Abstract
The circle system has been in use for more than a 100 years, whereas the first clinical application of an anaesthetic reflector was reported just 15 years ago. Its functional basis relies on molecular sieves such as zeolite crystals or activated carbon. In a circle system, the breathing gas is rebreathed after carbon dioxide absorption; a reflector on the other hand specifically retains the anaesthetic during expiration and resupplies it during the next inspiration. Reflection systems can be used in conjunction with intensive care ventilators and do not need the permanent presence of trained qualified staff. Because of easy handling and better ventilatory capabilities of intensive care ventilators, reflection systems facilitate the routine use of volatile anaesthetics in intensive care units. Until now, there are three reflection systems commercially available: the established AnaConDa™ (Sedana Medical, Uppsala, Sweden), the new smaller AnaConDa-S™, and the Mirus™ (Pall Medical, Dreieich, Germany). The AnaConDa consists only of a reflector which is connected to a syringe pump for infusion of liquid sevoflurane or isoflurane. The Mirus represents a technical advancement; its control unit includes a gas and ventilation monitor as well as a gas dispensing unit. The functionality, specific features, advantages and disadvantages of both systems are discussed in the text.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Historical development of anaesthesia systems
The history of modern anaesthesia was born with the Ether Day in Boston 170 years ago. William T. G. Morton used sulfuric ether to enable a pain-free surgical removal of a neck mass. The following decades were characterised by testing different medications such as sulfuric ether, chloroform, N2O, ethyl ether, and ethyl chloride.
The aim of the technical development was the reduction of anaesthetic consumption and targeted administration to the patient as well as keeping the ambient air pure. Yet, a consequent evaluation and progression was not attained for a long time.
As early as in the first publicly tested anaesthesia, William T. G. Morton applied a glass globe that can be referred to as a half-open system (Fig. 1b): inside the globe was a sponge impregnated with ether. The patient breathed in the vapour through a mouthpiece. The exhaled air was channelled past the globe via a valve. This prevented an uncontrolled emission of the ether vapours in the ambient air as well as rebreathing of carbon dioxide [1]. At the same time, Morton also used a simple cotton cloth soaked in ether that he held in front of the patient’s mouth and nose, a so-called absolutely open system (Fig. 1a).
In the period that followed, rebreathing systems such as those from Clover and Ombrédanne were applied [2]. These made it possible to reduce the consumption of anaesthetic agents and shorten the time of induction—however, without a carbon dioxide absorber and without supplying additional oxygen which particularly during the administration of N2O lead to hypoxia. Ever since Hickman’s investigations, it had been known that “controlled asphyxia” shows anaesthetizing effects [3, 4]. It did not take long before the first deaths were registered. The first known casualty of anaesthesia was Hannah Greener, a 15-year-old girl from Newcastle [5].
On the other hand, the description of the circle system by Dräger and Roth in 1902 presents a real milestone (Fig. 1c) [6]. An admixture of oxygen and carbon dioxide absorption through calcium oxide made a safe rebreathing of the anaesthesia gas possible. Through this “half-closed system”, the reduction of anaesthetic consumption and the precise administration to the patient as well as the assurance of indoor air quality were accomplished. Without anaesthetic gas scavenging, the latter was of great importance. Respiratory depression caused by anaesthetics could be compensated through ventilation with a manual resuscitator or with a ventilation bag operated with compressed air.
The first description of a reflection system as an alternative to the circle system was made in 1989 through Thomasson et al. [7] (Fig. 1d). A plastic container with a capacity of 110 ml and filled with 60 ml Zeolite granules served as a reflection filter. Zeolites are microporous aluminosilicate minerals that can absorb molecules in suitable sizes (molecular sieve). In order to maintain the end-tidal concentration at 1.5 vol.%, 40 vol.% isoflurane was intermittently injected into a test lung only during the inspiration. The test lung was ventilated through a classical intensive care unit (ICU) ventilator without a circle system. The reflection filter achieved a 51% reduction of anaesthetic consumption, yet the applied Zeolite also reflected carbon dioxide so that the authors did not consider applying it to patients [7].
