Abstract
Beijing is one of the largest and most densely populated cities in China. PM2.5 (fine particulates with aerodynamic diameters less than 2.5 μm) pollution has been a serious problem in Beijing in recent years. To study the temporal and spatial variations in the chemical components of PM2.5 and to discuss the formation mechanisms of secondary particles, SO2, NO2, PM2.5, and chemical components of PM2.5 were measured at four sites in Beijing, Dingling (DL), Chegongzhuang (CG), Fangshan (FS), and Yufa (YF), over four seasons from 2012 to 2013. Fifteen chemical components, including organic carbon (OC), elemental carbon (EC), K+, NH4 +, NO3 −, SO4 2−, Cl−, Al, Ca, Fe, Mg, Na, Pb, Si, and Zn, were selected for analysis. Overall, OC, SO4 2−, NO3 −, and NH4 + were dominant among 15 components, the annual average concentrations of which were 22.62 ± 21.86, 19.39 ± 21.06, 18.89 ± 19.82, and 13.20 ± 12.80 μg·m−3, respectively. Compared with previous studies, the concentrations of NH4 + were significantly higher in this study. In winter, the average concentrations of OC and EC were, respectively, 3 and 2.5 times higher than in summer, a result of coal combustion during winter. The average OC/EC ratios over the four sites were 4.9, 7.0, 8.1, and 8.4 in spring, summer, autumn, and winter, respectively. The annual average [NO3 −]/[SO4 2−] ratios in DL, CG, FS, and YF were 1.01, 1.25, 1.08, and 1.12, respectively, which were significantly higher than previous studies in Beijing, indicating that the contribution ratio of mobile source increased in recent years in Beijing. Analysis of correlations between temperature and relative humidity and between SOR ([SO4 2−]/([SO4 2−] + [SO2])) and NOR ([NO3 −]/([NO3 −] + [NO2])) indicated that gas-phase oxidation reactions were the major formation mechanism of SO4 2− in spring and summer in urban Beijing, whereas slow gas-phase oxidation reactions and heterogeneous reactions both occurred in autumn and winter. NO3 − was mainly formed through year-round heterogeneous reactions in urban Beijing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of the social economy and continuously increasing energy consumption, air pollution has become a serious problem in China in recent years (Chan and Yao 2008; Lin et al. 2008; Rose et al. 2010; Yang et al. 2009; Zhang et al. 2012). Beijing has a population of 16 million within an area of 16,800 km2, making it one of the largest and most densely populated cities in China. Beijing is located at the northwestern border of the North China Plain and is bound by mountains on the north, east, and west. Many heavily populated industrialized cities are located near Beijing to the southwest and southeast (Xu et al. 2011). Unfavorable geographical conditions and rapid growth in traffic emissions and regional pollutant emissions have made Beijing one of the most seriously polluted cities in China. The latest research (Yang et al. 2015) showed that about 28 % ~ 36 % of PM2.5 in Beijing was transported from the exterior region. Among the local emissions, the main sources of PM2.5 were vehicle (31.1 %), coal consumption (22.4 %), industrial production (18.1 %), dust (14.3 %) and others (14.1 %), respectively.
Particulate matter (PM), especially PM2.5 (fine particles with aerodynamic diameters less than 2.5 μm), plays important roles in atmospheric visibility reduction, acid deposition, and climate change (Yan et al. 2008; Garland et al. 2009; Ma et al. 2011). PM2.5 also has significant adverse health effects. Exposure to high concentrations of PM2.5 has been found to result in increased hospitalizations and higher mortality rates (Michaels and Kleinman 2000; Dockery 2001; Schwartz et al. 2002; Zhang et al. 2010; Zheng et al. 2015). PM2.5 is a complex mixture of elemental carbon (EC), organic carbon (OC), nitrate (NO3 −), sulfate (SO4 2−), ammonium (NH4 +), mineral dust, sea salt, and heavy metals (Hueglin et al. 2005). OC and EC affect the extinction coefficient for light scattering and absorption, which can contribute up to 30 % of the total extinction (Chan et al. 1999). The strong absorption capacity of EC has a significant impact on aerosol radiative forcing, which can lead to global warming (Menon et al. 2002; Ramanathan et al. 2001; Bond et al. 2013). Water-soluble ions such as SO4 2−, NO3 −, and NH4 + are hydrophilic and can promote the formation of cloud condensation nuclei, which significantly influence visibility and climate (Yao et al. 2002; Wang et al. 2005).
