) or large (1 + mm). No fruits were observed to possess both large and small holes. The hole size categorization was chosen to differentiate herbivory by seed beetles (indicated by small holes) which specialize on Astragalus plants, from herbivory by all other insects (indicated by larger holes). While these seed beetles could be one of several beetle groups, for this dataset we infer all beetles with this exit hole to be from genus Acanthoscelides (Chrysomelidae: Brucidae), as these were the only insects of any kind that were reared from surveyed fruits. For the larger fruit holes, it was not possible to determine which insect herbivores were causing this damage as it could be several—these are referred to as non-specialists henceforth, because while it is unclear if they are generalists, they do not represent the dominant seed predators on A. lentiginosus.
For the fruit herbivory analysis, varieties A. lentiginosus wilsonii and A. lentiginosus maricopae were excluded, as these varieties have seed pods that dehisce (open naturally) and therefore would not require exit holes by most insect herbivores. For A. lentiginosus sierrae only swainsonine measurements were taken due to a limited number of fruits at the time of collection.
Seed herbivory was measured in a maximum of 50 seeds per plant and the proportion of seeds with herbivory was recorded. For this, seeds were compressed using a fingernail: seeds with beetle herbivory become hollowed out and crush easily, whereas seeds without beetle herbivory remain intact. All seed herbivory was presumed to be from Acanthoscelides beetles for the same reasons as explained for small exit holes above. We only find Acanthoscelides reared from intact fruits, and they are known to be dominant seed predators on these species (Green and Palmbald 1975; Combs et al. 2011, 2013).
Statistical Analyses
All statistical analyses as well as data visualization in this study were performed using R version 4.2.0 (R core team). Because swainsonine concentration ranges from very small (but non-zero) concentrations to multiple-fold increases, for all statistical analyses using swainsonine concentration as a variable, we used a log(x + 1) transformation to better represent variation in this compound.
For analyzing swainsonine concentration as a product of abiotic variables and Astragalus lentiginosus variety, we used the nlme package of R (Pinheiro et al. 2022) to construct linear mixed effect models. The model includes A. lentiginosus variety, date of collection (in day of the year), elevation, latitude, and bioclimatic variables for annual mean temperature (BIO1), annual mean precipitation (BIO12), and precipitation seasonality (BIO15) as fixed effect variables. Collection site was a random effect to account for pseudo replication of abiotic variables introduced by having multiple individuals from the same geographic location (i.e. same value for all variables). The model used transformed swainsonine concentration as the response variable. The model uses all individuals quantified for swainsonine, including the sizeable proportion that had no trace (i.e. 0.0 ug/mg) of swainsonine. We then ran an ANOVA on the model to determine the statistical contribution of each variable to variation in swainsonine concentration.
For the herbivory models, we used binomial generalized linear models where each plant was one row of data and included both count of seeds/fruit herbivorized as well as count that were intact. In this way, each seed or fruit was individually scored dichotomously, but tied to a particular plant in the glm model. All three models used the same three independent variables, log-transformed swainsonine concentration, host variety, and collection site. Collection site was a fixed effect in these models as there is no pseudo replication of other variables and collection site represents a real ecogeographic variable that might lead to variation in herbivore presence or intensity. The three models then only differed in the response variable, the seed model compared seeds crushed vs. not, and fruit models split by exit hole size: one model compared number of fruits with presence of small holes to all other fruits (including those with large holes) and the second model compared number of fruits with large holes compared to all others (including those with small holes). We then ran an Analysis of Deviance (an ANOVA for glm models) on each model to assess statistical contributions of these variables to each metric of herbivory. All code used in analyses can be found on Github (github.com/grillolab/Alentiginosus-swain) and can be replicated using the supplementary info in this publication or in that repository.
Results
Swainsonine Varies by Variety and site, but not Abiotic Variables
In total, we quantitated swainsonine concentration in 487 individual Astragalus lentiginosus plants across 18 varieties and 59 collection sites (Table 1). Of these 487 plants, 335 possessed swainsonine (69% of all plants), with the remaining 152 with no detectable swainsonine. Swainsonine concentration in plants that contained the compound ranged from trace detection (0.081 ug/mg) to high concentrations (26.15 ug/mg maximum – var. salinus), with a mean of 3.50 ug/mg (Fig. 2). There was broad variation among taxonomic varieties, ranging from two varieties (sierrae and antonius) with no detectable swainsonine to varieties maricopae, salinus, vitreus, and palans all with mean concentrations above 7 ug/mg. Interestingly, every variety had at least one plant with no swainsonine present (Fig. 2A). There was a similarly wide variation among and within collection sites, with 7 sites showing no swainsonine for any plants, while the site with the highest mean concentration (site 19–51, 15.80 ug/mg) also had one plant without any swainsonine (Figs. 1B and 2B).