In 2001, Enlund et al. described the use of the reflection method in clinical practice for the first time. Instead of Zeolite, they used activated carbon material. Their “anaesthetic agent-saving device”, likewise with a volume of 110 ml, additionally contained a porous hollow rod as an evaporator. Liquid isoflurane was infused into the hollow rod with a syringe pump. Sixteen patients with arthroscopic cruciate ligament reconstruction were ventilated with a half-open anaesthesia system (Bain-System): eight with and eight without the device. The average duration of the anaesthesia amounted to about 2 h; the end-tidal isoflurane concentration was 0.5 vol.%. The ventilation was adjusted in such a way that the end-tidal carbon dioxide concentration remained in the normal range. Compared to the half-open system, 40% isoflurane could be saved through the reflection [8]. A year later, the same authors published the use of the anaesthetic agent-saving device with 0.9 vol.% sevoflurane in a similar clinical setting. This time, the consumption correlated with that of the control group ventilated with a circle system with a fresh gas flow (FGF) of 1.5 l/min [9].
2 Physical principles of anaesthetic reflection
Molecular sieves are substances with a large adsorptive capacity for gases or dissolved molecules. They have a large inner surface with pores of uniform size and are used in technology for purification processes e.g. as oxygen concentrators.
Natural molecular sieves consist of zeolite (aluminium silicate crystals) and carbon. Through chemical modifications, the size of their pores can be varied so that they can specifically adsorb certain molecules, such as volatile anaesthetics. In the first description of an anaesthetic-reflection-system, Zeolite was applied, but it reflected a relatively great amount of carbon dioxide so that the application to patients was not considered [7]. The newer reflection systems are based on activated carbon where some degree of cross-reactivity is also present.
Absorption of molecules is unconstrained so that they can desorb again when partial pressure diminishes. In fact, the total amount of volatile anaesthetics taken up by a reflector is small, approximately 0.7 ml liquid isoflurane with AnaConDa™ (Sedana Medical, Uppsala, Sweden), in relation to the amount taken up by the body, approximating to 7 ml isoflurane at 1 vol.% [10].
In the working range of a reflector, the reflection efficiency, i.e. the number of molecules resupplied divided by the number of molecules exhaled by the patient, is fairly constant and amounts to approximately 90% with AnaConDa. However, when the number of anaesthetic molecules exhaled is too large and contains a large expired volume and/or a high concentration, the capacity of the reflector may be exceeded and surplus molecules will pass the reflector and be lost for the patient; thereby, efficiency will diminish [11].
3 Comparison of the anaesthesia systems: rebreathing versus reflection
The consumption of anaesthetics in the circle system is defined by the level of the fresh gas flow (FGF). The fresh gas functions as a carrier gas for wash-in of the volatile anaesthetic. In a simplified model, the FGF must however leave the circle system again. In doing so, it washes the patient’s exhalation out again. In a pharmacokinetic point of view, the FGF represents the pulmonary clearance of the anaesthetic (Fig. 2).
Rebreathing versus reflection: with the circle system, anaesthetic consumption is primarily determined through the fresh gas flow (FGF) which, on one hand, washes in the anaesthetic and, on the other hand, washes it out again. With the reflection system, up to 90% of the anaesthetic is reflected. The wash-out flow is the minute volume (MV), however the wash-out concentration at 90% reflection is only about one-tenth of the patient concentration (cpat). If the FGF of the rebreathing system amounts to a tenth of the minute volume, anaesthetic consumption with both systems will be roughly similar
In a reflection system such as in the ACD, up to 90% of the exhaled anaesthetic is retained during expiration and resupplied during the next inspiration. Ten percent of the anaesthetic is lost with each expiration. As a result, the anaesthetic concentration on the ventilator side of the reflector amounts to approximately one-tenth of the patient’s concentration. Multiplying one-tenth with the respiratory minute volume yields a theoretical flow that corresponds to the pulmonary clearance analog to the FGF of a circle system (Fig. 2).
Considering the characteristics of the circle and the reflection systems in an ICU, the benefits of the latter predominate (Table 1). The purchase or provision of an anaesthesia ventilator is connected with high technical effort, large space requirements, and high costs. The ventilation characteristics of a common bag-in bottle ventilator are worse than that of an ICU ventilator which becomes obvious especially with augmented spontaneous breathing modes. With a circle system, more mechanical components and a large compressible volume lead to higher trigger latencies, although modern anesthesia machines do compensate for this compressible volume in order to give a more accurate tidal volume. Maximal flow generation and leakage compensation are also inferior to ICU ventilators. The logistics of purchasing and storing as well as replacing and disposing of the carbon dioxide absorber together with the toxicity of dehydrated absorber impose obstacles. For induction and quick deepening of anaesthesia, the FGF must be increased, whereby the saving effects of low-flow-anaesthesia will be abated. With very low FGF, regular flushes are required in order to avoid accumulation of endogenous gases such as acetone, methane, or ethanol after previous consumption.