Since 2000, the number of studies of PM2.5 has gradually increased in various large city agglomerations in China such as the Pearl River Delta (Cao et al. 2004; Lai et al. 2007; Hagler et al. 2006; Duan et al. 2007; Hu et al. 2008; Liu et al. 2008), the Yangtze River Delta (Ho et al. 2011; Huang et al. 2012; Yang et al. 2005; Ding et al. 2013; Wang et al. 2008; Li et al. 2011a, b), and the Beijing–Tianjin–Hebei region (Wang et al. 2015a; Wang et al. 2015b). In Beijing, several studies have examined the general characteristics of PM2.5 chemical components and discussed their seasonal variations (He et al. 2001; Sun et al. 2004; Song et al. 2006). Also in Beijing, multiple studies have focused on correlations among PM2.5 components and the formation of secondary particles (Yao et al. 2002; Dan et al. 2004; Huang et al. 2006; Wang et al. 2009; Pathak et al. 2009; Ianniello et al. 2011). There have been several studies aimed at revealing the health effects of PM2.5 (Zhang et al. 2000; Guo et al. 2009; Kipen et al. 2010; Wu et al. 2010). Additionally, the aerosol number concentrations (Wu et al. 2007, 2008; Yue et al. 2009, 2011; Shen et al. 2011; Zhang et al. 2011; Gao et al. 2012), size distributions of aerosol chemical species (Yao et al. 2003; Cheng et al. 2009; Guo et al. 2010; Li et al. 2012), and aerosol optical characteristics and mixing states (Cheng et al. 2009; Deng et al. 2011a, b; Ma et al. 2012; Chen et al. 2012) have been discussed.
In this study, SO2, NO2, PM2.5, and chemical components of PM2.5 were measured at four sites in Beijing over four seasons. The main objectives of this paper were to (1) characterize the concentration levels and spatiotemporal variations in chemical components of PM2.5 in Beijing; (2) investigate the state of OC secondary pollution in Beijing; and (3) discuss the formation mechanisms of SO4 2− and NO3 − in urban Beijing.
2 Sampling and analysis
2.1 Sampling sites and occasions
Four sites, Dingling (DL), Chegongzhuang (CG), Fangshan (FS), and Yufa (YF), in different areas of Beijing were selected for this study, as shown in Fig. 1. DL is a background site in Beijing, which is located in the northern region of Beijing. CG is an urban environmental monitoring site in Beijing, which is located between 2nd ring and 3rd ring in Beijing. FS is a suburban environmental monitoring site in Beijing, which is located in the northeast of Fangshan district. YF is a regional transport pollution monitoring site in Beijing, which is located near the southern boundary in Beijing. Thus, the four sites can be a good representative of Beijing. Mass concentrations of gaseous pollutants and PM2.5 were measured by automatic monitoring instruments in the field (PM2.5 concentration data was only available in 2013). Chemical components of PM2.5 were manually sampled and analyzed in the laboratory. PNS 16 T-3.1/PNS 16 T-6.1 (Derenda, Germany) four-channel atmospheric particulate matter samplers with a flow of 16.67 L/min and Teflon membranes (Whatman, UK) were used to sample chemical components of PM2.5. Meteorological elements were also monitored at the CG site. The sampling period for the DL, CG, and FS sites was from August 2012 to July 2013. The sampling period for the YF site was from November 2012 to July 2013. A period of 5–7 continuous days was selected each month for sampling, and within that period, each site was monitored for 24 h.
2.2 Monitoring instruments and analysis methods
SO2 was monitored using a Thermo Fisher 43i pulsed UV fluorescence analyzer with a minimum detection limit of 0.5 × 10−9 (volume fraction) and accuracy of 1.0 × 10−9 (volume fraction). NO x was monitored using a Thermo Fisher 42C chemiluminescence NO–NO2–NO x analyzer with a minimum detection limit of 0.05 × 10−9 (volume fraction). PM2.5 was monitored with a Thermo Fisher 1405F analyzer with a tapered element oscillating microbalance (TEOM) monitoring mechanism with a minimum detection limit of 1.0 μg·m−3. OC and EC were analyzed with an RT-4 analyzer with a minimum detection limit of 0.2 μg·m−3 (Sunset Lab, USA). The analysis of OC and EC did not require any pre-treatment processes. Samples were stored under cryogenic refrigeration until use, and were then brought to room temperature before measurements. In the process of measurement, some OC tended to occur pyrolysis and be converted into EC at about 300 °C, thus, an optical detector was implied to solve this problem. A red laser was chose to be the source of the optical detector, and the strong absorption capacity of EC was applied to correct the errors caused by pyrolysis. In the end, a methane gas with known quantities was applied to calibrate the instrument (NIOSH 1996 and 1999). Water-soluble cations and anions were monitored using an ICS-2000 and an ICS-3000 ion chromatographic analyzer (Dionex), respectively. Sample filters and blank filters were digested in a clean Teflon-TFM digestion tank. Saturated boric acid solution (20 mL) was added to neutralize the excess F−. After another microwave digestion, the solution was brought to a 50-mL constant volume. Selenium was monitored with an AFS-9230 atomic fluorescence spectrometer (China), and other inorganic elements were monitored using an Intrepid II-XDL spectrometer (Thermo Fisher, USA). Ground meteorological elements were observed with a WXT520 meteorological instrument (Vaisala, Netherlands). Before each chemical component measurement, standard samples from the Institute for Environmental Reference Materials of the Ministry of Environmental Protection were applied to calibrate the instruments. A multi-point calibration was done every week. Parallel samples contributed at least 10 % of the total number of samples.