(A) Map showing collection sites for all Astragalus lentiginosus plants used in this study, with colors indicating variety. All sites had only one variety. (B) Map of swainsonine variation across sites. Pie chart on interior indicates the number of plants at a given site with (black) vs. without (white) swainsonine, while the outer ring indicates average swainsonine concentration at that site
The statistical model interrogating this variation using log-transformed swainsonine concentrations indicates that there is significant variation between A. lentiginosis varieties (F(17) = 3.45, P = 0.0009). However, there was no significant effects of elevation (F(1) = 0.0659, P = 0.799), latitude (F(1) = 0.13, P = 0.721), date of collection (F(1) = 0.923, P = 0.343), annual mean temperature (BIO1: F(1) = 0.471, P = 0.497), annual mean precipitation (BIO12: F(1) = 1.00, P = 0.324), or precipitation seasonality (BIO15: F(1) = 3.02, P = 0.091). Some of the abiotic variables display noticeable trends—there is a modest decrease in swainsonine concentrations with higher precipitation and seasonality. While trends in abiotic variables are observed, the lack of significance can be explained by the large variation in swainsonine concentration among plants of the same variety at a given site (Fig. 2). For example, while the plants with the highest overall mean concentrations can be found at higher latitudes, there are also plants with no swainsonine found at the same site.
Log-transformed swainsonine concentrations of individual Astragalus lentiginosus varieties separated by (A) variety and (B) collection site. Panel B) shows only collection sites for a subset of 4 varieties that showcases presence/absence as well as quantitative variation both within and between sites. Swainsonine concentration was found to significantly differ between varieties (F(17) = 3.45, P = 0.0009)
Relationships Between Herbivory and Swainsonine Concentration
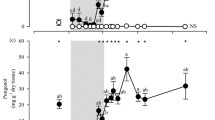
We quantified relationships between swainsonine concentration and several measures of herbivory across plants where both swainsonine concentration was measured and herbivory information was obtainable. Overall, we identified 2,813 seeds with evidence of herbivory out of a total 22,135 seeds (12.7%) assayed across 471 plants, with sizeable variation between varieties (Table 2). Our model revealed that A. lentiginosus variety (D(16) = 1036.03, P(χ²) < 0.001), collection site (D(40) = 1135.23, P(χ²) < 0.001) and log-transformed swainsonine concentration (D(1) = 174.14, P(χ²) < 0.001) significantly influenced seed herbivory, with higher swainsonine concentrations associated with higher levels of seed herbivory (Fig. 3A).
For fruits, herbivory was divided into two groups based on size of exit holes: small exit holes made by specialist seed beetles of the genus Acanthoscelides (Fig. 3B), and larger holes made by a variety of other insects. Out of 10,020 fruits evaluated from 395 plants, 1,628 (16.2%) had small exit holes while 1,177 (11.7%) had larger exit holes (Table 2). Separate analyses were performed for each exit hole size to independently assess the effect of swainsonine between herbivore types. Results for the small exit hole model mirrors those from seed herbivory, with significance for A. lentiginosus variety (D(14) = 567.38, P(χ²) < 0.001), collection site (D(35) = 961, P(χ²) < 0.001) and swainsonine concentration (D(1) = 63.35, P(χ²) < 0.001) and a positive relationship between swainsonine concentration and small exit holes in fruits (Fig. 3B). In contrast, while the model for large exit holes also showed a significant effect of A. lentiginosus variety (D(14) = 181.72 P(χ²) < 0.001) and collection site (D(35) = 339.49, P(χ²) < 0.001), there was no relationship between swainsonine concentration and presence of large exit hole herbivory (D(1) = 1.04, P(χ²) = 0.3075) (Fig. 3D).
Associations between various measures of herbivory (Y-axes) and swainsonine toxin concentration (X-axes) in Astragalus lentiginosus. Each point represents a single plant, its log-transformed swainsonine concentration and the proportion of it’s seed or fruit herbivorized. Panel (A) and (B) show the positive associations between swainsonine and herbivory by specialist Acanthoscelides seed beetle predators (shown panel C). Panel (A) shows positive relationship between proportion of seeds herbivorized and swainsonine concentration (D(1) = 174.14, P(χ²) < 0.001*), while panel (B) shows a similar pattern but with small exit holes characteristic of Acanthoscelides emergence (D(1) = 63.35, P(χ²) < 0.001*). Panel D) highlights the lack of association between toxin level and herbivory by non-seed beetle specialists (D(1) = 1.04, P(χ²) = 0.3075). Trend lines on panels A, B and D are y ~ x lines to show direction of relationship, and not actual statistical analyses, which were analyzed using binomial regression, not proportions
Discussion
Plant chemical defenses have been frequently shown to exhibit continuous or otherwise ecologically clinal variation, often associated with selective pressures from herbivores (Agrawal et al. 2012; Züst et al. 2012). However, these studies have been almost exclusively focused on endogenous plant toxin systems, and considerably less is known about the distribution and ecological role of toxins produced by endophytes (Clay 2014; Panaccione et al. 2014). Our survey of Astragalus lentiginosus includes nearly 500 plants thereby allowing us to robustly assess the geographic scale of variation for this compound. We identify considerable variation in swainsonine toxin concentrations among varieties and collection sites, and most notably a striking level of presence/absence variation at the local site level. Interestingly, swainsonine variation was not associated with a reduction in overall herbivory, but patterns differed by herbivore type. Our data revealed a positive association between swainsonine and specialist seed beetle predation, while other insect herbivores show no association. Here we discuss the insights that these results can provide on the eco-evolutionary forces that shape swainsonine toxin variation, while acknowledging the limits of this inference and identifying areas for continued research.
Overall, we identified a surprising level of swainsonine variation within and between both collection sites and taxonomic varieties of A. lentiginosus. There was considerable variation between taxonomic varieties, as multiple varieties had average concentrations higher than the maximum value of other varieties (Fig. 2). There was also considerable variation between collection sites within a variety, with some sites possessing the toxin in a single plant while nearby sites showing high prevalence and concentration. Most surprisingly, variation within sites was profound, with the large majority (78.8%) of sites containing at least one plant with no detectable levels of swainsonine that co-occurred with plants with varying levels of swainsonine concentration (Fig. 1).
