Abstract
Hemophagocytic lymphohistiocytosis (HLH) has a low incidence and high mortality. In order to improve our understanding of the clinical features and prognostic risk factors of adult HLH, we analyzed the clinical characteristics and prognostic risk factors of adult HLH and developed a prognostic model to predict the overall survival (OS) of adult HLH. The clinical characteristics and survival statistics of adult patients with HLH identified at The Second Affiliated Hospital of Chongqing Medical University between February 2012 and October 2020 were retrospectively analyzed to constitute the primary cohort, while patients between 25 October 2020 and 20 March 2023 were collected at the same institution as a validation cohort for the prospective study. A total of 142 patients met the inclusion criteria, with 72 and 70 in the primary cohort and validation cohort respectively. In the primary cohort, the median OS was 102 days, with 37.5%, 34.5%, and 28.7% 1-, 2-, and 3-year OS, respectively. Univariate analysis showed that age, interleukin-10 (IL-10), interleukin-2 receptor (IL-2R), prothrombin time (PT), and indirect bilirubin (IBiL) were correlated with prognosis. Multivariate analysis showed that IL-10 and PT were independent factors affecting OS in adult patients with HLH. A prognostic model consisting of IL-2R, PT, and IL-10 and a corresponding prognostic nomogram were developed adopting the principle of minimum value of Akaike information criterion(AIC). The model has a high prediction accuracy letter (C-index = 0.708). The AUC values of 1-year, 2-year, and 3-year were 0.826, 0.865, and 0.882, correspondingly. In the validation cohort, all patients were divided into high-risk and low-risk groups, and the risk of death was significantly higher in the high-risk group than in the low-risk group (p < 0.01). The calibration curve for the model shows that the Nomogram constructed in this study is very reliable to predict the OS of HLH patients. IL-10 and PT have significant prognostic value in adult HLH. The prognostic model and the nomogram built in this study can forecast the OS of adult HLH patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemophagocytic lymphohistiocytosis (HLH), also known as hemophagocytic syndrome (HPS),is characterized by the uncontrolled activation of cytotoxic T lymphocytes and natural killer cells induced by a variety of causes. Failure to manage the immune response will result in increased inflammatory cytokine release and macrophage activation. Hypercytokinemia and immune-mediated multiple organ system damages is a life-threatening hyperinflammatory syndrome rather than an independent disease. It is clinically distinguished by persistent fever, hepatosplenomegaly, coagulopathy, hypertriglyceridemia, hyperferritinemia, and hemophagocytosis [1] [2] [3]. HLH is arbitrarily classified into two types: familial hemophagocytic lymphohistiocytosis (FHL) and secondary hemophagocytic lymphohistiocytosis (sHLH). FHL is an autosomal recessive genetic illness that mostly affects youngsters. There is one case in every 30,000 to 50,000 newborns, and 70–80% of the cases occur in the first year after birth. At the same time, it occurs at any other age, induced by infection and diagnosed by genetic testing or positive family history [1] [4]. There are many reasons for sHLH, but HLH caused by infection is most common. In addition to common pathogens, tumours, and autoimmune diseases, sHLH can be caused by some rare pathogens, such as the influenza virus [5] [6]. Although the pathogenesis of sHLH is mainly unknown, the two forms of HLH, sHLH, and FHL, have similar clinical characteristics. Despite attempts to differentiate sHLH from FHL or reactive forms of FHL, the symptomatic presentations are highly overlapping [7]. HLH may affect anybody at any age, however most clinical recommendations, prospective research, and therapeutic trials are geared toward children [2] [8] [9].
Current studies have shown that HLH has the characteristics of rapid progress and high mortality, which seriously threatens the OS of HLH patients. It is required to create further methods that can swiftly estimate the prognosis of HLH patients in order to investigate significant clinical indicators to guide clinical treatment choices, make reasonable use of medical resources, optimize patients' interests, and aid improve patients’ clinical results. At present, many studies have found the related variables of poor prognostic factors of HLH, In a study by Xu Yang et al. [10], a nomogram model was constructed using PET/CT (positron emission tomography) as a variable along with clinical features and demonstrated that the model was more predictive of 6-month survival than either the clinical model or the Rad-score alone. In addition, the nomogram model showed better discrimination, calibration and clinical usefulness in both cohorts; however, in clinical work, many patients do not have PET/CT data prior to treatment due to the high price of PET/CT. Therefore, we conducted a retrospective study of adult HLH patients treated at our institution to analyze prognostic risk factors for OS and propose a nomogram model that can be calculated based on conventional clinical variables to help medical staff quickly and accurately evaluate the 1-year, 2-year, or even 3-year OS of patients with HLH.
