Abstract
The legume pod borer, Maruca vitrata, is a pantropical pest on leguminous crops. (E,E)-10,12-Hexadecadienal, (E,E)-10,12-hexadecadienol, and (E)-10-hexadecenal were described previously as sex pheromone components for this nocturnal moth. A blend of these components in a ratio of 100:5:5 attracted males in field trapping experiments in Benin, but not in Taiwan, Thailand, or Vietnam. This finding suggests geographic variation in the pheromone blend between Asian and West African populations of M. vitrata. We, therefore, determined the pheromone compositions of single pheromone glands of females from the three Asian regions and from Benin by gas chromatography—mass spectrometry. Additionally, we compared the responses of males from Taiwan and Benin to calling females and to gland extracts of females from both regions in laboratory no-choice and two-choice assays. Chemical analysis revealed the presence of (E,E)-10,12-hexadecadienal and (E,E)-10,12-hexadecadienol, as well as the absence of (E)-10-hexadecenal in all four populations. The relative amounts of the detected compounds did not vary significantly among the insect populations. The behavioral bioassays showed that Taiwanese and Beninese males were similarly attracted to females from both regions, as well as to their gland extracts. As a result, we did not find geographic variation in the sexual communication system of M. vitrata between West African and Asian insect populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The legume pod borer, Maruca vitrata (F.) (Lepidoptera: Crambidae), causes severe damage on economically important leguminous crops throughout the tropics and subtropics (Sharma et al. 1999), and it is a major pest on yard long bean (Vigna unguiculata spp. sesquipedalis) in Thailand and Vietnam (Schreinemachers et al. 2014). This pest is controlled mainly by synthetic insecticides (Schreinemachers et al. 2014; Srinivasan et al. 2013), which are not very effective because the larvae are exposed only for a short time after hatching before they start feeding on plant organs internally (Sharma et al. 1999). Since development of insecticide resistance has been reported for M. vitrata (Ekesi 1999; Ulrichs et al. 2001), more specific and targeted use of insecticides is necessary to reduce its overuse.
Pheromone traps are a valuable tool in crop protection for species-specific pest monitoring and are used to perform target-oriented control measures. The sex pheromone of M. vitrata has been studied for more than a decade. Initial work described (E,E)-10,12-hexadecadienal (EE10,12-16:Ald) as the major sex pheromone component and (E,E)-10,12-hexadecadienol (EE10,12-16:OH) as a minor component (Adati and Tatsuki 1999; Downham et al. 2003). However, EE10,12-16:OH did not increase male response of a M. vitrata population from Ghana in a behavioral bioassay (Adati and Tatsuki 1999), while males of a mixed insect population from Benin, Nigeria, India, and Taiwan approached EE10,12-16:Ald as an attractant in wind tunnel assays only when 5 % of EE10,12-16:OH was added (Downham et al. 2003). In addition to these two compounds, Downham et al. (2003) suggested the presence of a monounsaturated hexadecenal in gland extracts from the mixed M. vitrata population, based on gas chromatography—electroantennographic detection (GC/EAD). Comparing a range of synthetic monounsaturated hexadecenals, (E)-10-hexadecenal (E10-16:Ald) elicited the strongest electroantennographic (EAG) response from male antennae. Subsequent field trapping experiments revealed that a blend containing 100:5:5 EE10,12-16:Ald : EE10,12-16:OH : E10-16:Ald was the most attractive synthetic lure in Benin (Downham et al. 2003, 2004). However, this blend did not attract any males in field trapping experiments in Taiwan (Schläger et al. 2012), Thailand, or Vietnam (Srinivasan et al. 2015). These findings suggest geographic variation in the pheromone blend between Asian and Beninese M. vitrata populations. Geographic variation has been reported recently in two different Chinese populations: females from Wuhan produced a pheromone ratio of 100:12.1:79.5 EE10,12-16:Ald : EE10,12-16:OH : E10-16:Ald, whereas a ratio of 100:0.7:10.3 was found in gland extracts from females from Huazhou (Lu et al. 2013).