Moreover, two taxonomic varieties (var. antonius and var. sierrae) possessed no detectable swainsonine in any specimens, whereas the remaining 16 varieties included plants with and without swainsonine. Interestingly, the two varieties without swainsonine were the only “mountain island” varieties—varieties that are found exclusively at high elevation sites with very narrow geographic distributions, often separated by lower elevation regions. It is possible that these ranges—being more isolated and distinct in geology—have different selective pressures, either by overall relaxed herbivory patterns, or stricter need for resources that could otherwise by used by the endophyte for toxin production.
Plant chemical defenses are diverse, the vast majority of which are synthesized as secondary metabolites produced by the plant at some cost to growth or other forms of fitness (Erb and Kliebenstein 2020; Whitehead et al. 2021). Ample evidence of such endogenous defenses often show continuous geographic variation that matches gradients of herbivory, abiotic variables that influence fitness, or a combination of the two (Agrawal et al. 2012; Züst et al. 2012; Moreira et al. 2018). To the best of our knowledge, presence/absence variation as we have identified here—at all levels including the local spatial scales—does not occur for plant derived endogenous toxins. Comparatively, toxins produced by fungal endosymbionts are currently limited to just three plant families—Convolvulaceae, Fabaceae, and Poaceae (Quach et al. 2023). While considerable research has been conducted, largely in grasses, to investigate endosymbiont toxins (reviewed Rudgers and Clay 2007; Clay and Schardl 2002; Schardl et al. 2006), this area of inquiry is still quite understudied and additional fungal derived secondary defensive compounds are likely to be found. Previous surveys on the geographic distribution of endophytic toxins are limited to Poaceae, very few of which have investigated toxin distributions at local scales. Work in tall fescue (Lolium arundinaceum) shows broad worldwide distribution of both the plant and its endophyte (Neotyphodium coenophialum), and global sampling reveals approximately 90% infection rates; although data on geographic toxin variation was absent (Rudgers and Clay 2007; Ball et al. 1991). One study examining sleepygrass (Achnatherum robustum) endophytes identified a pattern of presence/absence of alkaloid toxins among plant populations (but not within population), and this pattern was clinal in nature, with lower toxicity radiating from a focal population with high toxicity (Faeth et al. 2006).
Despite limited investigation to date, presence/absence variation may be a common feature of fungal derived defensive compounds. Thus, such toxins are fundamentally distinct from endogenous plant toxins in the scale of geographic variation. This extreme level of variation, as presented here, undoubtedly impacts the eco-evolutionary outcomes between plant hosts and insect herbivores and should be taken into account when studying tripartite interactions (i.e. plants, fungal endophytes, insects) in these ecologically and economically important systems.
The forces that maintain the high degree of swainsonine variation within and between sites and taxonomic varieties remain unclear. In locoweeds (Astragalus and Oxytropis) swainsonine levels have been shown to be largely attributed to fungal genotype and are consistent across environmental and plant-host conditions as revealed through common garden and cross-inoculation experiments (Cook et al. 2012). Therefore, the swainsonine variation that we have identified is not likely to be solely shaped by environmental variables. Likewise, we found here that A. lentiginosus varieties significantly differ in overall swainsonine level, highlighting that genotypic differences inherent to the plants also play a role in its variation. Yet, several studies on swainsonine levels that have found a consistent pattern of plants that contain detectable levels of swainsonine, and plants absent the compound (Cook et al. 2009, 2011). It is yet unclear if this is due to presence/absence variation in swainsonine production controlled by fungal genotype, or if it is simply the presence and absence of the fungus itself. Our results here reinforce that this pattern is widespread across geography and among taxonomic varieties but does not provide clarity on ecological pressures or dynamics maintaining this variation. Future endophyte-culturing studies across varieties would be critical in better understanding if this phenomenon is dictated primarily by abundance of endophyte or by plant genotypic variation.
Much of our understanding of naturally occurring variation in plant endogenous toxins revolves around the cost of defense (e.g. Optimal Defense Theory, Resource Availability Hypothesis; Coley 1987; Stamp 2003). Moreover, association with fungal endophytes is expected to come at a cost that is outweighed by the benefits that plant receives from the interaction (Clay and Schardl 2002; Davitt et al. 2010). There is ample evidence in other endophytic fungi-plant toxin systems that these associations provide protection from various vertebrate and insect herbivores (Omacini et al. 2001; Brem and Leuchtmann 2001; Siegel and Bush 1996, Clay et al. 2005). Other work has expanded on this idea by using a cost-benefit analysis to determine that fungal endophytes increase plant fitness and defense production with beneficial endophytes, while other endophytes can act as parasites to plant growth and defense (Vannette and Hunter 2011). Studies in the locoweeds Astragalus and Oxytropis have shown that the Alternaria endosymbiont plays little to no benefit for drought and nitrogen stress resistances (Vallotton et al. 2012; Klypina et al. 2017). Additional studies investigating nitrogen use in these plants have shown that while swainsonine production is positively impacted by presence of nitrogen-fixing rhizobia, toxin production is not nitrogen-limited by rhizobia or added soil nitrogen (Delaney et al. 2011; Barillas et al. 2007).
