Abstract
Purpose
Hemophagocytic lymphohistiocytosis (HLH) is a severe disease with high mortality. The purpose of this investigation was to build models to predict 30-day death in total and subgroup HLH patients based on available and cheap laboratory parameters.
Method
The research contained 431 adults HLH patients from January 2015 to September 2021 in the hospital. Logistic regression and receiver operating characteristic (ROC) were utilized to build models.
Results
Results suggested that age, ferritin, lymphocyte (LY), international normalized ratio (INR), thrombin time (TT), globulin, uric acid (UA), chloride, activated partial thromboplastin time (APTT), aspartate aminotransferase (AST), triglycerides (TG), total bilirubin (TB), and indirect bilirubin (IB) were independent factors in HLH and subgroups. Then, models adapted to patients with different underlying diseases were established based on these factors. Area under curve (AUC) of these models was excellent: HLH patients: 0.838 (p < 0.001); infection-associated HLH (I-HLH) patients: 0.913 (p < 0.001); malignancy-associated HLH (M-HLH): 0.921 (p < 0.001) and 0.809 (p < 0.001) for two or more different etiologies-associated HLH (Mix-HLH patients). In addition, UA, TT, and chloride were firstly confirmed as independent factors in adult HLH.
Conclusion
Four models depending on biomarkers that available and affordable in clinical practice were built. With these models, high-risk patients with different underlying diseases could be easily identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a severe disease, accompanied with persistent fever, high levels of ferritin, splenomegaly, cytopenia, cytokine storm, and multi-organ dysfunction [1]. Cytokine storm coming from activated natural killer (NK) and/or cytotoxic T cells was the direct cause of HLH [2]. HLH could be developed at any age [3]. In Japan, from 2001 to 2005, adult patients account for 40% of the total HLH [4]. In adults patients, infection and malignancy are the most commonly reported underlying diseases [5]. In addition, when HLH developed, one or more organ functions would be affected (such as liver, kidney, and coagulation disorders). Thus, the prognosis varies widely [6, 7].
Elevated post-ferritin, interleukin-10, Epstein-Barr virus (EBV) infection, fibrinogen (FIB), platelet (PLT) and lactate dehydrogenase (LDH), blood routine parameters, and their ratios are risk markers and models that included some of these indicators have excellent predictive ability to forecast the outcomes of patients with HLH [3, 8,9,10]. To malignancy-induced HLH patients, the prognosis is also poor [11, 12]. However, prognostic efficiency and efficacy would be low as these indexes may be expensive and/or have low sensitivity/specificity. Thus, feasible, non-invasive, and reasonably predictive models are needed.
In clinical practice, routine blood test, biochemical test, and coagulation test are the most common, readily available, and measured tests. In models, combinations of multi-factors possess markedly better presentation than a single marker [13]. Thus, the purpose of this investigation was to build models to predict 30-day death in total and subgroup HLH patients based on available and cheap factors.
Methods
Patients and Data
The research contained 431 adults with HLH (meeting 5 or more of the 8 HLH-2004 criteria) [14] from January 2015 to September 2021 in the hospital. Inclusion criteria: (1) age ≥ 18 years; (2) newly diagnosed HLH; (3) complete clinical results. Exclusion criteria: patients with unknown outcome. Data such as age, gender, clinical signs, admission laboratory parameters, etiology, medicine, and outcomes.
The primary outcome was defined as death within 30 days when HLH was initially confirmed. The research was consistent with the Declaration of Helsinki and permitted by the hospital (Nanjing, China) (2019-SR-066).
Statistical Methods
Differences in categorical biomarkers (frequencies), continuous parameters (means ± SD), and non-parametric indexes (medians (ranges)) were evaluated by the Chi square test, ANOVA, T-test, or Mann-Whitney U test as appropriate. Logistic regression was employed to seek out biomarkers of 30-day mortality. Parameters (p < 0.10) on univariate analysis were included in the multivariable analysis. Receiver operating characteristic (ROC) was utilized to judge the performance of models in predicting adverse events and selected the ideal points for each of the models and parameters. p-value <0.05 was considered statistically significant. Data analysis was performed by IBM SPSS 21.0 statistical software (IBM SPSS Version 21.0. Armonk, NY).