Since the ratio of the pheromone components is crucial to design efficient lures (Baker 2008), the aim of this study was to determine whether there is geographic variation in the sexual communication of M. vitrata, specifically between Asian (Taiwan, Thailand, and Vietnam) and West African (Benin) populations. Single female pheromone gland extracts were analyzed by gas chromatography–mass spectrometry (GC/MS), and pheromone components were identified and quantified by comparison to all authentic stereoisomers of the three described pheromone compounds for M. vitrata. Male preference was assessed by performing wind tunnel experiments to compare responses of males from Taiwan and Benin to females and gland extracts from the two populations.
Methods and Materials
Insects
Larvae and pupae of M. vitrata were obtained from laboratory colonies at AVRDC -The World Vegetable Center in Taiwan and Thailand; the Vietnam Academy of Agricultural Sciences in Ha Noi, Vietnam; and the International Institute of Tropical Agriculture in Cotonou, Benin. At the Leibniz Institute of Vegetable and Ornamental Crops in Großbeeren (IGZ), Germany, the larvae were reared individually in small plastic cups (37 ml, Market Grounds GmbH & Co. KG, Hamburg, Germany) for at least one generation on artificial cowpea diet until pupation. The diet was prepared as described in Jackai and Raulston (1988), with three modifications. We added saccharose to the diet, but did not add dried and pulverized cowpea leaves, and used kanamycin (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) as antibiotic. The pupae were transferred to acrylic glass or glass cages where the emerging moths mated. For oviposition, females were placed singly in small plastic cups (37 ml). For experiments, pupae were placed separately in plastic cups (37 ml) directly after pupation and reared under a 14 L:10D photoperiod at 25 °C and 80 % relative humidity in a controlled environment chamber (Vötsch, Balingen, Germany). After emergence, moths were kept under the same conditions, and were provided with a 10 % honey solution from a cotton dental roll (Apo-discounter, Markkleeberg, Germany) as food.
Pheromone Gland Extraction
Pheromone glands were excised from 4 to 5-d-old, unmated female moths 5–6 h into scotophase. Behavioral observations confirmed calling behavior of females from Benin and Taiwan during this time. Glands were transferred singly to a 150 μl conical glass insert (Macherey-Nagel GmbH & Co. KG, Düren, Germany) containing 50 μl hexane (≥98.0 %, Merck KGaA, Darmstadt, Germany) with 1 ng pentadecane (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) as internal standard. Glands were extracted for 30–40 min at room temperature in 1.5 ml brown glass vials (Macherey-Nagel GmbH & Co. KG, Düren, Germany) to prevent photoisomerization of conjugated diene systems (Cork et al. 1988; Ideses and Shani 1988; Cork 2004). Samples were analyzed immediately by gas chromatography coupled with mass spectrometry (GC/MS) or were stored at −80 °C for up to 2 d until analysis.
Reference Compounds
The synthetic standards EE- (isomeric purity: 98 %), ZZ10,12-16:Ald (95 %), EE- (98 %), and ZZ10,12-16:OH (93 %) were purchased from Pherobank, Wageningen, Netherlands. EZ- (97 %), ZE10,12-16:Ald (97 %), EZ- (87 %), ZE10,12-16:OH (92 %), Z- (78 %), and E10-16:Ald (96 %) were obtained from the Biocontrol Research Laboratories, Bangalore, India. The isomeric purity was determined by GC/MS analysis. All synthetic compounds were verified using NMR spectroscopy at the Max Planck Institute for Chemical Ecology (MPICE).