A disadvantage of the reflection system is that with the current commercially available medical products, AnaConDa and Mirus, a relatively large device dead space of 100 ml must be inserted between the Y-piece and the endotracheal tube.
It may be considered as a further disadvantage that the application of the AnaConDa- and also of the Mirus™-system is only possible through a combination of several medical devices with possible sources of error.
To come to conclusion about what systems to use in the ICU: all classical anaesthesia ventilators known to the authors require the permanent presence of trained, qualified staff, as specified in the operating instructions. This cannot be accomplished in an ICU. On the other hand, all devices used in conjunction with reflection systems are approved for stand-alone use.
4 The commercial systems: AnaConDa™ and Mirus™
AnaConDa™ (anaesthetic conserving device, Sedana Medical, Uppsala, Sweden) was the first medical product that permitted the efficient administration of volatile anaesthetics by the reflection principle (Fig. 3a). After the first description of the prototype [8], Sackey et al. published a randomised controlled trial in 2004 where AnaConDa was implemented for inhalation sedation [12]. Critically ill patients were sedated up to 96 h: 20 with isoflurane and 20 with midazolam. The wake-up time after isoflurane was noticeably shorter and the new sedation method was described as safe and effective. Ever since, many studies were performed about inhalation sedation with AnaConDa and it became a routine procedure to sedate selected patients in many European intensive care units [13, 14].
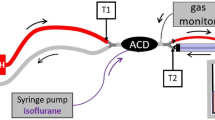
A The anaesthetic conserving device (AnaConDa™, Sedana Medical, Uppsala, Sweden), a: photograph, b: reflection scheme. 1: Ventilation hoses with y-piece, 2: infusion line (connection to the syringe pump with liquid isoflurane or sevoflurane), 3: evaporator (porous plastic cylinder on which anaesthetics vaporise from its surface that is visible as a white column in the transparent plastic casing), 4: patient’s end of the device with tube extension, 5: gas sampling port with sampling line leading to the gas monitor, 6: anaesthetic reflector hidden in the black part of the plastic covering. Reflection scheme: approximately 90 of the 100 exhaled gas molecules are absorbed on the reflector and resupplied at the next inhalation. About 10% pass through the reflector and are thereby lost for the patient. Should the concentration be maintained, these gas molecules must be replaced by the continuous infusion. B The recently commercially available Mirus™ system (Pall Medical, Dreieich, Germany) contains a control unit (a: Mirus™-Controller) which is connected with a multi-lumen-cable (b: depicted: cut open) to the Mirus™-Exchanger (c). Similar to that of the AnaConDa™, it is inserted between the y-piece and ventilation tube. According to the terminology of the manufacturer, the Mirus™-Exchanger consists of the Mirus™-Filter (light blue—equivalent to a common heat moisture exchanger) and the Mirus™-Reflector. This contains a cotton-like coating of active carbon fibres—the actual anaesthetic reflector (d)
AnaConDa-S (Sedana Medical), a smaller version of AnaConDa, has been introduced recently with an internal volume of only 50 ml for use in patients with small tidal volumes [15]. This compares favourably with the internal volumes of common heat moisture exchangers which are in the order of 35–50 ml.
In 2014, the first description of a technically further developed reflection system Mirus™ (Pall Medical, Dreieich, Germany) followed [16]. The system contains a control unit which incorporates the ventilation and gas monitor as well as its own gas dispensing unit with target control of the end-tidal anaesthetic concentration (Fig. 3b). The administration of isoflurane, sevoflurane, or desflurane is thereby possible. The control unit is connected to the reflector with a multi-lumen-cable that is placed between the y-piece and the endotracheal tube.
Table 2 summarises the differences between both commercially available systems: The Mirus™ system has high investment costs yet a longer usability of the disposable reflector which can be applied on different patients up to 7 days, provided that the pre-connected heat moisture exchanger is replaced. A direct comparison of the sedation costs cannot be made at this point since it is common practice that the prices for the Mirus™ controller and reflector as well as for the anaesthetics are individually negotiated with the hospitals. Mirus™ can also apply desflurane, but a different version of the control unit is necessary for each volatile anaesthetic. While the syringe pump of the AnaConDa continually injects the anaesthetic, the Mirus™ utilises the early inspiratory flow phase in order to inject the anaesthetic as saturated vapour. This injection method is supposed to save anaesthetic, however, consumption still appeared quite high. When administering 3.5 vol.% desflurane to a critically ill patient over 24 h, 1260 ml liquid desflurane were used [17].