Results
Characteristics
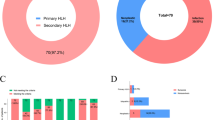
In total, 431 subjects with HLH (252 males, 179 females) were recruited in the retrospective research. The overall mean age was 52.2 ± 16.5 years. Almost all patients were accompanied with fever (> 90%); EBV infection was remarked in 42.92% of the patients. The overall mortality was 29.7%. Other clinical and laboratory parameters covered splenomegaly (208, 48.26%), lymph node enlargement (200, 46.40%), hemophagocytosis (139, 32.25%), and hyperferritin (2358.9 (15.2, 15,000)). Underlying diseases of HLH were listed as follows: infectious diseases (named infection-associated HLH (I-HLH)) (150, 34.56%); malignancies (named malignancy-associated HLH (M-HLH)) (80, 18.56%); mixed connective tissue disease (named MCTD-HLH) (6, 1.39%); unidentified disease (named Unclear-HLH) (33, 7.66%); and two or more different etiologies-associated HLH which could not be classified into any of the above groups (named Mix-HLH) (162, 37.59%). Other features of the total and subgroups of HLH patients are presented in Table 1. In addition, we also listed and compared the types of infections and malignancies in I-HLH, M-HLH, and Mix-HLH (Supplementary table 1).
Independent Parameters for Total or Subgroup HLH
Based on the follow-up results, patients were assigned to survivors and nonsurvivors. Compared with survivors, there was a distinctly difference in nonsurvivors in the following laboratory data: age, ferritin, EBV infection, lymphocyte (LY), neutrophil, hemoglobin, PLT, prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT), FIB, thrombin time (TT), d-dimer, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), direct bilirubin (DB), indirect bilirubin (IB), triglycerides (TG), albumin, globulin, urea nitrogen (UREA), creatinine (CREA), uric acid (UA), chloride for total HLH (all p < 0.10); age, ferritin, EBV infection, LY, PLT, APTT, FIB, TT, d-dimer, ALT, AST, TB, DB, TG, UREA, chloride for I-HLH patients (all p < 0.10); ferritin, EBV infection, LY, neutrophil, PLT, PT, INR, APTT, FIB, TT, ALT, AST, TB, DB, IB, TG, globulin, UREA, CREA for M-HLH patients (all p < 0.10); age, ferritin, PLT, PT, INR, APTT, TT, d-dimer, AST, TB, DB, IB, TG, UREA, CREA, UA for Mix-HLH patients (all p < 0.10) (Table 2).
To select the significant independent markers, ROC was employed to discover the optimal value for each of the continuous factors and models. Then, we reset these parameters in Table 2 with p < 0.10 (except for EBV infection, as not all patients were tested for EBV) into categorical parameters.
On univariate and multivariable logistics regression analyses for HLH patients: age (hazard ratio (HR): 2.990, 95% confidence interval (CI): 1.657–5.397, p < 0.001), ferritin (HR: 4.264, 95% CI: 2.513–7.238, p < 0.001), LY (HR: 0.391, 95% CI: 0.232–0.659, p < 0.001), INR (HR: 2.513, 95% CI: 1.382–4.568, p = 0.003), TT (HR: 2.077, 95% CI: 1.193–3.616, p = 0.010), globulin (HR: 0.560, 95% CI: 0.301–0.848, p = 0.010), UA (HR: 2.637, 95% CI: 1.394–4.988, p = 0.003), and chloride (HR: 0.479, 95% CI: 0.285–0.806, p = 0.006) remained as independent factors of 30-day death. Model was set up depending on these markers (Table 3) (Table 4).
After adjusted confounding factors for I-HLH patients: age (HR: 8.813, 95% CI: 2.602–29.848, p < 0.001), LY (HR: 0.235, 95% CI: 0.078–0.710, p = 0.010), APTT (HR: 5.028, 95% CI: 1.709–14.799, p = 0.003), TT (HR: 3.736, 95% CI: 1.233–11.321, p = 0.020), AST (HR: 3.656, 95% CI: 1.219–10.965, p = 0.021), TG (HR: 4.016, 95% CI: 1.356–11.894, p = 0.012), and chloride (HR: 0.214, 95% CI: 0.074–0.615, p = 0.004) were still in the multivariate model. A model was developed (Table 3).
On univariate and multivariable exploration for M-HLH patients: ferritin (HR: 53.839, 95% CI: 7.772–372.961, p < 0.001), LY (HR: 0.086, 95% CI: 0.016–0.467, p = 0.011), and TB (HR: 12.326, 95% CI: 1.765–86.063, p = 0.005) were confirmed as independent indexes. A multivariate model was formed (Table 3).
On univariate and multivariable logistics regression analyses for Mix-HLH patients: ferritin (HR: 2.215, 95% CI: 1.009–4.864, p = 0.048), LY (HR: 0.387, 95% CI: 0.175–0.859, p = 0.020), APTT (HR: 4.075, 95% CI: 1.470–11.294, p = 0.007), IB (HR: 2.516, 95% CI: 1.129–5.609, p = 0.024), TG (HR: 3.120, 95% CI: 1.037–9.386, p = 0.043), and UA (HR: 4.680, 95% CI: 1.918–11.418, p = 0.001) persisted as significant factors of 30 days mortality. A multivariate model was created (Table 3).