Chemical Analysis of Female Gland Extracts
Single gland extracts were analyzed by GC/MS using selected ion monitoring (SIM) to increase sensitivity. This method is suitable to detect trace compounds, such as minor pheromone compounds. A glass syringe (10 μl, Hamilton, Höchst, Germany) was rinsed × 10 with acetone (≥99.8 %, Merck KGaA, Darmstadt, Germany) and × 10 with hexane (≥98.0 %, Merck KGaA, Darmstadt, Germany), after which 1 μl octane (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was taken up into the syringe. The gland extract was reduced under a gentle stream of nitrogen to 2–4 μl, and transferred with the syringe to a 150 μl glass insert (Macherey-Nagel GmbH & Co. KG, Düren, Germany) in a 1.5 ml brown crimp-capped vial (Macherey-Nagel GmbH & Co. KG, Düren, Germany). Samples were analyzed using an Agilent 7890A gas chromatograph equipped with a Supelcowax column (60 m × 0.25 mm ID, 0.25 μm film thickness, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and coupled with an Agilent 5975C mass selective detector (MSD). The carrier gas was helium at a constant flow of 1.2 ml/min. Samples were analyzed by injecting the entire volume (i.e., 3–5 μl) in splitless mode at 250 °C inlet temperature. The oven program was optimized for separation of all standard compounds: 70 °C held for 1 min, increased at 5 °C/min to 240 °C and held for 10 min to clean the column. SIM was conducted focusing on unique or most abundant ions of the internal standard and the pheromone components: pentadecane (m/z 57, 212), hexadecenal (m/z 55, 69, 238), hexadecadienal, (m/z 67, 81, 236), and hexadecadienol (m/z 67, 81, 238). Each ion was monitored individually at the expected retention times of the compounds and their isomers. Retention times were determined by injecting multicomponent standard mixtures containing 0.1, 0.5, 1, 2.5, 5, and 7.5 ng of each component together with 1 ng of the internal standard. The quantity of each compound in single gland extracts was determined by relating its peak area to the internal standard and correcting for the differential responsiveness of the MSD to the compound. Pheromone quantities below the detection limit (<0.01 ng) were scored as zero, and such samples were excluded from statistical analysis. Additionally, one combined and concentrated gland extract of 19 M. vitrata females from Taiwan was analyzed under the same conditions.
Male Attraction in Wind Tunnel Assays
Cross-attraction between Asian and West African M. vitrata populations was determined with the populations from Benin and Taiwan only. Wind tunnel assays were conducted by using both live female moths and their gland extracts as source of pheromone. The custom-made wind tunnel (80 cm long × 35.5 cm high × 37.5 cm wide) was made of glass, except for one long side which was made of polypropylene with two closable windows to be able to exchange the moths and gland extracts (mechanical workshop, MPICE). An airflow of ~0.03 m/s was generated by an axial fan (REW 150/2, axial in-duct fan 150 mm, 6 in 1 PH, Helios, Villingen-Schwenningen, Germany) in the wind tunnel. Incoming air was cleaned using active charcoal (double filter for range hoods, Ewald Wolf Kunststoffwerk GmbH & CO. KG, Weißenburg in Bayern, Germany). A strip of light-emitting diodes (λ = 625 nm, Barthelme GmbH & Co, Nürnberg, Germany) was placed on top of the tunnel to provide illumination. Experiments were conducted in an environment–controlled climate chamber (Johnson Controls International, Essen, Germany) at 25 °C and 80 % relative humidity. Every experimental day before scotophase, the wind tunnel was cleaned with 70 % ethanol (≥99.8 % with 1 % methyl ethyl ketone, Carl Roth GMBH + CO. KG, Karlsruhe, Germany) while air was flowing through the tunnel.
Females and males were placed in the chamber for at least 1.5 h prior to the experiment. Containers with confined females were covered by a layer of active charcoal (double filter for range hoods, Ewald Wolf Kunststoffwerk GmbH & CO. KG, Weißenburg in Bayern, Germany) to prevent contaminations of pheromone components inside the climate chamber. Bioassays were performed between 5 and 10 h into the scotophase. Four- to 6-d-old males were released individually from small plastic cups (37 ml) 52 cm downwind from the pheromone source. If a male did not take flight within 10 min, he was scored as a non-responder. After taking flight, moth behavior was observed for up to 10 min. Males from Benin and Taiwan were tested alternately and each individual was tested only once. The male response was divided into the following behavioral categories: (1) taking flight but not orienting toward the source of stimuli; (2) oriented flight, ending in hovering in front of the pheromone source; and (3) pheromone source contact.