3 Results and discussion
3.1 Annual average concentrations of chemical components
After the laboratory analyses, 15 chemical components with relatively higher concentrations in PM2.5 were selected for further analysis, annual average concentrations of which are shown in Table 1. Overall, OC, SO4 2−, NO3 −, and NH4 + were dominant among 15 components, the annual average concentrations of which in the four sites were 22.62 ± 21.86, 19.39 ± 21.06, 18.89 ± 19.82, and 13.20 ± 12.80 μg·m−3, respectively. For most components, the lowest concentrations were observed at the DL site, reflecting the characteristics of the site’s urban background. The sum of the annual average concentrations of the 15 selected chemical components at the DL site was 64.61 μg·m−3. The sum of the annual average concentrations of the 15 selected chemical components at the YF site was 116.32 μg·m−3, 1.8 times that of the DL site. Overall, the concentrations of PM2.5 chemical components were higher in the south and lower in the north. The concentration of OC was the highest among the 15 components in DL, FS, and YF, accounting for 24.2 %, 24.4 %, and 30.7 % of the summed concentrations, respectively. NO3 − had the highest concentration among the 15 components in CG, accounting for 23.3 % of the summed concentrations. The CG site was close to Chegongzhuang West Road and Capital Stadium Road, where NO2 emitted by motor vehicles is converted into NO3 − through atmospheric chemical reactions (Xie et al. 2000; Fu et al. 2001; Carslaw 2005).
Figure 2 shows the average concentrations of the 15 chemical components over four seasons. Metal elements accounted for a relatively small proportion of PM2.5, and their concentrations were higher in winter and lower in summer. The most abundant metal element was Si, concentration of which were higher in spring and summer, this may have been caused by sand and dust storms that occur in Beijing in the spring and summer (Zhang et al. 1993). Concentrations of the water-soluble ions K+ and Cl− were highest in winter and lowest in summer. The sampling period in February, 2013 was a few days after the Spring Festival in China. The discharge of fireworks has a significant effect on K+ (Cheng et al. 2013, 2014; Chang et al. 2011). Also, the burning of fossil fuels in winter facilitates the formation of organic and inorganic substances containing Cl−. Concentrations of the water-soluble ions NH4 + and SO4 2− were highest in winter and lowest in autumn. Coal combustion in winter increases the emission of NH3, and lower temperatures favor the formation of particulate inorganic nitrogen salts from NH3, NO x , and HNO3 (O’Dowd et al. 2000; Schlünzen and Meyer 2007; Elminir 2005; Watson et al. 1994). Coal combustion in winter also increases the emission of SO2, and the haze that frequently occurs in winter in Beijing promotes the formation of SO4 2− from SO2 (Sun et al. 2006; Galindo et al. 2008). The concentration of NO3 −, another water-soluble ion, was highest in spring and lowest in autumn. Studies have reported that the dust that invades Beijing carries particulate inorganic nitrogen components (Zhang and Iwasaka 1999). Additionally, coarse particles in dust, such as CaCO3, provide a reaction medium for NO3 −, NH4 +, Na+, and Ca+, facilitating the formation of NO3 −.
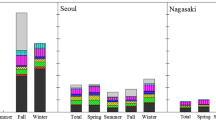
Figure 3 shows the annual average concentrations of OC, EC, and water-soluble ions observed in this study and previous studies from 2001 to 2010 (Tan et al. 2009; Wang et al. 2005; Ho et al. 2006; Shon et al. 2012; Pathak et al. 2009; Zhao et al. 2013; Yang et al. 2011; Hagler et al. 2006; Kim et al. 2007; Qin et al. 2006). Concentrations of OC, NO3 −, and SO4 2− in this study were significantly higher than monitoring results for Beijing, Lanzhou, Hong Kong, Shanghai, New York, and Seoul from the period 2001–2006, whereas they were similar to monitoring results for Shijiazhuang and Tianjin from 2010. The average concentration of NH4 + was higher than all previously reported levels, as shown in Fig. 3 and Table 2.