Patients and methods
Data collection
The Second Affiliated Hospital of Chongqing Medical University’s electronic medical record database was searched for patients diagnosed with “hemophagocytic syndrome” and “hemophagocytic lymphohistiocytosis.” With a minimum follow-up time of 1 year, between 2012-2-25 and 2020-10-25, 72 individuals with a principal diagnosis of HLH were included to form the primary cohort, and from October 2020 to March 2023, 70 patients who met the inclusion criteria formed the validation cohort. The HLH-2004 criteria [1] were often utilized to aid in the diagnosis of HLH. Patients were eligible for HLH if they were (1) above the age of 18 and (2) met at least five of the eight criteria given by the Histiocyte Society in 2004. Exclusion criteria included (1) patients under the age of 18, (2) patients with a history of treatment prior to admission, (3) patients who did not meet the diagnostic criteria of HLH-2004, and (4) incomplete clinical information. All adult patients, including these terms, were manually examined according to the 2004 revised HLH diagnostic criteria. The clinical and laboratory data of all patients with HLH were reviewed retrospectively. Demographic data (age, sex), symptoms (hemophagocytosis, highest body temperature, jaundice, rash, altered mental status, oedema, lymph node enlargement, hepatomegaly, splenomegaly, ascites, pleural effusion, pneumonia), and laboratory indicators before treatment (Serum ferritin, interleukin-10, interleukin-2 receptor, prothrombin time and indirect bilirubin, high-density lipoprotein) were collected. Telephone calls and examining medical records are used as a means of follow-up to obtain the survival status of all HLH patients. The institutional and national research committees' ethical guidelines and the 1964 Helsinki Declaration and its later modifications or equivalent ethical principles were followed in this retrospective study. Overall survival (OS) is defined as the time elapsed between the day the patient was admitted to the hospital and death for any cause or the end of follow-up.
Statistical methods
SPSS software 23.0, GraphPadPrism8.0.1, and R3.6.1 are used for data analysis and sketching. We employed multiple imputations (MI) based on five replications and a chained equation approach technique in the R MI process to account for missing data. Classified variables are stated as frequency and percentage (n%), and continuous variables are expressed as mean ±standard deviation (SD), median (range), or median (interquartile range, IQR). The patients were separated into two groups based on the clinical follow-up results: survivors and non-survivors. We employed the Kruskal-Wallis rank-sum test, the chi-square test, and the Fisher exact test for inter-group comparison. At the same time, we analyzed the clinical indexes of adult HLH by univariate and multivariate analysis. The prognostic risk model of OS was established by multivariate Cox regression analysis. The risk factors obtained from univariate Cox regression analysis(P<0.05)were contained in the multivariate Cox proportional regression model as prognostic indicators. By combining all the prognostic indicators and adopting the principle of minimum value of Akaike information criterion(AIC), the prognostic model of the best combination was obtained. The HLH risk score formula will be calculated using the regression coefficient of the prognostic model: Riskscore(patient) = ∑ Coefficient(Riskfactor) ∗ Value(Riskfactor) [11]. All patients were divided into two groups based on their median risk score: high-risk and low-risk. When constructing the survival curve of risk factors for HLH, the Kaplan-Meier technique was utilized; analysis of the prognostic difference between groups was carried out using the Log-Rank methodology. The receiver operating characteristic (ROC) curve was used to evaluate the HLH model’s accuracy in predicting 1-year, 2-year, and 3-year overall survival. To determine if the anticipated probability and the actual probability are estimated by the model fit, the Hosmer-Lemeshow goodness-of-fit test was utilized. All of the significance tests were bilateral, and P≤0.05 was considered statistically significant.
By the way, in all of our patients, treatment followed the HLH-04 regimen, which included etoposide, dexamethasone, and cyclosporine (CSA) within 1–8 weeks after diagnosis, with some patients (with neuropsychiatric symptoms) receiving intrathecal injections of methotrexate and prednisone; patients with secondary HLH may be completely cured after 8 weeks of treatment, while some patients with secondary HLH and familial HLH that are not in remission are recommended to continue treatment with etoposide, dexamethasone, and CSA, and to receive hematopoietic stem cell transplantation (HSCT) when appropriate.
Results
Basic clinical characteristics of adult patients with HLH
Clinical characteristics of survival group and death group of adult HLH before treatment
A total of 142 patients met the inclusion criteria, 72 in the primary cohort and 70 in the validation cohort. Table 1 lists the clinical characteristics of patients in the primary cohort and the validation cohort. In the main cohort, 49 (68%) patients died, with a male to female ratio of 0.94, The median age of the first diagnosis of adult HLH was 58 years old, 62 years old in the death group and 46 years old in the survival group. Among the clinical symptoms collected, all patients had fever, the majority of them had an irregular high fever, with a peak temperature of 39.0 ~ 40.2°C, lasting from 1 week to several months. The remaining more common symptoms were pneumonia (dry and wet sounds in the lungs, chest X-ray or chest CT indicating pneumonia) (80.6%), hemophagocytosis (77.8%), enlarged lymph nodes (65.3%), pleural effusion (62.5%), and splenomegaly (58.3%).
In laboratory tests, 95.8% of cases showed ferritin levels beyond 500 g/L and 59.7% of cases had ferritin levels over 1500 g/L. The remaining frequent indicators were raised IL-10 (84.7%), decreased HDL (93.1%), IL-2R levels more than 2400 U/ml (87.5%), and Hemophagocytosis in bone marrow (77.8%).