No-choice Assay with Live Females
Four- to 6-d-old females were caged singly inside a translucent, horizontally placed plastic cup (473 ml) near the upwind end of the tunnel in the middle of the cross section. The bottom of the plastic cup and the lid consisted of gauze to enable airflow through the plastic cup. The female was observed continuously during the experiment for showing calling behavior (extruding her ovipositor) and was replaced if she did not call.
No-choice Assay with Gland Extracts
Gland extracts prepared as described above from 4 to 5-d-old virgin females also were used as a pheromone source. These gland extracts were used in the experiment within 24 h to make sure that the pheromone compounds would not have degraded. Gland extracts, which were not used on the same day, were stored in sealed, brown vials (Macherey-Nagel GmbH & Co. KG, Düren, Germany) at −80 °C. Up to 10 glands from females belonging to the same population were extracted together in hexane for 30 to 40 min. Gland extracts of 2 to 29 females were merged and adjusted to a concentration of one gland equivalent in 5 μl, which was applied on a 1 cm2 triangle of filter paper (VWR International GmbH, Dresden, Germany). This paper was suspended from a wire at the upwind end of the tunnel. Filter paper with 5 μl pure hexane served as a control.
Two-choice Bioassay
Females from Benin and Taiwan or the corresponding gland extracts prepared as described above were offered simultaneously in the wind tunnel to males from both populations. The distance between the plastic cups with females in the wind tunnel was 1 cm; filter papers with gland extracts were placed 10 cm apart. Experiments were conducted as described above.
Statistical Analysis
Statistical analysis was conducted using SAS 9.4. Pheromone quantities and pheromone ratios were log(x + 0.01) transformed and analyzed using the non-parametric Kruskal-Wallis test with Bonferroni correction for pairwise multiple comparisons because data were not always normally distributed. To analyze behavioral data, the number of male moths recorded for each behavioral category in the wind tunnel was subjected to a two-tailed 2 × 2 Fisher’s exact test.
Results
Chemical Analysis of Pheromone Gland Extracts
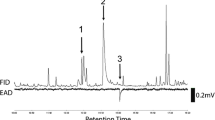
GC/MS analyses revealed that EE10,12-16:Ald and EE10,12-16:OH were present in gland extracts of all four M. vitrata populations (Fig. 1). EE10,12-16:Ald was the major pheromone component accounting for 91.4 % (for Benin) to 92.4 % (for Thailand) of the pheromone blend. The minor component EE10,12-16:OH was always present in gland extracts of females from Benin, but could not be detected in 19 % (7) of the Vietnam samples, in 9 % (7) of the Thailand samples, or in 2 % (1) of the Taiwan samples. E10-16:Ald, previously described as a pheromone component, was not detected in any sample. Analysis of a pooled and concentrated gland extract of 19 females from Taiwan confirmed that the amount of this compound was below the detection level (data not shown). Amounts of the stereoisomers of the three pheromone components also were below the detection limit.
GC/MS selected ion chromatograms of a single gland extract of Maruca vitrata (Benin; top) and the multicomponent standard mixture (1 ng/μl; inverted). Dotted lines indicate monitoring of a new group of selected ions. Peaks highlighted by a grey color correspond to compounds reported to be sex pheromone components for M. vitrata
The total amount of pheromone detected per gland differed significantly between the populations (P ≤ 0.001). Females from Benin (N = 60) and Taiwan (N = 59) produced significantly more pheromone compared to females from Thailand (N = 71) and Vietnam (N = 30), and females from Vietnam produced the least (Fig. 2a). The ratio of EE10,12-16:Ald to EE10,12-16:OH also differed among populations (Kruskal-Wallis test, P = 0.04). However, pairwise multiple comparisons with Bonferroni correction showed no difference between specific populations (P = 0.39 - 1) (Fig. 2b).