3.2 OC and EC
Both OC and EC concentrations were higher in winter and lower in summer, as shown in Fig. 4. The average concentration of OC over the four sites was 13.49 ± 6.00 μg·m−3 in summer, whereas in winter, it was almost three times higher at 39.30 ± 29.00 μg·m−3. The average concentration of EC over the four sites was 2.12 ± 0.92 μg·m−3 in summer and 5.22 ± 3.89 μg·m−3 in winter, 2.5 times higher. It can be concluded that coal combustion in winter significantly influenced OC and EC. In addition, the low boundary height caused by temperature inversion was another factor that influenced OC and EC. Among the four sites, the concentrations of EC were highest at the FS site in summer, autumn, and winter. The FS site was close to traffic trunks located outside the Fifth Ring Road, and heavy diesel vehicles were allowed on the road, increasing EC emissions.
The OC/EC ratio was applied to discern transformation characteristics of carbonaceous aerosols and to identify secondary sources of particulate matter. Most EC is produced from the incomplete combustion of carbonaceous fuels. Therefore, EC is considered a tracer of direct anthropogenic emissions (Strader et al. 1999). OC comes from both primary organic carbon (POC) that is directly emitted from pollution sources and secondary organic carbon (SOC) that is formed through photochemical reactions (Cabada et al. 2004). Studies have shown that when the OC/EC ratio is >2, SOC is formed in the atmosphere (Chow et al. 1996; Castro et al. 1999; Cao et al. 2009). In addition to photochemical reactions, biomass burning emits large quantities of OC, but relatively little EC, also resulting in high OC/EC ratios (Zhang et al. 2007).
The OC/EC ratio remained high throughout the entire year, as shown in Fig. 5. The average OC/EC ratios for the four seasons were 4.9, 7.0, 8.1, and 8.4, in the spring, summer, autumn, and winter, respectively. The OC/EC ratio was relatively low in the spring, and there were small differences among the four sites. The OC/EC ratio increased in the summer due to increased sunlight intensity and temperature. In summer, among the four sites, both OC and EC concentrations were lowest at the DL site, whereas the OC/EC ratio was the highest. This is likely due to the fact that EC concentrations for many of the samples from DL were <1.0 μg·m−3 in the summer, resulting in high OC/EC ratios. The monitoring results for DL were similar to those from a regional background site in Europe (Novakov et al. 2005).
3.3 Formation of SO4 2− and NO3 −
3.3.1 [NO3 −]/[SO4 2−] ratios
The [NO3 −]/[SO4 2−] ratio was applied to characterize the relative contributions of stationary sources and mobile sources (Arimoto et al. 1996). The [NO3 −]/[SO4 2−] ratios were lower in summer and winter and higher in spring and autumn, as shown in Fig. 6. Coal combustion in winter increased SO2 emissions. High temperatures, high humidity, and strong solar radiation in summer favor the formation of SO4 2−. The distribution of NO3 − across the gas phase, particle phase, and different sizes of particulate matter is related to temperature, humidity, SO4 2− concentration, and the concentration of crustal elements in the particulate matter. The combination of these potential factors causes seasonal variations in the [NO3 −]/[SO4 2−] ratio. The annual average [NO3 −]/[SO4 2−] ratios in DL, CG, FS, and YF were 1.01, 1.25, 1.08, and 1.12, respectively, which were significantly higher than previous studies in Beijing, indicating that the contribution ratio of mobile source increased in recent years in Beijing. At the CG site, the contribution of mobile source was higher than other sites due to its special location (Table 3).
3.3.2 Formation mechanisms of SO4 2− and NO3 −
The main sources of SO4 2− and NO3 − are photochemical reactions of SO2 and NO2, respectively. Therefore, concentrations of SO4 2− and NO3 − are related to the oxidation efficiency of SO2 and NO2. The sulfur and nitrogen oxidation ratios (SOR and NOR, respectively) were applied to characterize the conversion efficiency of SO2 and NO2 (Sun et al. 2006; Sun et al. 2014; Sun et al. 2015), as follows:
Previous studies have demonstrated that the SOR is lower than 0.1 in pollutants that are discharged directly, such as vehicle exhaust (Pierson et al. 1979). Truex et al. (1980) and Ohta and Okita (1990) reported that when the SOR is higher than 0.1, SO2 participates in photochemical reactions through which SO4 2− is formed. The SOR values were higher than 0.1 throughout all four seasons, reflecting obvious photochemical reactions of SO2 in Beijing, as shown in Fig. 7. Among the four seasons, the SOR was significantly higher in the summer, likely due to the fact that higher temperatures are favorable for the transformation from SO2 to SO4 2− (Quan et al. 2008; McMurry P and Wilson J 1983). The NORs were significantly lower than the SORs. The NOR was highest in the spring at the CG site, but it was highest in summer at the other three sites. The annual average SORs were 0.433, 0.383, 0.438, and 0.460 at the DL, CG, FS, and YF sites, respectively. The annual average NORs were 0.269, 0.186, 0.223, and 0.305 at the DL, CG, FS, and YF sites, respectively. Among the four sites, the NO3 − concentration was highest at the CG site; however, the NOR was the lowest.