We further explored the distribution of mortality. Figure 1 showed the percentage of mortality by the cumulative number of independent biomarkers. The more risk factors, the higher the mortality.
Performance of Models
ROC was hired to estimate the performance of these clinical models. AUC of these models was listed as follow: HLH patients: 0.842 (95% CI 0.804–0.875, p < 0.001); I-HLH patients: 0.913 (95% CI 0.856–0.953, p < 0.001); M-HLH: 0.921 (95% CI 0.838–0.969, p < 0.001), and 0.809 (95% CI 0.740–0.866, p < 0.001) for Mix-HLH patients (Fig. 2).
Discussion
In this study, models for total or subgroup HLH patients were established based on admission laboratory results. LY was an independent marker for total or subgroup HLH patients. For HLH patients, age, ferritin, LY, INR, TT, globulin, UA, and chloride were the most stable factors and the combination of them is the most predictive. For I-HLH patients, age, LY, APTT, TT, AST, TG, and chloride were the most stable factors and the combination of them is the most predictive. For M-HLH patients, ferritin, LY, and TB were the most stable factors and the combination of them is the most predictive. For Mix-HLH patients, ferritin, LY, APTT, UB, TG, and UA were the most stable factors and the combination of them is the most predictive.
Laboratory markers could be useful in monitoring the prognosis of HLH patients. It is reported that old age, low LY, elevated ferritin, high post-treatment ferritin, TB, triglycerides, and AST were positively related with adverse events such as early death, long prognosis in adult HLH patients, or malignancy-associated HLH [3, 8, 15,16,17,18,19]. Similar results were also concluded in our research. Furthermore, to pediatric HLH patients, INR > 1.5, APTT, TB > 19 μmol/L, and globulin < 20 g/L were risk factors for 7 days and within 30 days prognosis [20]. In this investigation, similar results were gained in adult HLH patients.
Serum bilirubin from the metabolism of heme may be effective in protecting the body from oxidant inflammation and cancers in colorectal cancer [21, 22]. Previous research has described that elevated IB was a risk factor in mortality in the acute phase of ischemic stroke patients [23]. UA, an end product of purine, plays a key role in antioxidants [24,25,26]. Chloride ion, a plentiful anion in the extracellular fluid, is an essential part of many functions to work with the body [27]. A high level of serum chloride (≥ 105.4 mmol/L) is an adverse prognosis marker for IgAN patients [28]. Our study also provides clinical data on the prognosis role of the above parameters in adult HLH patients. In addition, the thrombin time (TT), a common coagulation index, has rarely been studied in many diseases. In this research, we firstly found that high TT was an independent and risk factor for HLH patients in 30-day death.
In this research, we built four models based on admission laboratory results. Firstly, we divided the total patients into I-HLH, M-HLH, and Mix-HLH groups according to the underlying diseases. Then, univariate and multivariable logistics regression analyses were applied to select the independent factors to predict 30-day mortality in total and subgroups. We found that independent factors were different in different groups with different values. Therefore, the choice of which laboratory marker should be the primary outcome may depend on the underlying disease. For example, LY is an independent factor to total and subgroup HLH. Ferritin is an independent factor in total, M-HLH, and Mix-HLH. Age, TT, and chloride are independent factors to total and I-HLH.
Strengths of the investigation included that data were from a single center with large samples. Patients were evaluated as total and subgroups with biomarkers that are available and affordable in clinical practice. UA, TT, and chloride were firstly confirmed as independent factors in adult HLH.
As a retrospective research, selection bias is unavoidable. Internal and external validation is lacking. In addition, less information on treatment and genetic testing is included. We encourage these findings to be tested in other studies with multi-centers.
In conclusion, we built four models with biomarkers that are available and affordable in clinical practice. With these models, high-risk patients with different underlying disease could be easily identified.
Data availability
The data and materials can be found from the corresponding author.
References
Albeituni S, Verbist KC, Tedrick PE, Tillman H, Picarsic J, Bassett R, et al. Mechanisms of action of ruxolitinib in murine models of hemophagocytic lymphohistiocytosis. Blood. 2019;134(2):147–59.
Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125(19):2908–14.
Huang J, Yin G, Duan L, Tian T, Xu J, Wang J, et al. Prognostic value of blood-based inflammatory biomarkers in secondary hemophagocytic lymphohistiocytosis. J Clin Immunol. 2020;40(5):718–28.
Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86(1):58–65.
Mehta P, Cron RQ, Hartwell J, Manson JJ, Tattersall RS. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2(6):e358–67.
Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–16.
La Rosee P, Horne A, Hines M, von Bahr GT, Machowicz R, Berliner N, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–77.
Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG. Development and validation of the prognostic value of ferritin in adult patients with hemophagocytic lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):71.