Comparisons (mean ± SE) of the pheromone amount (a) and ratio (b) of Maruca vitrata females from populations of Benin (N = 60), Taiwan (N = 59), Thailand (N = 71), and Vietnam (N = 30). Bars with the same letter are not significantly different according to Kruskal-Wallis test followed by Bonferroni correction (P ≤ 0.05)
Behavioral Experiments with Live Females
In no-choice experiments, males from Benin (N = 48) (Fig. 3a) and Taiwan (N = 52) (Fig. 3b) responded similarly to calling females from both regions, although significantly more males from Benin took flight in tests with females from Taiwan (Fig. 3a). In two-choice assays, males from Benin (N = 41) did not discriminate between females from Taiwan or Benin (Fig. 3c), while M. vitrata males from Taiwan (N = 63) hovered significantly more often in front of their own females. There was no difference regarding the frequencies of source contact (Fig. 3d, Table S1).
Behavioral responses of Maruca vitrata males from Benin (no-choice: a two-choice assay: c) and Taiwan (no-choice: b two-choice assay: d) towards calling females from Benin or/and Taiwan given as percentages. Symbols in the same behavioral category with different letters were significantly different according to Fisher’s exact test, two tailed (P ≤ 0.05)
Behavioral Experiments with Gland Extracts.
In control experiments with pure solvent, no hovering or source contact was observed (data not shown). In no-choice assays, males from Benin responded equally to gland extracts from both regions (N = 81) (Fig. 4a). This time, males from Taiwan (N = 80) hovered significantly more often in front of gland extracts of females from Benin compared to gland extracts from Taiwan, but they contacted both sources equally often (Fig. 4b, Table S2). In two-choice assays, both males from Benin (N = 36) and Taiwan (N = 35) did not discriminate between gland extracts from both regions (Fig. 4c, d).
Behavioral responses of Maruca vitrata males from Benin (no-choice: a two-choice assay: c) and Taiwan (no-choice: b two-choice assay: d) towards pheromone gland extracts from females from Benin or/and Taiwan applied on filter paper. Symbols in the same behavioral category with different letters were significantly different according to Fisher’s exact test, two-tailed (P ≤ 0.05)
Discussion
In previous work on the sex pheromone of female M. vitrata, up to three compounds, EE10,12-16:Ald, EE10,12-16:OH and E10-16:Ald have been reported as potential pheromone components. Downham et al. (2003) first reported E10-16:Ald but only could detect it in GC/EAD and EAG analyses of pheromone gland extract from females from a mixed population from Benin, Nigeria, India, and Taiwan, and could not detect it in GC/MS analyses. Downham et al. (2003, 2004) found that a blend of the three components in a ratio of 100:5:5, respectively, was the most attractive synthetic lure in wind tunnel assays and field trapping experiments in Benin. We only detected EE10,12-16:Ald and EE10,12-16:OH in our GC/MS analyses of pheromone gland extracts and E10-16:Ald was not detected (<0.01 ng) in any of our samples. Similarly, Adati and Tatsuki (1999) did not detect E10-16:Ald in gland extracts of female M. vitrata from Ghana, but, recently, Lu et al. (2013) detected E10-16:Ald in single female gland extracts from two Chinese M. vitrata populations by GC/MS. The aldehyde constituted 10.3 % of the pheromone gland extract of females from Huazhou, while in females from Wuhan E10-16:Ald was the major component at 79.5 %. In field studies in China, the number of males caught also was highest when E10-16:Ald was added: in Huazhou, a 100:10:10-blend (EE10,12-16:Ald : EE10,12-16:OH : E10-16:Ald) attracted the maximum number of males, whereas in Wuhan a 100:10:80-blend was most attractive (Lu et al. 2013).