Many mechanisms have been reported for the formation of SO4 2− from SO2, such as gas-phase reactions of SO2 and OH radicals, aqueous transformation processes (metal-catalyzed oxidation or H2O2/O3 oxidation), and in-cloud processes (Ziegler 1979; Meng and Seinfeld 1994; Xiu et al. 2004). The formation of SO4 2− and NO3 − is related to many factors, including the concentrations of precursors, concentrations of oxidant in gas- and liquid-phases, characteristics of preexisting aerosol particles, and meteorological conditions. The gas-phase conversion of SO2 to SO4 2− initiated by OH radicals is strongly a function of temperature (Seinfeld 1986; Radojević 1992), whereas heterogeneous reactions are closely related to humidity and particulate matter load (Liang and Jacobson 1999). Currently, studies of heterogeneous reactions on particle surfaces are in the preliminary stages compared with studies of homogeneous reactions. The Chinese scientist Zhu and his research group (Zhu et al. 2011; Shang et al. 2010; Xu et al. 2010; Ye et al. 2010; Tang et al. 2010; Li et al. 2011a, b) have conducted related studies on atmospheric heterogeneous reaction processes on particle surfaces, confirming the importance of heterogeneous reactions in the formation of secondary particles of PM2.5 in Beijing.
Meteorological data were only available at the CG site. The Spearman correlation coefficients between the SOR and NOR and the temperature and relative humidity (RH) were calculated (Table 4) to suggest formation mechanisms of SO4 2− and NO3 −, following the evaluation protocols of Yao et al. (2002) and Wang et al. (2009). In spring and summer, the SOR and temperature had a strong positive correlation, whereas the SOR and RH had no significant correlation, suggesting that a possible oxidation mechanism of SO2 to SO4 2− was the local gas-phase oxidation of SO2 by OH radicals, followed by condensation or absorption into the particle phase. The correlations of SOR with temperature and RH are close in autumn and winter. Thus, the formation mechanisms of SO4 2− included both slow gas-phase oxidation and heterogeneous reactions in the autumn and winter in urban Beijing. The NOR and temperature had no significant correlation during any season. Meanwhile the NOR and RH had strong positive correlations in spring, autumn, and winter, suggesting that heterogeneous reactions played major roles in the formation of NO3 − throughout the year in urban Beijing. Additionally, studies have shown that NO3 − is formed by gas-phase oxidation when NH4 + is in excess of SO4 2− (i.e., [NH4 +]/[SO4 2−] > 1.5; Pathak et al. 2004, 2009). However, the [NH4 +]/[SO4 2−] ratios at the CG site were 0.73, 0.62, 1.26, and 0.67 in the spring, summer, autumn, and winter, respectively, suggesting that NH4 + was not in excess of SO4 2−. Thus, gas-phase oxidation cannot explain the high NO3 − concentrations observed in urban Beijing. However, the analysis is based on the overall situation of the whole year. In some heavy air pollution process, the rapid increase of concentration of PM2.5 was related to more complex chemical reaction mechanism.
Laboratory and field experiments have shown that under conditions of high NO x concentrations, the heterogeneous hydrolysis reaction of N2O5 is one of the main sources of NO3 − in particulate matter (Anttila et al. 2006; Martinez et al. 2000; Hu and Abbatt 1997; McLaren et al. 2004; Hallquist et al. 2003). Two factors are favorable for the heterogeneous hydrolysis reaction of N2O5 to NO3 − on particle surfaces of existing SO4 2−. PM2.5 in Beijing has a large surface area and strong hygroscopicity resulting from the high concentration of PM2.5 and water-soluble ions in PM2.5. The formation and heterogeneous hydrolysis reaction of N2O5 include the R1–R3 reactions (Mentel et al. 1999; Underwood et al. 2001; Seisel et al. 2005; Bauer et al. 2004; Bian and Zender 2003). R1 is the speed-control reaction. HNO3 formed by R3 has high reactivity and participates in chemical reactions (R4–R7) to form secondary particulate matter such as surface nitrates (Hanisch and Crowley 2001; Goodman et al. 2001). In our monitoring results, the NORs were lower than the SORs; this may be related to the different formation mechanisms of SO4 2− and NO3 −, a question that warrants further study.