Distribution of primary diseases in 72 adult patients with HLH
There was no history of HLH in any of the 72 adult patients. Figure 1A shows that there were two cases of primary HLH (2.8%) and 70 cases of secondary HLH (97.2%). As shown in Fig. 1B, the most common cause of secondary HLH (50%) is an infection, followed by malignancies (27.2%), autoimmune-related disorders (11.4%), and idiopathic HLH (11.4 %). Tumor-associated HLH was diagnosed in 19 instances (26.4%), among them, there were 18 cases of hematological diseases, mainly lymphoma, and only 1 case of solid tumor. Thirty-five patients (48.6%) were diagnosed with the infection-associated HLH, virus infection was predominant, accounting for 48.6%, and some infection-related patients were discharged or died without knowing the source of infection. The autoimmune disease-associated HLH was diagnosed in eight patients (11.1%), there were 4 cases of other connective tissue diseases, and the other 4 cases were polymyositis, rheumatoid arthritis, systemic lupus erythematosus and mixed connective tissue diseases. In eight patients, the idiopathic HLH was discovered (11.1%). There were two individuals (2.8%) with the primary HLH, one of whom had Familial HLH type 2 (PRF1 gene mutation-positive) and the other had Chediak-Higashisyndrome (LYST gene mutation-positive). Detailed etiologic data can be found in the supplementary table.
Etiological distribution and diagnostic index compliance of the primary cohort; (A) classification of primary and secondary HLH in 72 adult patients with HLH; (B) classification of patients with secondary HLH; (C) positive rate of diagnosed indicators in 72 patients with HLH; (D) the number of adult HLH patients between different groups
The positive rate of HLH-2004 diagnostic index in patients with HLH
Because the NK cell activity test was not performed at our hospital, the diagnosis of this group of patients satisfied 5 of the other seven HLH-2004 diagnostic criteria. Figure 1C shows the rate of coincidence for each diagnostic index. The positive rates of seven HLH-2004 diagnostic criterion indicators in 72 individuals with HLH were studied. It was found that the coincidence rate for fever reached 100%, and the remaining diagnostic indicators with high coincidence rate were serum ferritin, IL-2R, and hemophagocytosis, with 95.8%, 87.5%, and 77.8%, respectively.
Survival and prognosis
Survival of HLH under the different etiological classification
We obtained the prognosis of the patients through telephone follow-up and medical records. The median OS was 102 days, and the 1 -, 2-, and 3-year OS were 37.5%, 34.5%, and 28.7%, respectively. The median survival time in the infection group was 128 days, including 23 cases of death, 8 cases of EB virus infection, 2 cases of cytomegalovirus infection, 4 cases of bacterial infection, 1 case of lymph node tuberculosis, 3 cases of fungal infection, 1 case of mycoplasma pneumonia infection, and 4 cases of unexplained infection, whereas, in the rheumatism immune group (8 cases), the median survival time was 58 days, 5 cases died (8 cases). The tumour group’s median survival period (19 cases) was 69 days, and 14 patients died (As shown in Fig. 1D).
Comparison of laboratory results before treatment between survival group and death group
When SF, IL-10, IL-2R, HDL, PT, and IBIL were compared between the two groups, it was shown that there was no significant difference in SF and IBIL (Table 2). The median IL-10 in the dead group [mean±SD 300.3 ±287.0 pg/ml] was greater than that in the survival group [mean±SD 60.3 ±60.7pg/ml], P<0.001, and the median IL-2R in the death group [mean±SD 6244.3 ±2153.7U/L] was higher than that in the survival group [mean±SD 4050.6 ±2229.8U/L], P<0.001. The median PT of the dead group [mean(IQR) 15.5 (14.05–17.95)s] was higher than that of the survival group [mean(IQR) 14.3 (13.5–15.1)s], but the median HDL of the survival group [mean±SD 0.6 ±0.2s] was higher than that of the dead group [mean±SD 0.5 ±0.5mmol/L], P=0.012 (Fig. 2A–D).
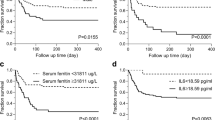
Survival analysis of the primary cohort. (A–D) Comparison of laboratory findings—HDL, IL-10, IL-2R, and PT among Survivor group and Non-survivor group; (E–H) Kaplan-Meier survival curve of HLH patients with different risk factors; (I) univariate Cox regression analyses of OS with HLH; (J) multivariate Cox regression analyses of OS with HLH. [HDL: high-density lipoprotein; IBiL: indirect bilirubin; IL-10(IL10):interleukin-10; IL-2R(IL2R):interleukin-2 receptor; PT: prothrombin time; SF: serum ferritin]
Kaplan-Meier curve showed that the survival rate of HLH patients with PT>15s, IL-2R> 5543.5306 U/ml, IL-10>223.7pg/ml, HDL≤0.5mmol/L before treatment was substantially lower than that of patients with PT≤15s, IL-2R≤5543.5306 U/ml, IL-10≤223.7pg/ml, HDL>0.5mmol/L (Fig. 2E–H).