In our study, the ratio of both detected pheromone components, EE10,12-16:Ald to EE10,12-16:OH, did not vary among the investigated M. vitrata populations. EE10,12-16:OH was a minor compound and ranged from 10.1 % in females from Thailand to 11.5 % in females from Taiwan, compared to EE10,12-16:Ald (100 %). Pheromone ratios are known to be affected by the time of gland extraction in the scotophase as well as the age of the female (Delisle and Royer 1994; Kamimura and Tatsuki 1993), thus making it difficult to compare different studies. However, variations in the extraction times could at least partly explain differences in relative amounts of the pheromone components. A combined gland extract of M. vitrata females from Ghana contained 3–4 % of EE10,12-16:OH (Adati and Tatsuki 1999). The shorter extraction time of the pheromone glands (10 min) compared to our extraction time (30–40 min) possibly led to a lower amount of the alcohol. EE10,12-16:OH is more polar than EE10,12-16:Ald and might need more time to dissolve in the nonpolar solvent hexane. Downham et al. (2003) also found a lower percent of the alcohol (2–5 %) in a pooled gland extract of the mixed M. vitrata population, where they used a shorter extraction time (5–10 min), albeit with a brief sonication during the solvent extraction. Lu et al. (2013) extracted single pheromone glands of Chinese M. vitrata females for a longer time period (30 min) and found a similar percentage of EE10,12-16:OH (12.1 %) in females from Wuhan. Females from Huazhou contained the lowest proportion of the alcohol (0.7 %) detected in a M. vitrata population so far.
We did not perform pheromone collection by air entrainment because of the low pheromone amounts detected in individual glands (0.03–6.3 ng/female). Downham et al. (2003) collected volatiles by air entrainment from 1 to 2 calling M. vitrata females on activated charcoal (5 mg; 0.01 mm particle size) or from 12 to 22 females on Porapak Q (50–80 mesh; 100 mg). These authors detected only EE10,12-16:Ald, although in much lower amounts compared to the merged gland extract (0.5–2 ng/female). However, subsequent experiments revealed that only 44 % of synthetic EE10,12–16:Ald were recovered from activated charcoal filters, but over 90 % from Porapak Q filters (Downham et al. 2003).
In our behavioral assays, M. vitrata males from Taiwan and Benin responded similarly to females from both regions and to the corresponding gland extracts. The behavioral response of male M. vitrata to live females and gland extracts was comparable, which verifies our extraction method for the chemical pheromone blend analysis. Visual cues also may affect male response, as males were hovering longer in front of calling females compared to gland extracts applied on filter paper.
Together, our results do not support the hypothesis of geographic variation in the sexual communication between Asian and West African M. vitrata populations. However, this conclusion is drawn with caution, because in artificial wind tunnel experiments Agrotis ipsilon (Hufnagel) males also were equally attracted to geographically distinct females with significantly different pheromone blends (Gemeno et al. 2000), while recent field experiments in China did show geographic variation in attraction of A. ipsilon males (Du et al. 2015).
It is possible that additional, unidentified pheromone components are essential for the attraction of M. vitrata males, because reported trap catches of M. vitrata males generally are very low. For example, in a period of eight weeks the 100:5:5-blend attracted only a total of 33.1 males per trap in Benin (Downham et al. 2003). In China, only 19.5 males in total per trap were caught in four weeks (Lu et al. 2013). In contrast, we caught up to 89 males in traps baited with live females in one night (unpublished results). However, traps baited with the 100:5:5-blend attracted significantly more M. vitrata males than traps baited with two virgin females in field trapping experiments in Benin (Downham et al. 2003). The authors assumed that females were not constantly releasing pheromones compared to the synthetic lure. In our experiments, only females caged in large plastic cups (250 ml) attracted males, while females confined in smaller cages (96 or 37 ml) did not attract any males. A similar effect might be responsible for the low attractiveness of live females used as baits in field experiments in Benin.
Another possible explanation for the low trap catches with synthetic pheromone blends is the presence of isomeric impurities that may inhibit male attraction. EE10,12-16:Ald and EE10,12-16:OH contain conjugated diene systems that are susceptible to photoisomerization induced by sunlight (Cork 2004). Pheromone lures for field studies in Benin (Downham et al. 2003), China (Lu et al. 2013), Taiwan (Schläger et al. 2012), Thailand, and Vietnam (Srinivasan et al. 2015) were not formulated with UV-stabilizers to prevent photoisomerization. Adati and Tatsuki (1999) reported that M. vitrata males were more attracted to purified EE10,12-16:Ald (isomeric purity 99 %) than to unpurified EE10,12-16:Ald (92 %) in behavioral bioassays. Furthermore, the presence of stereoisomers reduced male attraction, especially when adding EZ10,12-16:Ald to EE10,12-16:Ald (Adati and Tatsuki 1999). In field bioassays with Earias vittella (F.), the addition of an isomer (EZ10,12-16:Ald) to the major pheromone component EE10,12:16Ald also significantly reduced trap catches (Cork et al. 1988). However, trap catches of M. vitrata males in field studies in Benin were not affected by different isomeric purities of EE10,12-16:Ald and EE10,12-16:OH (73 %, 80 %, 91 %, or >99 %) (Downham et al. 2004). Additionally, pheromone lures for field trapping experiments in Benin were wrapped in aluminium foil to prevent photoisomerization (Downham et al. 2003, 2004), but again there was no difference between male trap catches with protected or exposed pheromone dispensers (Downham et al. 2004).