4 Conclusions
OC, SO4 2−, NO3 −, and NH4 + were dominant among 15 components, the annual average concentrations of which in the four sites were 22.62 ± 21.86, 19.39 ± 21.06, 18.89 ± 19.82, and 13.20 ± 12.80 μg·m−3, respectively. Concentration of OC and EC in winter were significantly higher than those in summer, indicating that coal combustion greatly influenced OC and EC. The annual average [NO3 −]/[SO4 2−] ratios in DL, CG, FS, and YF were 1.01, 1.25, 1.08, and 1.12, respectively, which were significantly higher than previous studies in Beijing, indicating that the contribution ratio of mobile source increased in recent years in Beijing. Correlation analyses between temperature and RH, and SOR and NOR showed that gas-phase oxidation reactions were a major mechanism of SO4 2− formation in the spring and summer in urban Beijing, whereas slow gas-phase oxidation reactions and heterogeneous reactions were both important in autumn and winter. Heterogeneous reactions were the main formation mechanism of NO3 − year round in urban Beijing.
Reference
Anttila T., Kiendler-Scharr A., Tillmann R., et al.: On the reactive uptake of gaseous compounds by organic-coated aqueous aerosols: theoretical analysis and application to the heterogeneous hydrolysis of N2O5 [J]. J Phys Chem A. 110, 10435–10443 (2006)
Arimoto R., Duce R.A., Savoie D.L., et al.: Relationships among aerosol constituents from Asia and the North Pacific during PEM-West A [J]. J. Geophys. Res. Atmos. 101(1984–2012), 2011–2023 (1996)
Bauer S.E., Balkanski Y., Schulz M., et al.: Global modeling of heterogeneous chemistry on mineral aerosol surfaces: influence on tropospheric ozone chemistry and comparison to observations [J]. J. Geophys. Res. Atmos. 109(D2), 1984–2012 (2004)
Bian H., Zender C.S.: Mineral dust and global tropospheric chemistry: relative roles of photolysis and heterogeneous uptake [J]. J. Geophys. Res. Atmos. 108(D21), 1984–2012 (2003)
Bond T.C., Doherty S.J., Fahey D.W., et al.: Bounding the role of black carbon in the climate system: A scientific assessment [J]. J. Geophys. Res. Atmos. 118(11), 5380–5552 (2013)
Cabada J.C., Pandis S.N., Subramanian R., et al.: Estimating the secondary organic aerosol contribution to PM2.5 using the EC tracer method special issue of aerosol science and technology on findings from the fine particulate matter supersites program [J]. Aerosol Sci. Technol. 38, 140–155 (2004)
Cao J.J., Lee S.C., Ho K.F., Zou S.C., Fung K., Li Y., Watson J.G., Chow J.C.: Spatial and seasonal variations of atmospheric organic carbon and elemental carbon in Pearl River Delta region, China. Atmos. Environ. 38, 4447–4456 (2004)
Cao J., Shen Z., Chow J.C., et al.: Seasonal variations and sources of mass and chemical composition for PM10 aerosol in Hangzhou, China[J]. Particuology. 7(3), 161–168 (2009)
Carslaw D.C.: Evidence of an increasing NO2/NO x emissions ratio from road traffic emissions [J]. Atmos. Environ. 39(26), 4793–4802 (2005)
Castro L.M., Pio C.A., Harrison R.M., et al.: Carbonaceous aerosol in urban and rural European atmospheres: estimation of secondary organic carbon concentrations[J]. Atmos. Environ. 33(17), 2771–2781 (1999)
Chan C.K., Yao X.: Air pollution in mega cities in China [J]. Atmos. Environ. 42(1), 1–42 (2008)
Chan Y.C., Simpson R.W., McTainsh G.H., et al.: Source apportionment of visibility degradation problems in Brisbane (Australia) using the multiple linear regression techniques[J]. Atmos. Environ. 33(19), 3237–3250 (1999)
Chang S.C., Lin T.H., Young C.Y., et al.: The impact of ground-level fireworks (13 km long) display on the air quality during the traditional Yanshui Lantern Festival in Taiwan [J]. Environ. Monit. Assess. 172(1–4), 463–479 (2011)