Univariate and multivariate analysis of prognostic factors for HLH
A univariate Cox analysis results revealed that an increase in age, IL10, IL-2R, PT, and IBiL levels was associated with a drop in survival rate. Age, IL-10, IL-2R, PT, and IBiL were investigated further as model candidate indicators. Multivariate analysis showed that IL-10(hazard ratio = 1.002; 95% CI: 1.000–1.003; P = 0.014), PT(hazard ratio = 1.262; 95% CI: 1.086–1.465; P = 0.002) were independent prognostic index for patients (Fig. 2I–J).
Development and Assessment of the performance of a nomogram for OS prediction in HLH
Construction of a prognostic model for adult HLH
The principle of minimum value of Akaike information criterion(AIC) is adapted to obtain the best combination of prognostic models. When the AIC value was minimum (AIC=338.67), the model was composed of three indexes: IL-2R, IL10, and PT. However, our multivariate regression analysis showed that IL-10 and PT were independent prognostic indicators, while IL-2R was not. However, in the process of literature collection, we found that both Thomas Wimmer [12] and Shuyan Yao’s [13] retrospective studies suggested that IL-2R was an independent prognostic indicator. The results of multivariate analysis were (HR= 2.22; 95% CI: 1.28–3.87; P = 0.005) and (HR= 2.362; 95% CI: 1.556–3.587; P = < 0.001), a prospective study in Dominik Bursa [14] also showed IL-2R as an independent prognostic indicator, with multivariate results (HR= 1.001; 95% CI: 1.000–1.001; P = 0.008). And in the analysis of the survival group versus the survivor group in the validation cohort, we found that IL-2R, IL10 and PT were clinically significant between the two groups (p<0.05) (Fig. 4F–H). Therefore, we could not rule out whether the difference between the model with the lowest AIC and the results of multivariate analysis was due to the small sample size. So, we use the prognosis model composed of IL-2R, IL10, and PT for risk assessment. The prognostic risk score formula for HLH was calculated using the prognostic model’s regression coefficient (Table 3): Riskscore = 0.00015 ∗ IL2R + 0.00189 ∗ IL10 + 0.25572 ∗ PT. The risk score of each HLH patient was computed according to the risk score formula.
Survival analysis curve of model high-risk group and low-risk group
With the median risk score value of 1.025 as the cutoff value, all patients were divided into a high-risk group (risk score >1.025) and a low-risk group (risk score ≤ 1.025). As shown in Fig. 3A, log-rank findings showed that patients with high-risk scores had a poorer prognosis than patients with low-risk ratings (P<0.001).
Nomogram validation in the primary cohort. (A) The survival curve of HLH patients with high risk vs low risk; (B) nomogram for predicting 1-year, 2-year, and 3-year OS; Performance of the HLH model. (C–E) The area underneath the ROC curve’s receiver operating curve (AUC) value corresponds to 1 year, 2 years, and 3 years of the HLH model. (F–H) Calibration curves for 1-, 2-and 3-year OS, which are indicative of predictive accuracy
Construction of nomogram corresponding to adult HLH prognostic mode
According to the prognosis model constructed by IL-2R, IL-10, and PT, nomograms for OS prediction in HLH were built to quickly predict the 1-year, 2-year, and 3-year OS of HLH patients. The total score was obtained by adding each item score of IL-2R, IL-10, and PT of HLH patients. The corresponding probability of the total score on the scale was 1-year, 2-year, and 3-year OS (Fig. 3B).
Performance evaluation and validation of prognostic model
A total of 70 patients who met the criteria were included in the validation cohort and the clinical data collected were substituted into our prognostic risk score formula, showing that the mortality rate was significantly higher in the high-risk group than in the low-risk group, P<0.01 (Fig. 4E).
Nomogram validation in the validation cohort. (A–B) The area underneath the ROC curve’s receiver operating curve (AUC) value corresponds to 1 year and 2 years of the HLH model; (C–D) calibration curves for 1- and 2-year OS, which are indicative of predictive accuracy; (E) the survival curve of HLH patients with high risk vs low risk; (F–H) Kaplan-Meier survival curve of HLH patients with different risk factors
The area-under-the-curve (AUC) value of the time-dependent receiver operating characteristic (ROC) curves was applied to test the predictive ability of the HLH prognostic model. The area under the curve > 0.75 was considered to have sufficient discrimination. A 3-year OS study was not conducted due to the short time span of the validation cohort collection. As shown in Fig. 3C–E, the AUC for the primary cohorts of 1-year, 2-year, and 3-year were 0.826 (95% confidence intervals [CI]: 0.730–0.923; P < 0.001), 0.865 (95% CI: 0.772–0.958; P < 0.001), and 0.882 (95% CI: 0.794–0.969; P < 0.001), respectively, and the AUC for the validation cohort was 0.755 at 1-year and 0.764 at 2-years (Fig. 4A–B).