These results, combined with our result of extensive presence/absence variation, present a conundrum, as we lack evidence for strong negative or positive benefits of Alternaria association with Astragalus. It therefore remains unknown if there is a cost for locoweeds to maintain association with Alternaria fungal endophytes or what the beneficial ecological role might be. Additional work in these plant species directly measuring fitness of the plants (including in size of plant, fecundity, etc.) associated with swainsonine levels and the absence and presence of herbivores would be very useful for further insights on why these species have ubiquitous endophytic associations.
We hypothesized that swainsonine is a plant defense against seed predation. While it is well established that swainsonine is a potent neurotoxin for domesticated mammals, impacts on invertebrates have not been previously described. Through our survey of fruit and seed herbivory we did not find an association between swainsonine levels and large fruit exit holes that are indicative of herbivory by non-specialist insects. We did identify a positive relationship between both small fruit exit holes and seed predation which are caused by specialist Acanthoscelides beetles. It is important to note that while our survey of herbivory was large in scale, encompassing thousands of fruits and seeds across a broad geographic area, this analysis is best viewed as a “snapshot” of insect herbivory at a single time point. The results may very well differ if sampling at different time points throughout the season or across years; therefore, herbivory results should be interpreted with some caution.
Our findings suggest that swainsonine is not an effective defense against herbivory by various insects that cause large exit holes. However, the overall incidence of large exit holes was quite low and swainsonine, or perhaps some other endogenous chemical, may indeed function as an effective deterrent causing non-specialist insects to avoid A. lentiginosus. Given our hypothesis that swainsonine serves as a chemical defense against herbivory, it is somewhat surprising that we identified a positive correlation between both measures (i.e., small fruit exit holes and seed predation) of specialist Acanthoscelides beetle herbivory. That is, plants with higher swainsonine experienced greater levels of seed predation, contrary to expectations of this toxin being an effective defense for seed predation. While this may seem surprising, this finding may be indicative of trait matching or phenotypic escalation where Acanthoscelides beetles have evolved resistance to swainsonine through an on-going coevolutionary arms race (Berenbaum and Zangerl 1998). Regions with high levels of herbivory and swainsonine may reflect co-evolutionary hotspots as described by Thompson (2005). Spatial and temporal variation in herbivory could potentially give rise to the high degree of swainsonine variation that we have identified, particularly if the costs of maintaining association with the fungal endophytes are high. For instance, in years of high insect herbivory, we would expect there to be selection to increase association with high-toxin chemotype Alternaria, and selection to associate with low-toxin chemotypes when insect herbivory is low. Observed high levels of toxin and chemotype variation even at local levels may be in part maintained by the seed bank which delays response to selection from insect herbivores. Nonetheless, our results provide the first documented association between swainsonine and insect seed predation, albeit in a somewhat unexpected direction. Manipulative insect rearing experiments as well as long term field monitoring studies are necessary to more rigorously test the hypothesis that swainsonine acts as a defense against insect predation in Astragalus.
Given that our data represent a snapshot of herbivory it is important to consider alternative hypotheses and scenarios. First, swainsonine, and association with fungal endophytes that produce it, may indeed serve as a plant chemical defense but our sampling occurred too early in the season. Overall, our measures of insect predation were much less than published reports in related Astragalus species (Green and Palmbald 1975; Combs et al. 2011, 2013). Astragalus lentiginosus spans disparate ecogeographic habitats, and at the time of sampling, some sites possessed only ripe fruits and dried plants, whereas others were in early stages of fruit ripening. Under laboratory conditions, Acanthoscelides beetles can have multiple generations as long as ripe seeds are available (Morse personal observation). It is thus possible that sampling at the end of the growing season may yield different results. An important caveat to consider is that while the seed coat of Astragalus harbors the endophyte and swainsonine, it is unclear if these beetles actually directly interact with the toxin and sequester it in some form or another (Erb and Robert 2016), or mechanically burrow past it to consume the toxin-absent seed inside. More in-depth experiments are necessary to understand the relationship between specialist seed beetles and swainsonine. An alternative hypothesis may be that swainsonine acts primarily as a foliar defense against herbivory. When the toxic endophyte is present in locoweeds, swainsonine is present throughout all plant tissues (Cook et al. 2011). Predictions from the Optimal Defense Hypothesis (Stamp 2003) would suggest the seed coat would have the highest toxin concentrations to defend against insect seed predation. But perhaps defending foliar or other tissue is more important to these plants, and more explicit tests of foliar herbivory would be needed to dissect this. Or perhaps swainsonine functions in some other capacity beyond herbivore resistance. While other toxin producing plant fungal endophytes have been shown to confer defense against herbivores (Omacini et al. 2001; Brem and Leuchtmann 2001; Siegel and Bush 1996, Clay et al. 2005) other benefits to plant hosts have been documented. Most prominently, several studies have shown benefits of fungal endophytes for tolerance to abiotic stressors, including drought and heat stress as well as nutrient acquisition in poor environments (Zhang et al. 2011; Lewis et al. 1997; Marks and Clay 1996; Malinowski and Belensky 2000)—but the generality of these benefits has been recently challenged (Decunta et al. 2021). Another benefit of fungal endophytes and/or their toxins is defense against diseases imposed by other fungi, as seen in Ephicloë systems (Pérez et al. 2020), but this would still not explain extensive presence/absence variation as seen here. Specific to Astragalus, we noted above that Alternaria shows no benefit for drought and nitrogen stress resistances (Vallotton et al. 2012; Klypina et al. 2017), but it is possible that Astragalus’ association with Alternaria endophytes may be driven in part by benefits to survival unrelated to herbivory. More work evaluating fitness of Astragalus species associated with both presence and absence of the endophyte—both in laboratory and field settings—is needed to disentangle these overlapping hypotheses.