In summary, our studies revealed no significant differences in the relative amounts of the three compounds previously reported as components of the female sex pheromone in extracts of pheromone glands of female M. vitrata from colonies originating in Taiwan, Thailand, Vietnam, or Benin. Furthermore, in our behavioral assays, M. vitrata males from Taiwan and Benin responded similarly to females from both regions and to the corresponding gland extracts. Hence, we do not support the hypothesis that there is geographic variation in sex pheromone blends among M. vitrata populations from Asia and West Africa although a recent study indicated the presence of different putative subspecies in M. vitrata in Asia and sub-Saharan Africa based on mitochondrial cytochrome oxidase I (COI) gene sequences (Periasamy et al. 2015). Future research should focus on the possibility of additional pheromone components that are essential to attract males, and on the verification of pheromone purity and stability under field conditions.
References
Adati T, Tatsuki S (1999) Identification of female sex pheromone of the legume pod borer, Maruca vitrata and antagonistic effects of geometrical isomers. J Chem Ecol 25:105–115
Baker TC (2008) Balanced olfactory antagonism as a concept for understanding evolutionary shifts in moth sex pheromone blends. J Chem Ecol 34:971–981
Cork A (2004) Pheromone manual. Natural Resources Institute, Chatham Maritime ME4 4TB, UK
Cork A, Chamberlain DJ, Beevor PS, Hall DR, Nesbitt BF, Campion DG, Attique MR (1988) Components of female sex pheromone of spotted bollworm, Earias vittella F. (Lepidoptera: Noctuidae): identification and field evaluation in Pakistan. J Chem Ecol 14:929–945
Delisle J, Royer L (1994) Changes in pheromone titer of obliquebanded leafroller, Choristoneura rosaceana, virgin females as a function of time of day, age, and temperature. J Chem Ecol 20:45–69
Downham MCA, Hall DR, Chamberlain DJ, Cork A, Farman DI, Tamò M, Dahounto D, Datinon B, Adetonah S (2003) Minor components in the sex pheromone of legume pod-borer: Maruca vitrata development of an attractive blend. J Chem Ecol 29:989–1011
Downham MCA, Tamò M, Hall DR, Datinon B, Adetonah S, Farman DI (2004) Developing pheromone traps and lures for Maruca vitrata in Benin, West Africa. Entomol Exp Appl 110:151–158
Du Y, Feng B, Li H, Liu C, Zeng J, Pan L, Yu Q (2015) Field evaluation of Agrotis ipsilon (Lepidoptera: Noctuidae) pheromone blends and their application to monitoring moth populations in China. Environ Entomol 44:724–733
Ekesi S (1999) Insecticide resistance in field populations of the legume pod-borer, Maruca vitrata Fabricius (Lepidoptera: Pyralidae), on cowpea, Vigna unguiculata (L.), Walp in Nigeria. Int J Pest Manag 45:57–59
Gemeno C, Lutfallah AF, Haynes KF (2000) Pheromone blend variation and cross-attraction among populations of the black cutworm moth (Lepidoptera: Noctuidae). Ann Entomol Soc Am 93:1322–1328
Ideses R, Shani A (1988) Chemical protection of pheromones containing an internal conjugated diene system from isomerization and oxidation. J Chem Ecol 14:1657–1669
Jackai LEN, Raulston JR (1988) Rearing the legume pod borer Maruca testulalis (Geyer) (Lepidoptera: Pyralidae) on artificial diet. Trop Pest Manag 34:168–172
Kamimura M, Tatsuki S (1993) Diel rhythms of calling behavior and pheromone production of oriental tobacco budworm moth, Helicoverpa assulta (Lepidoptera: Noctuidae). J Chem Ecol 19:2953–2963