Hosmer-Lemeshow test shows that the P values of Nomogram predicted the 1-year, 2-year, and 3-year survival probability are 0.404, 0.989, and 0.964, respectively, and P values of 0.057 and 0.266 for the validation cohort's 1- and 2-year survival probability, respectively, which are all greater than 0. 05, indicating that the difference between the model's predicted and actual observed values is not statistically significant, and the prediction model has good calibration ability. The HLH model’s calibration curves for the potential of OS after one, two, and three years show the difference between anticipated and actual results (Fig. 3F–H and Fig. 4C–D). The results show that the predicted results are very close to the actual results, which means that the Nomogram constructed in this study is very reliable to predict the 1-year, 2-year, and 3-year survival probability of HLH patients.
It is worth noting that the C index of our prognostic model was C-index=0.708 (95CI:0.6296–0.7864), indicating that our model has good predictive value. Based on our AUC, calibration curve results, C-index of the model, and evaluation of the validation cohort, the HLH prognostic model and the Nomogram have an excellent ability to predict the OS of adult HLH patients.
Discussion
HLH is a rare but potentially fatal immune disorder syndrome. The disease is characterized by uncontrolled activation and proliferation of macrophages and lymphocytes, which eventually leads to a severe cytokine storm, followed by tissue infiltration and secondary multiple organ system failures.
In a Chinese retrospective analysis of people aged two months to 79 years old, the most prevalent mutation was UNC13D (29 per cent), followed by Lyst (21 %), PRF1 (17 %), and STXBP2 (10 %) [15]. Primary HLH accounted for just two instances (2.8%) of the 72 patients in our research. One instance tested positive for a PRF1 mutation, while the other tested positive for a Lyst gene mutation. The key reason for the discrepancy was that the individuals were adults. According to South Korean research, EBV viral infection is the leading cause of secondary HLH [16], The most prevalent kind of secondary HLH in Japanese research of 567 individuals with HLH was EB virus (EBV) associated HLH, followed by other infections or lymphoma related HLH [8], these results are consistent with the findings of our study.
Previous studies have shown that HLH has the characteristics of low morbidity, but serious illness and high mortality. Shabbir et al. diagnosed 18 adult HLH from 2004 to 2009, with a mortality rate of 72%. After diagnosis, the median survival time was 35 days [17]. Parikh et al. reviewed 15 years of treatment at the Mayo Clinic (1996–2011) and pointed out that 62 of the 250 adults suspected of HLH encountered the 2004 HLH diagnostic criteria were contained in the final analysis. After a median of 42 months of follow-up, 41 patients (66%) died, with the whole group having a median overall survival of 2.1 months [18]. According to a study on adult HLH management by Otrock, 73 patients encountered the diagnostic criterion of HLH-2004, with a median OS of 7.67 months and a 1-year OS of 48% [19]. In fatal HLH, death usually appears in the first 4-8 weeks from multiple organ failure, bleeding, or sepsis [20]. Sébastian Rivière et al. carried out 6-year multicenter research at three French tertiary health centers. The medical records of 312 individuals suspected of having HLH were reviewed retrospectively. The one-month death rate (within one month after diagnosis) was 20% [21]. The median survival period of adult HLH was 102 days in our 9-year retrospective analysis, the 1-year adult HLH overall survival rate was 37.5%, the 2-year overall survival rate was 34.5%, and the 3-year overall survival rate was 28.7%.
In HLH patients, abnormal activation of T cells, natural killer (NK) cells, and macrophages in patients lead to excessive secretion of cytokines such as interferon-γ (IFN--γ), tumour necrosis factor-α (TNF- α), interleukin-6 (IL-6), and interleukin-10 (IL-10), which can lead to hypercytokinemia to cause systemic organ function damage and even death [3]. Since there are so many inflammatory factors involved in HLH, are there any factors that affect the survival and prognosis of patients with HLH? It was found that the serum concentration of IL-10 in HLH increased significantly [22] [23]. Shi-LongYang et al. divided the patients with HLH into two groups according to the level of cytokines and found that the high IL-10 group had lower hemoglobin and platelet count, suggesting that IL-10 is a cytokine related to cytopenia [24]. Studies have found that in both children and adults with HLH, the increased level of newly diagnosed IL-10 can be used as a tool for early diagnosis of HLH and an independent risk factor for overall poor survival of HLH patients [25,26,27,28]. Malignancy-associated HLH (M-HLH) has a poor prognosis with a mortality rate >80%, while elevated IL-10 was found to be strongly associated with M-HLH in the study by Xu Yang et al. [29]. (p=0.007), so it can be further inferred that elevated IL-10 is associated with poor prognosis in patients with HLH. Our study also found that IL-10 is a poor prognostic factor for the long-term overall survival of adult patients with HLH.
IL-10 is mainly produced by monocytes, macrophages, helper T cells, and dendritic cells, while cytotoxic T cells, B cells, NK cells, mast cells, and granulocytes have also been found to produce IL-10 [30]. Studies have indicated that IL-10 has multiple immunosuppressive effects. IL-10 inhibits the release of pro-inflammatory mediators such as TNF-α, Interleukin-1β(IL-1 β), IL-6, Interleukin-8(IL-8), granulocyte colony-stimulating factor(G-CSF), and granulocyte-macrophage colony-stimulating factor(GM-CSF)by acting on antigen-presenting cells(APC), like monocytes and macrophages. Meantime, it can down-regulate the expression of II major histocompatibility complex (MHC) antigens on monocytes to inhibit antigen presentation [31] [32] [33] [34]. In addition, studies have indicated that IL-10 can directly inhibit Th2 cells and memory helper T(Th)17 cells. Moreover, IL-10 also stimulates regulatory T (Treg) cells and other immune cells [35] [36] [37] [38]. However, the function of IL-10 in HLH is still unclear.