Finally, beyond swainsonine correlations, it is possible that there are plant secondary metabolites that differ among A. lentiginosus varieties that influence patterns of herbivory seen here. These metabolites may function as either plant defenses against herbivores or attractants for pollinators and can often have complex relationships ecologically (Nelson and Whitehead 2021). Future studies that examine such compounds would help to provide a more comprehensive understanding of the chemodiversity of this species and perhaps provide insight on ecological functions of swainsonine.
Conclusion
Compared to endogenous plant-chemical defense systems, geographic variation in endophyte-produced toxin systems is severely understudied—a critical gap in our understanding of plant-symbiont interactions and some of the most common and important coevolutionary relationships in nature. Here we provide a large-scale survey of toxin variation and herbivory in a legume-fungal endophyte system aimed at bridging this gap. Our results show a relatively unique pattern of geographic toxin variation dominated by presence/absence at the level of taxonomic variety, population, and local scales. This pattern is in stark contrast to the vast majority of patterns seen in endogenous plant defenses, subverting the classical expectation that plant toxins are a response to clinal variation in herbivore activity. This relationship is further confounded by our survey of herbivory, which found a positive relationship between toxins and specialist seed predators, and a lack of association between toxin level and other insects. Together, these results raise many questions and hypotheses about the underlying mechanisms responsible for the extensive variation in endophytic toxins in Astragalus and plant-endophyte systems more broadly. Here and elsewhere, herbivory is assumed to be an important driver of toxin prevalence in plants, but this may not be the case in every system.
Overall, our results highlight that much more research is needed in the area of fungal-endophyte toxin variation, with particular emphasis on identifying ecological roles that are driving patterns of association and toxin levels. Further research, both in the field and laboratory, connecting plant fitness, endophyte association, toxin variation, and herbivory levels could be transformational in our understanding of plant-symbiont relationships, and coevolution more broadly.
Data Availability
Data is provided within the manuscript or supplementary information files.
References
Agrawal AA, Hastings AP, Johnson MTJ, Maron JL, Salminen JP (2012) Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338(6103):113–116. https://doi.org/10.1126/science.1225977
Ball D, Lacefield GD, Hoveland CS (1991) The Tall Fescue Endophyte. Agric Nat Resour Publications, 33. https://uknowledge.uky.edu/anr_reports/33
Barillas JRV, Paschke MW, Ralphs MH, Child RD (2007) White locoweed toxicity is facilitated by a fungal endophyte and nitrogen-fixing bacteria. Ecology 88(7):1850–1856
Baucom DL, Romero M, Belfon R, Creamer R (2012) Two new species of Undifilum, fungal endophytes of Astragalus (locoweeds) in the United States. Botany 90(9):866–875
Berenbaum MR, Zangerl AR (1998) Chemical phenotype matching between a plant and its insect herbivore. Proc Natl Acad Sci USA 95(23):13743–13748. https://doi.org/10.1073/pnas.95.23.13743
Braun K, Romero J, Liddell C, Creamer R (2003) Production of swainsonine by fungal endophytes of locoweed. Mycol Res 107(8):980–988. https://doi.org/10.1017/S095375620300813X
Brem D, Leuchtmann A (2001) Epichloë grass endophytes increase herbivore resistance in the woodland grass Brachypodium sylvaticum. Oecologia 126(4):522–530. https://doi.org/10.1007/s004420000551
Brodie ED Jr, Ridenhour BJ, Brodie ED III (2002) The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evolution 56(10):2067–2082
Christenhusz MJM, Byng JW (2016) The number of known plants species in the world and its annual increase. Phytotaxa 261(3):201–217. https://doi.org/10.11646/phytotaxa.261.3.1
Clay K (2014) Defensive symbiosis: a microbial perspective. Funct Ecol 28(2):293–298. https://doi.org/10.1111/1365-2435.12258
Clay K, Schardl C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160(4 SUPPL). https://doi.org/10.1086/342161
Clay K, Holah J, Rudgers JA (2005) Herbivores cause a rapid increase in hereditary symbiosis and alter plant community composition. Proc Natl Acad Sci USA 102(35):12465–12470. https://doi.org/10.1073/pnas.0503059102
Coley P (1987) Interspecific variation in Plant Anti-herbivore properties: the role of Habitat Quality and Rate of Disturbance. New Phytol 106:251–263. https://doi.org/10.1111/j.1469-8137.1987.tb04693.x