Lu PF, Qiao HL, Luo YQ (2013) Female sex pheromone blends and male response of the legume pod borer Maruca vitrata (Lepidoptera: Crambidae), in two populations of mainland China. Z Naturforsch 68:416–427
Periasamy M, Schafleitner R, Muthukalingan K, Ramasamy S (2015) Phylogeographical structure in mitochondrial DNA of legume pod borer (Maruca vitrata) population in tropical Asia and sub-Saharan Africa. PLoS One 10, e0124057
Schläger S, Ulrichs C, Srinivasan R, Beran F, Bhanu KRM, Mewis I, Schreiner M (2012) Developing pheromone traps and lures for Maruca vitrata in Taiwan. Gesunde Pflanz 64:183–186
Schreinemachers P, Srinivasan R, Wu MH, Bhattarai M, Patricio R, Yule S, Quang VH, Hop BTH (2014) Safe and sustainable management of legume pests and diseases in Thailand and Vietnam: a situational analysis. Int J Trop Insect Sci 34:88–97
Sharma HC, Saxena KB, Bhagwat VR (1999) The legume pod borer, Maruca vitrata: Bionomics and management. Information Bulletin no. 55. Patancheru 502 324. International Crops Research Institute for the Semi-Arid Tropics, Andhra Pradesh
Srinivasan R, Yule S, Chang JC, Periasamy M, Lin MY, Hsu YS, Schafleitner R (2013) Towards developing a sustainable management strategy for legume pod borer, Maruca vitrata on yard-long bean in Southeast Asia. In: Holmer R, Linwattana G, Nath P, Keatinge JDH (eds) Proceedings of the Regional Symposium on High Value Vegetables in Southeast Asia: Production, Supply and Demand (SEAVEG2012), 24–26 January 2012, Chiang Mai, Thailand. AVRDC—The World Vegetable Center, Publication No. 12–758. AVRDC – The World Vegetable Center, Taiwan. pp 76–82
Srinivasan R, Lin MY, Su FC, Yule S, Khumsuwan C, Hien T, Hai VM, Khanh LD, Bhanu KRM (2015) Use of insect pheromones in vegetable pest management: Successes and struggles. In: Chakravarthy AK (ed) New horizons in insect science: Towards sustainable pest management. Springer, India, pp 231–237
Ulrichs C, Mewis I, Schnitzler WH, Burleigh JR (2001) Effectivity of synthetic insecticides against Maruca vitrata F. and the parasitoid Bassus asper CHOU & SHARKEY in the Philippines. Mitt Dtsch Ges Allg Angew Entomol 13:279–282
Acknowledgments
We thank Lin Mei-ying and Yang Li-hua from the AVRDC-The World Vegetable Center, Taiwan; Sopana Yule from the AVRDC-The World Vegetable Center, Thailand; Manuele Tamò and Benjamin Datinon from the International Institute of Tropical Agriculture, Benin; Vu Manh Hai, and Le Duc Khanh from the Vietnam Academy of Agricultural Sciences, Vietnam for the constant supply of test insects. We also acknowledge the help and support of Annett Platalla for the chemical analysis and Andrea Maikath for maintenance of the insect colonies at the Leibniz Institute of Vegetable and Ornamental Crops in Großbeeren. The work was funded by Federal Ministry for Economic Cooperation and Development (BMZ) through Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ, GTZ): 09.7860.1-001.00. Stefanie Schläger is grateful for financial support from the FAZIT Stiftung.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Schläger, S., Beran, F., Groot, A.T. et al. Pheromone Blend Analysis and Cross-Attraction among Populations of Maruca vitrata from Asia and West Africa. J Chem Ecol 41, 1155–1162 (2015). https://doi.org/10.1007/s10886-015-0653-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0653-z