PT is the primary biomarker to evaluate the blood coagulation state of a person. The prolongation of serum PT may lead to abnormal blood coagulation. In the study, Gao and his colleagues found a significant difference in PT between the HLH death group and the survival group [39]. At the same time, it is reported that the prolongation of PT> 3 seconds is an independent poor prognostic factor in patients with HLH [40]. Our study also found that prolonged PT was connected to poor prognosis in patients with HLH. Cox regression analysis showed that PT could be used as a risk index for predicting OS in patients with HLH. Meanwhile, the construction of a risk prognosis model combined with IL-10 and IL-2R could significantly improve the predictive efficiency.
In the study of 116 patients with sHLH, Qiong Bin et al. discovered that neutrophils <0.5 × 109/ L, total bilirubin more than twice the standard upper limit and albumin ≤ 20g/L were independent risk factors for early death caused by HLH [41]. Sameer A Parikh et al. also found that low serum albumin level is a significant predictor of poor survival in patients with HLH and found that the survival rate in patients with tumour-related HLH was significantly worse [18]. Jinzhou et al. have demonstrated that high levels of serum ferritin (SF>1050 μ g / L) after treatment are associated with a higher risk of death and a poor overall survival rate [42]. MengxinLu et al. also found that male mental state changes, high levels of serum ferritin and IL-6 were four independent predictors of poor prognosis in HLH [43]. A study of 48 children with HLH found that hemoglobin and triglycerides were significantly associated with 180-day mortality [44].
Jinzhou et colleagues gathered 136 adult patients with HLH in 2020 and used multivariate binary logistic regression to build a 1-year survival model consisting of platelets, ferritin, and Alanine aminotransferase to predict the prognosis of HLH patients better. The model's AUC under the ROC curve is 0.842 [45]. MengxinLu et colleagues gathered 107 adult HLH patients in 2021 and used multivariate analysis to create a clinical predicting model comprised of gender, mental state changes, ferritin, and IL-6, and 48 adult HLH patients were collected to validate the predictive model, which had an AUC value of 0.8628 [43]. In this work, we also created a novel clinical prediction model that can predict the 1-year survival rate and the 2-year and 3-year survival rates, with a comparatively high AUC value. Simultaneously, the model is made up of three readily accessible indicators: IL-10, PT, and IL-2R. In comparison to the previous two models, our model can concurrently forecast and predict the 1-year, 2-year, and 3-year OS of HLH patients, and the probability we want can be achieved very easily and logically through the line chart. This model's predictive Nomogram offers the benefits of simplicity, intuition, great practicability, and ease of clinical application, among other things. Furthermore, our study reveals that the adult HLH model's prediction findings are accurate and reliable. Our study does, of course, have certain flaws. First and foremost, given the low prevalence of HLH, we do not have enough HLH cases to evaluate the model fruit externally. Second, our analysis excluded platelets, hemoglobin, white blood cells, and other cytokines, which may be possible prognostic markers of HLH; In addition, among all our follow-up patients, the loss of follow-up rate reached 20%. According to our understanding, some patients were transferred to hospitals for treatment because they were not satisfied with the treatment plan. All the above reasons may affect the accuracy of our model.
To sum up, this retrospective study found that PT and IL-10 before treatment can be used as independent biomarkers of poor prognosis in adult patients with HLH. At the same time, our prognostic model composed of age, PT, and IL-10 can predict 1-year, 2-year, and 3-year OS with reasonable accuracy.
Data availability
The data and materials can be found from the corresponding author.