Combs JK, Reichard SH, Groom MJ, Wilderman DL, Camp PA (2011) Invasive competitor and native seed predators contribute to rarity of the narrow endemic Astragalus sinuatus Piper. Ecol Appl 21(7):2498–2509. https://doi.org/10.1890/10-2344.1
Combs JK, Lambert AM, Reichard SH (2013) Predispersal seed predation is higher in a rare species than in its widespread sympatric congeners (Astragalus, Fabaceae). Am J Bot 100(11):2149–2157. https://doi.org/10.3732/ajb.1300238
Cook D, Gardner DR, Ralphs MH, Pfister JA, Welch KD, Green BT (2009) Swainsoninine concentrations and endophyte amounts of undifilum oxytropis in different plant parts of oxytropis sericea. J Chem Ecol 35(10):1272–1278. https://doi.org/10.1007/s10886-009-9710-9
Cook D, Gardner DR, Grum D, Pfister JA, Ralphs MH, Welch KD, Green BT (2011) Swainsonine and endophyte relationships in astragalus mollissimus and astragalus lentiginosus. J Agric Food Chem 59(4):1281–1287. https://doi.org/10.1021/jf103551t
Cook D, Grum DS, Gardner DR, Welch KD, Pfister JA (2013) Influence of endophyte genotype on swainsonine concentrations in Oxytropis sericea. Toxicon 61(1):105–111. https://doi.org/10.1016/j.toxicon.2012.10.018
Cook D, Gardner DR, Pfister JA (2014) Swainsonine-containing plants and their relationship to endophytic fungi. J Agric Food Chem 62(30):7326–7334. https://doi.org/10.1021/jf501674r
Cook D, Gardner DR, Roper JM, Ransom Cv, Pfister JA, Panter KE (2016a) Fungicide treatment and clipping of Oxytropis sericea does not disrupt swainsonine concentrations. Toxicon 122:26–30. https://doi.org/10.1016/j.toxicon.2016.09.012
Cook D, Gardner DR, Lee ST, Pfister JA, Stonecipher CA, Welsh SL (2016b) A swainsonine survey of North American Astragalus and Oxytropis taxa implicated as locoweeds. Toxicon 118:104–111
Davitt AJ, Stansberry M, Rudgers JA (2010) Do the costs and benefits of fungal endophyte symbiosis vary with light availability? New Phytol 188(3):824–834. https://doi.org/10.1111/j.1469-8137.2010.03428.x
Decunta FA, Pérez LI, Malinowski DP, Molina-Montenegro MA, Gundel PE (2021) A systematic review on the effects of Epichloë fungal endophytes on drought tolerance in cool-season grasses. Front Plant Sci 12:644731
Delaney KJ, Klypina N, Maruthavanan J, Lange C, Sterling TM (2011) Locoweed dose responses to nitrogen: positive for biomass and primary physiology, but inconsistent for an alkaloid. Am J Bot 98(12):1956–1965. https://doi.org/10.3732/ajb.1100133
Ehrlich P, Raven P (1964) Butterflies and plants: a study in Coevolution. Evolution 18(4):586–608
Emerick JC, DeMarco LS (1990) Geobotany of selenium. USGS Surv 1064:37–41
Erb M, Kliebenstein DJ (2020) Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional Trichotomy1[OPEN]. Plant Physiol 184(1):39–52. https://doi.org/10.1104/PP.20.00433
Erb M, Robert CA (2016) Sequestration of plant secondary metabolites by insect herbivores: molecular mechanisms and ecological consequences. Curr Opin Insect Sci 14:8–11
Faeth SH, Gardner DR, Hayes CJ, Jani A, Wittlinger SK, Jones TA (2006) Temporal and spatial variation in alkaloid levels in Achnatherum robustum, a native grass infected with the endophyte Neotyphodium. J Chem Ecol 32(2):307–324. https://doi.org/10.1007/s10886-005-9003-x
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37(12):4302–4315. https://doi.org/10.1002/joc.5086
Folk RA, Charboneau JLM, Belitz M, Singh T, Kates HR, Soltis DE, Soltis PS, Guralnick RP, Siniscalchi CM (2024) Anatomy of a mega-radiation: Biogeography and niche evolution in Astragalus. Am J Bot 1–19. https://doi.org/10.1002/ajb2.16299
Gardner DR, Molyneux RJ, Ralphs MH (2001) Analysis of swainsonine: extraction methods, detection, and measurement in populations of locoweeds (Oxytropis spp). J Agric Food Chem 49(10):4573–4580
Gardner DR, Romero J, Ralphs MH, Creamer R (2004) Correlation of an Endophytic fungus (Alternaria spp.) with the presence of swainsonine in lambert locoweed (Oxytropic lambertii). In Poisonous plants and related toxins
Green TW, Palmbald IG (1975) Effects of Insect seed predators on Astragalus cibarius and Astragalus utahensis (Leguminosae). Ecology 56(6):1435–1440. https://doi.org/10.2307/1934711
Grum DS, Cook D, Gardner DR, Roper JM, Pfister JA, Ralphs MH (2012) Influence of seed endophyte amounts on swainsonine concentrations in Astragalus and oxytropis locoweeds. J Agric Food Chem 60(33):8083–8089. https://doi.org/10.1021/jf3024062
James LF, Nielsen DB, Panter KE (1992) Impact of poisonous plants on the livestock industry. J Range Manag 45(1):3–8. https://doi.org/10.2307/4002517
Janzen D (1971) Seed predation by animals. Annu Rev Ecol Syst 2:465–492
Johnson CD (1970) Biosystematics of the Arizona, California, and Oregon species of the seed Beetle: Genus Acanthoscelides Schilsky (Coleoptera: Bruchidae). University of California Press