Gas monitors may display the end-tidal anaesthetic concentration as too high when using AnaConDa. This phenomenon has previously been described by our group [18]. At the end of the expiration, carbon-dioxide containing air remains in the device. The anaesthetic is further infused through the evaporator, and a cloud with high concentration evolves. At the next inhalation, this cloud together with carbon-dioxide containing air passes the gas sampling port. The monitor interprets this high concentration as end-tidal because it coincides with a high carbon-dioxide concentration decreasing immediately thereafter. In the authors’ clinic, it is therefore common practice to assess the mean value of the displayed end-tidal and inspiratory concentrations (which is normally monitored as too low) as patient concentration. This measurement error is avoided in the Mirus™ system since the allocation to the phases of the respiratory cycle is not made according to the carbon-dioxide concentration but according to the actual flow. In a technical evaluation of the Mirus™ system with a test lung, a very good consistency between the end-tidal concentration displayed by the Mirus™ controller with that of an external gas monitor based on high-resolution single breath measurements was found [16].
With Mirus™, the end-tidal concentration can be controlled automatically. In the technical evaluation, the target concentration was well achieved, yet with cyclical fluctuations of 20% around the target and a periodicity of 2.6 min [16]. However, no fluctuations of the sedation depth were observed, neither clinically nor through monitoring of the bispectral index [17].
With AnaConDa, the end-tidal concentration is primarily determined through the ratio of the infusion rate divided by the respiratory minute volume. Consequently, an increase in the spontaneous breathing activity leads to a decrease of the concentration and to a higher degree of alertness. In clinical practice, many patients who are sedated with the AnaConDa-System are relatively deeply sedated; nevertheless, in most cases, spontaneous breathing is well maintained. It remains to be seen whether target control of the end-tidal concentration with the Mirus™ system will lead to sedation closer to the threshold of consciousness (MACawake). The MACawake stands at 30–40% of the classical “minimal alveolar concentration” (MAC) at which half of the patients do not move after noxious stimulation such as skin incision at steady state.
One limitation of the reflection systems has been their large internal volume of 100 ml for AnaConDa and Mirus, and 50 ml for AnaConDa-S. In addition to this volumetric dead space, some carbon dioxide is also reflected back to the patient similar to volatile anaesthetics. The tidal volume increase, necessary to overcome this carbon dioxide reflection and to maintain normocapnia, has been called reflective dead space [19]. Reflective dead space under clinically relevant conditions has been quantified as 40 ml for the classical AnaConDa and 25 ml for AnaConDa-S [15] and Mirus [19]. Thus, total device dead space, the sum of volumetric and reflective dead space, amounts to 75 ml for AnaConDa-S, 125 ml for Mirus, and 140 ml for the classical AnaConDa system.
“Autopumping” is a term used to describe the formation of gas bubbles in the pumping syringe of the AnaConDa that leads to an uncontrolled release of anaesthetic to the patient. The following conditions facilitate the emerging of Autopumping: heat sources, gravitation (high positioning of the syringe pump) and the administration of isoflurane (boiling point 48 °C) versus sevoflurane (boiling point 58 °C) as well as the bubble formation by dissolved oxygen or nitrogen in the anaesthetic if this was priorly cooled (Caution: Don’t cool!) [18, 20]. Autopumping should be avoided at all costs. In the Mirus™ system, this risk does not exist.
When using the AnaConDa, other than autopumping, the following can lead to severe overdose: too large boluses, too high infusion rates (in relation to the minute ventilation), or a too high priming volume when prefilling the system. Filling of the syringe with liquid anesthetic is also tricky, and spilling and contamination of room air may occur.
When using the Mirus™ system, gas application may be interrupted through certain alarms or the absence of carbon dioxide, possibly leading to awakening of the patient.
5 Conclusion
Inhalation sedation has advanced to a routine procedure of sedation in selected patients in many European intensive care units. This was made possible through the introduction of reflection systems such as the AnaConDa system as an alternative to the circle system of classical anaesthesia ventilators. The Mirus™ system represents a technical advancement—nevertheless, its reflection characteristics seem to be inferior to those of the AnaConDa system.
References
Bigelow HJ. Boston Med Surg J 1846;25: 312.
Wawersik J. [The history of anesthesia apparatus: basic principles]. Der Anaesthesist. 1982;31(10):541–8.