References
Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S et al (2007) HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48:124–131. https://doi.org/10.1002/pbc.21039
Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X (2014) Adult haemophagocytic syndrome. Lancet 383:1503–1516. https://doi.org/10.1016/S0140-6736(13)61048-X
Al-Samkari H, Berliner N (2018) Hemophagocytic Lymphohistiocytosis. Annu Rev Pathol 13:27–49. https://doi.org/10.1146/annurev-pathol-020117-043625
Maakaroun NR, Moanna A, Jacob JT, Albrecht H (2010) Viral infections associated with haemophagocytic syndrome. Rev Med Virol 20:93–105. https://doi.org/10.1002/rmv.638
Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C (2007) Infections associated with haemophagocytic syndrome. Lancet Infect Dis 7:814–822. https://doi.org/10.1016/S1473-3099(07)70290-6
Lai W, Wang Y, Wang J, Wu L, Jin Z, Wang Z (2018) Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults and adolescents-a life-threatening disease: analysis of 133 cases from a single center. Hematology 23:810–816. https://doi.org/10.1080/10245332.2018.1491093
Filipovich AH (2009, 2009) Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology Am Soc Hematol Educ Program:127–131. https://doi.org/10.1182/asheducation-2009.1.127
Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I et al (2007) Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol 86:58–65. https://doi.org/10.1532/IJH97.07012
Daver N, McClain K, Allen CE, Parikh SA, Otrock Z, Rojas-Hernandez C et al (2017) A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer 123:3229–3240. https://doi.org/10.1002/cncr.30826
Yang X, Liu J, Lu X, Kan Y, Wang W, Zhang S, Liu L, Zhang H, Li J, Yang J (2021) Development and Validation of a Nomogram Based on 18F-FDG PET/CT Radiomics to Predict the Overall Survival in Adult Hemophagocytic Lymphohistiocytosis. Front Med (Lausanne) 8:792677. https://doi.org/10.3389/fmed.2021.792677
Simon N, Friedman J, Hastie T, Tibshirani R (2011) Regularization Paths for Cox's Proportional Hazards Model via Coordinate Descent. J Stat Softw 39:1–13. https://doi.org/10.18637/jss.v039.i05
Wimmer T, Mattes R, Stemmler HJ, Hauck F, Schulze-Koops H, Stecher SS, Starck M, Wendtner CM, Bojko P, Hentrich M, Nickel KE, Götze KS, Bassermann F, von Bergwelt-Baildon M, Spiekermann K (2023) sCD25 as an independent adverse prognostic factor in adult patients with HLH: results of a multicenter retrospective study. Blood Adv 7(5):832–844. https://doi.org/10.1182/bloodadvances.2022007953
Yao S, He L, Zhang R, Liu M, Hua Z, Zou H, Wang Z, Wang Y (2023) Improved hemophagocytic lymphohistiocytosis index predicts prognosis of adult Epstein-Barr virus-associated HLH patients. Ann Med 55(1):89–100. https://doi.org/10.1080/07853890.2022.2149850
Bursa D, Bednarska A, Pihowicz A, Paciorek M, Horban A (2021) Analysis of the occurrence of hemophagocytic lymphohistiocytosis (HLH) features in patients with sepsis: a prospective study. Sci Rep 11(1):10529. https://doi.org/10.1038/s41598-021-90046-4
Zhang J, Sun Y, Shi X, Zhang R, Wang Y, Xiao J et al (2020) Genotype characteristics and immunological indicator evaluation of 311 hemophagocytic lymphohistiocytosis cases in China. Orphanet J Rare Dis 15:112. https://doi.org/10.1186/s13023-020-01390-z
Koh KN, Im HJ, Chung NG, Cho B, Kang HJ, Shin HY et al (2015) Clinical features, genetics, and outcome of pediatric patients with hemophagocytic lymphohistiocytosis in Korea: report of a nationwide survey from Korea Histiocytosis Working Party. Eur J Haematol 94:51–59. https://doi.org/10.1111/ejh.12399
Shabbir M, Lucas J, Lazarchick J, Shirai K (2011) Secondary hemophagocytic syndrome in adults: a case series of 18 patients in a single institution and a review of literature. Hematol Oncol 29:100–106. https://doi.org/10.1002/hon.960
Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP 2014, Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin Proc 89:484–492. https://doi.org/10.1016/j.mayocp.2013.12.012
Otrock ZK, Eby CS (2015) Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol 90:220–224. https://doi.org/10.1002/ajh.23911
Créput C, Galicier L, Buyse S, Azoulay E (2008) Understanding organ dysfunction in hemophagocytic lymphohistiocytosis. Intensive Care Med 34:1177–1187. https://doi.org/10.1007/s00134-008-1111-y
Rivière S, Galicier L, Coppo P, Marzac C, Aumont C, Lambotte O et al (2014) Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med 127:1118–1125. https://doi.org/10.1016/j.amjmed.2014.04.034
Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y et al (1997) Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood 89:4100–4103
An Q, Hu SY, Xuan CM, Jin MW, Ji Q, Wang Y (2017) Interferon gamma and interleukin 10 polymorphisms in Chinese children with hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 64. https://doi.org/10.1002/pbc.26505
Yang SL, Xu XJ, Tang YM, Song H, Xu WQ, Zhao FY et al (2016) Associations between inflammatory cytokines and organ damage in pediatric patients with hemophagocytic lymphohistiocytosis. Cytokine 85:14–17. https://doi.org/10.1016/j.cyto.2016.05.022
Xu XJ, Tang YM, Song H, Yang SL, Xu WQ, Zhao N et al (2012) Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr 160:984–990.e1. https://doi.org/10.1016/j.jpeds.2011.11.046
Luo ZB, Chen YY, Xu XJ, Zhao N, Tang YM (2017) Prognostic factors of early death in children with hemophagocytic lymphohistiocytosis. Cytokine 97:80–85. https://doi.org/10.1016/j.cyto.2017.03.013