Chen Y.Q., Zhang Y., Zhang X.S.: Size distribution and seasonal variation of ions in aerosol at semi-urban site in Beijing [J]. Acta Ecol. Sin. 25(12), 3231–3236 (2005)
Chen J., Zhao C.S., Ma N., Liu P.F., Göbel T., Hallbauer E., Deng Z.Z., Ran L., Xu W.Y., Liang Z., Liu H.J., Yan P., Zhou X.J., Wiedensohler A.: A parameterization of low visibilities for hazy days in the North China Plain. Atmos. Chem. Phys. 12, 4935–4950 (2012). doi:10.5194/acp-12-4935-2012
Cheng Y.F., Berghof M., Garland R.M., Wiedensohler A., Wehner B., M¨uuller T., Su H., Zhang Y.H., Achtert P., Nowak A., P¨oschl U., Zhu T., Hu M., Zeng L.M.: Influence of soot mixing state on aerosol light absorption and single scattering albedo during air mass aging at a polluted regional site in northeastern China. J. Geophys. Res. 114, D00G10 (2009). doi:10.1029/2008JD010883
Cheng Y., Engling G., He K.B., et al.: Biomass burning contribution to Beijing aerosol[J]. Atmos. Chem. Phys. 13(15), 7765–7781 (2013)
Cheng Y., Engling G., He K., et al.: The characteristics of Beijing aerosol during two distinct episodes: Impacts of biomass burning and fireworks [J]. Environ. Pollut. 185, 149–157 (2014)
Chow J.C., Watson J.G., Lu Z., et al.: Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX [J]. Atmos. Environ. 30(12), 2079–2112 (1996)
Dan M., Zhuang G.S., Li X.X., Tao H.R., Zhuang Y.H.: The characteristics of carbonaceous species and their sources in PM2.5 in Beijing. Atmos. Environ. 38, 3443–3452 (2004)
Deng L.Q., Li H., Chai F.H., Lun X.X., Chen Y.Z., Wang F.W., Ni R.X.: The pollution characteristics of the atmospheric fine particles and related gaseous pollutants in the northeastern urban area of Beijing [J]. China Environ. Sci. 31(7), 1064–1070 (in Chinese) (2011a)
Deng Z.Z., Zhao C.S., Ma N., Liu P.F., Ran L., Xu W.Y., Chen J., Liang Z., Liang S., Huang M.Y., Ma X.C., Zhang Q., Quan J.N., Yan P., Henning S., Mildenberger K., Sommerhage E., Sch¨afer M., Stratmann F., Wiedensohler A.: Size-resolved and bulk activation properties of aerosols in the North China Plain. Atmos. Chem. Phys. 11, 3835–3846 (2011b). doi:10.5194/acp-11-3835-2011
Ding A.J., Fu C.B., Yang X.Q., et al.: Ozone and fine particle in the western Yangtze River Delta: an overview of 1 yr data at the SORPES station [J]. Atmos. Chem. Phys. 13(11), 5813–5830 (2013)
Dockery D.W.: Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ. Health Perspect. 109, 483–486 (2001)
Duan J.C., Tan J.H., Cheng D.X., Bi X.H., Deng W.J., Sheng G.Y., Fu J.M., Wong M.H.: Sources and characteristics of carbonaceous aerosol in two largest cities in Pearl River Delta region, China. Atmos. Environ. 41, 2895–2903 (2007)
Elminir H.K.: Dependence of urban air pollutants on meteorology [J]. Sci. Total Environ. 350(1), 225–237 (2005)
Fu L., Hao J., He D., et al.: Assessment of vehicular pollution in China [J]. J. Air Waste Manage. Assoc. 51(5), 658–668 (2001)
Galindo N., Nicolás J.F., Yubero E., et al.: Factors affecting levels of aerosol sulfate and nitrate on the Western Mediterranean coast[J]. Atmos. Res. 88(3), 305–313 (2008)
Gao J., Chai F.H., Wang T., Wang S.L., Wang W.X.: Particle number size distribution and new particle formation: new characteristics during the special pollution control period in Beijing. J. Environ. Sci. 24, 14–21 (2012)
Garland R.M., Schmid O., Nowak A., Achtert P., Wiedensohler A., Gunthe S.S., Takegawa N., Kita K., Kondo Y., Hu M., Shao M., Zeng L.M., Zhu T., Andreae M.O., Pöschl U.: Aerosol optical properties observed during Campaign of Air Quality Research in Beijing 2006 (CAREBeijing-2006): Characteristic differences between the inflow and outflow of Beijing city air. J. Geophys. Res. 114, D00G04 (2009). doi:10.1029/2008JD010780