Keys TE. Historical vignettes. Henry Hill Hickman (1800-1830). Anesth Analg. 1972;51(3):349
Smith WD. A history of nitrous oxide and oxygen anaesthesia IVD: Henry Hill Hickman in his time. Br J Anaesth. 1978;50(6):623–7.
Knight PR 3rd, Bacon DR. An unexplained death: Hannah Greener and chloroform. Anesthesiology. 2002;96(5):1250–3.
Anonymous. The Roth-Drager Oxygen and Chloroform Apparatus. Br Med J. 1907;1(2418):1067–8.
Thomasson R, Luttropp HH, Werner O. A reflection filter for isoflurane and other anaesthetic vapours. Eur J Anaesthesiol. 1989;6(2):89–94.
Enlund M, Wiklund L, Lambert H. A new device to reduce the consumption of a halogenated anaesthetic agent. Anaesthesia. 2001;56(5):429–32.
Enlund M, Lambert H, Wiklund L. The sevoflurane saving capacity of a new anaesthetic agent conserving device compared with a low flow circle system. Acta Anaesthesiol Scand. 2002;46(5):506–11.
Meiser A, Bellgardt M, Vogelsang H, Sirtl C, Weber T. [Functioning of the anaesthetic conserving device: aspects to consider for use in inhalational sedation]. Der Anaesthesist. 2010;59(11):1029–40. https://doi.org/10.1007/s00101-010-1779-6.
Meiser A, Bellgardt M, Belda J, Rohm K, Laubenthal H, Sirtl C. Technical performance and reflection capacity of the anaesthetic conserving device—a bench study with isoflurane and sevoflurane. J Clin Monit Comput. 2009;23(1):11–9. https://doi.org/10.1007/s10877-008-9158-4.
Sackey PV, Martling CR, Granath F, Radell PJ. Prolonged isoflurane sedation of intensive care unit patients with the Anesthetic Conserving Device. Crit Care Med. 2004;32(11):2241–6.
Jerath A, Parotto M, Wasowicz M, Ferguson ND. Volatile anesthetics: is a new player emerging in critical care sedation? Am J Respir Crit Care Med. 2016. https://doi.org/10.1164/rccm.201512-2435CP.
Misra S, Koshy T. A review of the practice of sedation with inhalational anaesthetics in the intensive care unit with the AnaConDa((R)) device. Indian J Anaesth. 2012;56(6):518–23. https://doi.org/10.4103/0019-5049.104565.
Bomberg H, Meiser F, Daume P, Volk T, Sessler DI, Groesdonk HV, Meiser A. Halving the volume of AnaConDa: evaluation of a new small-volume anesthetic reflector in a test lung model. Anesth Analg (accept for publication with revision). 2017.
Bomberg H, Glas M, Groesdonk VH, Bellgardt M, Schwarz J, Volk T, Meiser A. A novel device for target controlled administration and reflection of desflurane—the Mirus. Anaesthesia. 2014;69(11):1241–50. https://doi.org/10.1111/anae.12798.
Bomberg H, Groesdonk HV, Bellgardt M, Volk T, Meiser A. AnaConDa and Mirus for intensive care sedation, 24 h desflurane versus isoflurane in one patient. SpringerPlus. 2016;5:420. https://doi.org/10.1186/s40064-016-2065-0.
Meiser A, Laubenthal H. Inhalational anaesthetics in the ICU: theory and practice of inhalational sedation in the ICU, economics, risk-benefit. Best Pract Res Clin Anaesthesiol. 2005;19(3):523–38.
Bomberg H, Veddeler M, Volk T, Groesdonk HV, Meiser A. Volumetric and reflective device dead space of anaesthetic reflectors under different conditions. J Clin Monit Comput (accept for publication). 2017.
Henning JD, Bateman R. (2004) Excess delivery of isoflurane liquid from a syringe driver. Anaesthesia. 59(12):1251 (author reply 1251). https://doi.org/10.1111/j.1365-2044.2004.4021_1.x.
Acknowledgements
We thank Karen Schneider for critical revision of the language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AM has received honoraria from Sedana Medical (Uppsala Sweden) and honoraria for lectures as well as research funding from Pall Medical (Dreieich, Germany).
Informed consent
No human participants or animals were involved.
Rights and permissions
About this article
Cite this article
Bomberg, H., Volk, T., Groesdonk, H.V. et al. Efficient application of volatile anaesthetics: total rebreathing or specific reflection?. J Clin Monit Comput 32, 615–622 (2018). https://doi.org/10.1007/s10877-017-0096-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-017-0096-x