Li Z, Liu J, Man Y, Liu F, Gao L, Hu P et al (2021) Analysis of cytokine risk factors in the early death of patients with secondary phagocytic lymphocytic histiocytosis. Am J Transl Res 13:2388–2398
Zhou Y, Kong F, Wang S, Yu M, Xu Y, Kang J et al (2021) Increased levels of serum interleukin-10 are associated with poor outcome in adult hemophagocytic lymphohistiocytosis patients. Orphanet J Rare Dis 16:347. https://doi.org/10.1186/s13023-021-01973-4
Yang X, Lu X, Liu J, Kan Y, Wang W, Zhang S, Liu L, Li J, Yang J (2022) Development and Validation of 18F-FDG PET/CT-Based Multivariable Clinical Prediction Models for the Identification of Malignancy-Associated Hemophagocytic Lymphohistiocytosis. Korean J Radiol 23(4):466–478. https://doi.org/10.3348/kjr.2021.0733
Iyer SS, Cheng G (2012) Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 32:23–63. https://doi.org/10.1615/critrevimmunol.v32.i1.30
Fiorentino DF, Bond MW, Mosmann TR (1989) Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 170:2081–2095. https://doi.org/10.1084/jem.170.6.2081
de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174:1209–1220. https://doi.org/10.1084/jem.174.5.1209
de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C et al (1991) Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 174:915–924. https://doi.org/10.1084/jem.174.4.915
Neumann C, Scheffold A, Rutz S (2019) Functions and regulation of T cell-derived interleukin-10. Semin Immunol 44:101344. https://doi.org/10.1016/j.smim.2019.101344
Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM et al (2011) Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34:566–578. https://doi.org/10.1016/j.immuni.2011.03.018
Huber S, Gagliani N, Esplugues E, O'Connor W Jr, Huber FJ et al (2011) Th17 cells express interleukin-10 receptor and are controlled by Foxp3- and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34:554–565. https://doi.org/10.1016/j.immuni.2011.01.020
Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O'Connor W Jr et al (2011s) Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med 208:1027–1040. https://doi.org/10.1084/jem.20102149
Coomes SM, Kannan Y, Pelly VS, Entwistle LJ, Guidi R, Perez-Lloret J et al (2017) CD4(+) Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol 10:150–161. https://doi.org/10.1038/mi.2016.47
Gao WB, Shi MJ, Zhang HY, Wu CB, Zhu JH (2021) Relationship between marked hyperferritinemia and hemophagocytic lymphohistiocytosis. Beijing Da Xue Xue Bao 53:921–927
Zhou M, Li L, Zhang Q, Ma S, Sun J, Zhu L et al (2018) Clinical features and outcomes in secondary adult hemophagocytic lymphohistiocytosis. QJM 111:23–31. https://doi.org/10.1093/qjmed/hcx183
Bin Q, Gao JH, Luo JM (2016) Prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: an analysis of 116 cases. Ann Hematol 95:1411–1418. https://doi.org/10.1007/s00277-016-2727-6
Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG (2020) Development and validation of the prognostic value of ferritin in adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis 15:71. https://doi.org/10.1186/s13023-020-1336-6
Lu M, Xie Y, Guan X, Wang M, Zhu L, Zhang S et al (2021) Clinical analysis and a novel risk predictive nomogram for 155 adult patients with hemophagocytic lymphohistiocytosis. Ann Hematol 100:2181–2193. https://doi.org/10.1007/s00277-021-04551-7
Yu TY, Lu MY, Lin KH, Chang HH, Chou SW, Lin DT et al (2021) Outcomes and prognostic factors associated with 180-day mortality in Taiwanese pediatric patients with Hemophagocytic Lymphohistiocytosis. J Formos Med Assoc 120:1061–1068. https://doi.org/10.1016/j.jfma.2020.10.026
Zhou J, Zhou J, Wu ZQ, Goyal H, Xu HG (2020) A novel prognostic model for adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis 15:215. https://doi.org/10.1186/s13023-020-01496-4
Acknowledgements
The authors are very grateful to the patients and their families for their cooperation during our follow-up.
Funding
This study was sponsored by the Natural Science Foundation of Chongqing, China (grant no. 2021jcyj-msxmX0064).
Author information
Authors and Affiliations
Contributions
Qunxiang Qiu and Ying Chen designed the study. All the authors contributed to the generation, collection, assembly, analysis and/or interpretation of data. Qunxiang Qiu wrote the manuscript, Jianchuan Deng, Ying Chen and Dan Li revised the manuscript. All the authors have read manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The institutional and national research committees’ ethical guidelines and the 1964 Helsinki Declaration and its later modifications or equivalent ethical principles were followed in this retrospective study. The study is approved by the Ethics Committee of The Second Affiliated Hospital of Chongqing Medical University (Chongqing, China).
Consent for publication
All authors accept the terms and conditions of the editorial for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consistent contribution of Qunxiang Qiu, Dan Li, and Ying Chen
Supplementary information
ESM 1
(XLSX 14 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, Q., Li, D., Chen, Y. et al. Clinical features and prognostic risk prediction of adult hemophagocytic lymphohistiocytosis: a 9-year retrospective study. Ann Hematol 102, 2671–2682 (2023). https://doi.org/10.1007/s00277-023-05368-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05368-2