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (1999) Plant systematics: a phylogenetic approach. Ecología mediterránea 25(2):215
Kingsbury JM (1964) Poisonous plants of the United States and Canada. Poisonous Plants of the United States and Canada
Kingsolver JM, Service U, States AR, Insecta, Coleoptera (2004) U.S. Department of Agriculture, Agricultural Research Service
Klypina N, Pinch M, Schutte BJ, Maruthavanan J, Sterling TM (2017) Water-deficit stress tolerance differs between two locoweed genera (Astragalus and Oxytropis) with fungal endophytes. Weed Sci 65(5):626–638. https://doi.org/10.1017/wsc.2017.21
Knaus BJ (2010) Morphometric architecture of the most taxon-rich species in the U.S. Flora: Astragalus lentiginosus (Fabaceae). Am J Bot 97(11):1816–1826. https://doi.org/10.3732/ajb.0900145
Knaus BJ, Cronn RC, Liston A (2005) Genetic characterization of three varieties of Astragalus lentiginosus (Fabaceae). Brittonia 57(4):334–344. https://doi.org/10.1663/0007-196X(2005)057[0334:GCOTVO]2.0.CO;2
Lewis GC, Ravel C, Naffaa W, Astier C, Charmet G (1997) Occurrence of Acremonium endophytes in wild populations of Lolium spp. in European countries and a relationship between level of infection and climate in France. Ann Appl Biol 130(2):227–238. https://doi.org/10.1111/j.1744-7348.1997.tb06828.x
Liu H, Shao S, Schellenberg M (2017) A simple and fast procedure to determine 3-nitropropanoic acid and 3-nitropropanol in freeze dried Canadian milkvetch (Astragalus canadensis). Toxins 9(7):1–10. https://doi.org/10.3390/toxins9070204
Lu H, Quan H, Ren Z, Wang S, Xue R, Zhao B (2016) The genome of Undifilum oxytropis provides insights into swainsonine biosynthesis and locoism. Sci Rep 6:1–8. https://doi.org/10.1038/srep30760
Maassoumi AA (2020) A checklist of Astragalus in the world: new grouping, new changes, and additional species with augmented data (Issue August)
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40(4):923–940. https://doi.org/10.2135/cropsci2000.404923x
Marks S, Clay K (1996) Physiological responses of Festuca arundinacea to fungal endophyte infection. New Phytol 133(4):727–733. https://doi.org/10.1111/j.1469-8137.1996.tb01941.x
Maron JL, Agrawal AA, Schemske DW (2019) Plant–herbivore coevolution and plant speciation. Ecology 100(7):1–11. https://doi.org/10.1002/ecy.2704
Marquis R, Salazar D, Baer C, Reinhardt J, Priest G, Barnett K (2016) Ode to Ehrlich and Raven or how herbivorous insects might drive plant speciation. Ecology 97(11):2939–2951
Martinez A, Robles CA, Roper JM, Gardner DR, Neyaz MS, Joelson NZ, Cook D (2019) Detection of swainsonine-producing endophytes in Patagonian Astragalus species. Toxicon 171(September):1–6. https://doi.org/10.1016/j.toxicon.2019.09.020
Molyneux RJ, James LF (1982) Loco Intoxication: Indolizidine alkaloids of spotted locoweed (Astragalus lentiginosus). Science 216(4542):190–191
Molyneux RJ, James LF, Panter KE, Ralphs MH (1991) Analysis and distribution of swainsonine and related polyhydroxyindolizidine alkaloids by thin layer chromatography. Phytochem Anal 2(3):125–129
Molyneux RJ, James LF, Ralphs MH, Pfister JA, Panter KP, Nash RJ (1994) Polyhydroxy alkaloid glycosidase inhibitors from poisonous plants of global distribution: analysis and identification. Plant-Associated Toxins-Agricultural, Phytochemical and Ecological Aspects, pp 107–112
Moore BD, Andrew RL, Külheim C, Foley WJ (2014) Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol 201(3):733–750. https://doi.org/10.1111/nph.12526
Moreira X, Castagneyrol B, Abdala-Roberts L, Berny-Mier y Teran JC, Timmermans BGH, Bruun HH, Covelo F, Glauser G, Rasmann S, Tack AJM (2018) Latitudinal variation in plant chemical defences drives latitudinal patterns of leaf herbivory. Ecography 41(7):1124–1134. https://doi.org/10.1111/ecog.03326
Nelson AS, Whitehead SR (2021) Fruit secondary metabolites shape seed dispersal effectiveness. Trends Ecol Evol 36(12):1113–1123
Oldrup E, Mclain-Romero J, Padilla A, Moya A, Gardner D, Creamer R (2010) Localization of endophytic Undifilum fungi in locoweed seed and influence of environmental parameters on a locoweed in vitro culture system. Botany 88(5):512–521. https://doi.org/10.1139/B10-026
Omacini M, Chaneton EJ, Ghersa CM, Müller CB (2001) Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature 409(6816):78–81. https://doi.org/10.1038/35051070
Panaccione DG, Beaulieu WT, Cook D (2014) Bioactive alkaloids in vertically transmitted fungal endophytes. Funct Ecol 28(2):299–314. https://doi.org/10.1111/1365-2435.12076
Pérez LI, Gundel PE, Zabalgogeazcoa I, Omacini M (2020) An ecological framework for understanding the roles of Epichloë endophytes on plant defenses against fungal diseases. Fungal Biology Reviews 34(3):115–125
Pfister JA, Stegelmeier BL, Gardner DR, James LF (2003) Grazing of spotted locoweed (Astragalus lentiginosus) by cattle and horses in Arizona. J Anim Sci 81(9):2285–2293. https://doi.org/10.2527/2003.8192285x