Zhou Y, Kong F, Wang S, Yu M, Xu Y, Kang J, et al. Increased levels of serum interleukin-10 are associated with poor outcome in adult hemophagocytic lymphohistiocytosis patients. Orphanet J Rare Dis. 2021;16(1):347.
Li F, Yang Y, Jin F, Dehoedt C, Rao J, Zhou Y, et al. Clinical characteristics and prognostic factors of adult hemophagocytic syndrome patients: a retrospective study of increasing awareness of a disease from a single-center in China. Orphanet J Rare Dis. 2015;10:20.
Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin Proc. 2014;89(4):484–92.
Liu YZ, Bi LQ, Chang GL, Guo Y, Sun S. Clinical characteristics of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis. Cancer Manag Res. 2019;11:997–1002.
Li Y, Guo C, Huang C, Jing L, Huang Y, Zhou R, et al. Development and evaluation of the prognostic nomogram to predict refractive error in patients with primary angle-closure glaucoma who underwent cataract surgery combined with goniosynechialysis. Front Med (Lausanne). 2021;8:749903.
Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–31.
Zhao Y, Lu D, Ma S, Li L, Zhu J, Zhou, et al. Risk factors of early death in adult patients with secondary hemophagocytic lymphohistiocytosis a single-institution study of 171 Chinese patients. Hematology. 2019;24(1):606–12.
Meng M, Chen L, Zhang S, Dong X, Li W, Li R, et al. Risk factors for secondary hemophagocytic lymphohistiocytosis in severe coronavirus disease 2019 adult patients. BMC Infect Dis. 2021;21(1):398.
Li B, Guo J, Li T, Gu J, Zeng C, Xiao M, et al. Clinical characteristics of hemophagocytic lymphohistiocytosis associated with non-Hodgkin B-cell lymphoma: a multicenter retrospective study. Clin Lymphoma Myeloma Leuk. 2021;21(2):e198–205.
Zoref-Lorenz A, Murakami J, Hofstetter L, Iyer SP, Alotaibi AS, Mohamed SF, et al 2021 An improved index for diagnosis and mortality prediction in malignancy associated hemophagocytic lymphohistiocytosis. Blood
Bin Q, Gao JH, Luo JM. Prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: an analysis of 116 cases. Ann Hematol. 2016;95(9):1411–8.
Li X, Yan H, Zhang X, Huang J, Xiang ST, Yao Z, et al. Clinical profiles and risk factors of 7-day and 30-day mortality among 160 pediatric patients with hemophagocytic lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):229.
Jiraskova A, Novotny J, Novotny L, Vodicka P, Pardini B, Naccarati A, et al. Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer. Int J Cancer. 2012;131(7):1549–55.
Zucker SD, Horn PS, Sherman KE. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40(4):827–35.
SaghebAsl E, Taheraghdam A, Rahmani F, Javadrashid R, Golzari SEJ, Ghaemian N, et al. Determination of the predictive value of serum bilirubin in patients with ischemic stroke: a prospective descriptive analytical study. Adv Pharm Bull. 2018;8(4):715–9.
Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: a danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutionary considerations. Semin Nephrol. 2011;31(5):394–9.
Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–62.
Waugh WH. Inhibition of iron-catalyzed oxidations by attainable uric acid and ascorbic acid levels: therapeutic implications for Alzheimer’s disease and late cognitive impairment. Gerontology. 2008;54(4):238–43.
Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23(3):203–11.
Silverstein FS, Johnston MV. A model of methotrexate encephalopathy: neurotransmitter and pathologic abnormalities. J Child Neurol. 1986;1(4):351–7.
Funding
This study was supported by the National Natural Science Foundation of China (81302531), Natural Science Foundation of Jiangsu Province of China (BK20181492), the Talents Planning of Six Summit Fields of Jiangsu Province (2013-WSN-037), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX17_1287), Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX18_0435), the National Key Clinical Department of Laboratory Medicine of China in Nanjing, Key laboratory for Laboratory Medicine of Jiangsu Province (ZDXKB2016005), and by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
Hua-Guo Xu and Jun Zhou designed the study. All the authors contributed to the generation, collection, assembly, analysis, and/or interpretation of data. Jun Zhou wrote the manuscript; Hua-Guo Xu, Zhi-Qi Wu, and Tengfei Qiao revised the manuscript. All the authors have read the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study is in accordance with the Declaration of Helsinki and approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (Nanjing, China) (2019-SR-066).
Competing Interests
The authors declare no competing interests.
Consent for Publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, J., Wu, ZQ., Qiao, T. et al. Development of Laboratory Parameters-Based Formulas in Predicting Short Outcomes for Adult Hemophagocytic Lymphohistiocytosis Patients with Different Underlying Diseases. J Clin Immunol 42, 1000–1008 (2022). https://doi.org/10.1007/s10875-022-01263-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-022-01263-z