Pinheiro J, Bates D, Team RC (2022) nlme: linear and nonlinear mixed effects models. R package version 3.1–157. Website: https://Cran.Rproject.Org/Web/Packages/Nlme/Index. Html [Accessed 24 Feb 2022]
Pryor BM, Creamer R, Shoemaker RA, McLain-Romero J, Hambleton S (2009) Undifilum, a new genus for endophytic Embellisia oxytropis and parasitic helminthosporium bornmuelleri on legumes. Botany 87(2):178–194. https://doi.org/10.1139/B08-130
Quach QN, Clay K, Lee ST, Gardner DR, Cook D (2023) Phylogenetic patterns of bioactive secondary metabolites produced by fungal endosymbionts in morning glories (Ipomoeeae, Convolvulaceae). New Phytol 238(4):1351–1361. https://doi.org/10.1111/nph.18785
Ralphs MH, Graham D, Duff G, Stegelmeier BL, James LF (2000) Impact of locoweed poisoning on grazing steer weight gains. J Range Manag 53(1):86–90. https://doi.org/10.2307/4003397
Ralphs MH, Gardner DR, Graham JD, Greathouse G, Knight AP (2002a) Clipping and precipitation influences on locoweed vigor, mortality, and toxicity. J Range Manag 55(4):394–399. https://doi.org/10.2307/4003477
Ralphs MH, Creamer R, Baucom D, Gardner DR, Welsh SL, Graham JD, Hart C, Cook D, Stegelmeier BL (2008) Relationship between the endophyte Embellisia spp. and the toxic alkaloid swainsonine in major locoweed species (Astragalus and Oxytropis). J Chem Ecol 34(1):32–38. https://doi.org/10.1007/s10886-007-9399-6
Rudgers JA, Clay K (2007) Endophyte symbiosis with tall fescue: how strong are the impacts on communities and ecosystems? Fungal Biology Reviews 21(2–3):107–124. https://doi.org/10.1016/j.fbr.2007.05.002
Schardl CL, Panaccione DG, Tudzynski P (2006) Ergot alkaloids - Biology and Molecular Biology. Alkaloids 63(06):45–86. https://doi.org/10.1016/S1099-4831(06)63002-2
Siegel MR, Bush LP (1996) Defensive chemicals in Grass-Fungal. Phytochemical Divers Ecol Interact, 82–107
Stamp N (2003) Out of the quagmire of plant defense hypotheses. Q Rev Biology 78(1):23–55. https://doi.org/10.1086/367580
Thompson JN (1994) The coevolutionary process. 376. https://books.google.com/books/about/The_Coevolutionary_Process.html?id=InCAChmWM1QC
Thompson JN (2005) The Geographic Mosaic of Coevolution. University of Chicago Press
Vallotton AD, Murray LW, Delaney KJ, Sterling TM (2012) Water deficit induces swainsonine of some locoweed taxa, but with no swainsonine-growth trade-off. Acta Oecol 43:140–149. https://doi.org/10.1016/j.actao.2012.06.006
Vannette RL, Hunter MD (2011) Plant defence theory re-examined: nonlinear expectations based on the costs and benefits of resource mutualisms. J Ecol 99(1):66–76
Whitehead SR, Bass E, Corrigan A, Kessler A, Poveda K (2021) Interaction diversity explains the maintenance of phytochemical diversity. Ecol Lett 24(6):1205–1214. https://doi.org/10.1111/ele.13736
Williams MC, Barneby RC (1977) The Occurrence of Nitro-Toxins in North American Astragalus (Fabaceae) Published by: Springer on behalf of the New York Botanical Garden Press Stable URL: https://www.jstor.org/stable/2806203 REFERENCES Linked references are available on JSTOR for thi. 29(3), 310–326
Wu C, Han T, Lu H, Zhao B (2016) The toxicology mechanism of endophytic fungus and swainsonine in locoweed. Environ Toxicol Pharmacol 47(2016):38–46. https://doi.org/10.1016/j.etap.2016.08.018
Zhang X, xu, Li Cjie, Nan Zbiao (2011) Effects of salt and drought stress on alkaloid production in endophyte-infected drunken horse grass (Achnatherum Inebrians). Biochem Syst Ecol 39(4–6):471–476. https://doi.org/10.1016/j.bse.2011.06.016
Zhao M, Gao X, Wang J, He X, Han B (2013) A review of the most economically important poisonous plants to the livestock industry on temperate grasslands of China. J Appl Toxicol 33(1):9–17. https://doi.org/10.1002/jat.2789
Züst T, Heichinger C, Grossniklaus U, Harrington R, Kliebenstein D, Turnbull L (2012) Natural enemies Drive Geographic Variation in Plant defenses. Science 338(October):116–119
Acknowledgements
We thank A. Hidad. S. Aguilar, and M. Thariath for assistance with measuring herbivory rates, Loyola University Chicago for funding, and three anonymous reviewers for comments on the manuscript.
Author information
Authors and Affiliations
Contributions
JSD and MG wrote the main manuscript text. JSD carried out formal analysis and prepared figures. MS, DC, GM, and MG conceptualized and designed the experiment. MS, MG, and DC collected and quantified the data. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Davis, J., Scott, M., Cook, D. et al. Extensive Local Geographic Variation in Locoweed Toxin Produced by a Fungal Endophyte. J Chem Ecol (2024). https://doi.org/10.1007/s10886-024-01529-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10886-024-01529-3