Goodman A.L., Bernard E.T., Grassian V.H.: Spectroscopic study of nitric acid and water adsorption on oxide particles: Enhanced nitric acid uptake kinetics in the presence of adsorbed water [J]. J Phys Chem A. 105(26), 6443–6457 (2001)
Guo Y.M., Jia Y.P., Pan X.C., Liu L.Q., Wichmann H.-E.: The association between fine particulate air pollution and hospital emergency room visits for cardiovascular diseases in Beijing, China. Sci. Total Environ. 407, 4826–4830 (2009)
Guo S., Hu M., Wang Z.B., Slanina J., Zhao Y.L.: Sizeresolved aerosol water-soluble ionic compositions in the summer of Beijing: implication of regional secondary formation. Atmos. Chem. Phys. 10, 947–959 (2010). doi:10.5194/acp-10-947-2010
Guo Z.B., Bao C.X., Chen T.L., Chen T., Zhou F., Dong Q.Y., Lin M.Y.: Mass concentration characteristics and source apportionment of water-soluble inorganic ions in aerosol in Beijing during 2008 Beijing Olympic Games [J]. T Atmos Sci. 34(6), 683–687 (in Chinese) (2012)
Hagler G.S.W., Bergin M.H., Salmon L.G., Yu J.Z., Wan E.C.H., Zheng M., Zeng L.M., Kiang C.S., Zhang Y.H., Lau A.K.H., Schauer J.J.: Source areas and chemical composition of fine particulate matter in the Pearl River Delta region of China. Atmos. Environ. 40, 3802–3815 (2006)
Hallquist M., Stewart D.J., Stephenson S.K., et al.: Hydrolysis of N2O5 on sub-micron sulfate aerosols [J]. Phys. Chem. Chem. Phys. 5(16), 3453–3463 (2003)
Hanisch F., Crowley J.N.: Heterogeneous reactivity of gaseous nitric acid on Al2O3, CaCO3, and atmospheric dust samples: A Knudsen cell study [J]. J Phys Chem A. 105(13), 3096–3106 (2001)
He K.B., Yang F.M., Ma Y.L., Zhang Q., Yao X.H., Chan C.K., Cadle S., Chan T., Mulawa P.: The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 35, 4959–4970 (2001)
Ho K.F., Cao J.J., Lee S.C., et al.: Source apportionment of PM2.5 in urban area of Hong Kong[J]. J. Hazard. Mater. 138(1), 73–85 (2006)
Ho K.F., Ho S.S.H., Lee S.C., et al.: Summer and winter variations of dicarboxylic acids, fatty acids and benzoic acid in PM 2.5 in Pearl Delta River region, China[J]. Atmos. Chem. Phys. 11(5), 2197–2208 (2011)
Hu J.H., Abbatt J.P.D.: Reaction probabilities for N2O5 hydrolysis on sulfuric acid and ammonium sulfate aerosols at room temperature[J]. J Phy Chem A. 101(5), 871–878 (1997)
Hu M., Wu Z., Slanina J., et al.: Acidic gases, ammonia and water-soluble ions in PM2.5 at a coastal site in the Pearl River Delta, China[J]. Atmos. Environ. 42(25), 6310–6320 (2008)
Huang X.F., He L.Y., Hu M., Zhang Y.H.: Annual variation of particulate organic compounds in PM2.5 in the urban atmosphere of Beijing. Atmos. Environ. 40, 2449–2458 (2006)
Huang X.F., He L.Y., Xue L., et al.: Highly time-resolved chemical characterization of atmospheric fine particles during 2010 Shanghai World Expo [J]. Atmos. Chem. Phys. 12(11), 4897–4907 (2012)
Huebert B.J., Ming-Xing W., Wei-Xiu L.Ü.: Atmospheric nitrate, sulfate, ammonium and calcium concentrations in China [J]. Tellus B. 40(4), 260–269 (1988)
Hueglin C., Gehrig R., Baltensperger U., et al.: Chemical characterisation of PM2.5, PM10 and coarse particles at urban, near-city and rural sites in Switzerland [J]. Atmos. Environ. 39(4), 637–651 (2005)
Ianniello A., Spataro F., Esposito G., Allegrini I., Hu M., Zhu T.: Chemical characteristics of inorganic ammonium salts in PM2.5 in the atmosphere of Beijing (China). Atmos. Chem. Phys. 11, 10803–10822 (2011). doi:10.5194/acp-11-10803-2011
Kim B.M., Teffera S., Zeldin M.D.: Characterization of PM2.5 and PM10 in the South Coast Air Basin of Southern California: Part 1-Spatial variations [J]. J. Air Waste Manage. Assoc. 50(12), 2034–2044 (2000a)
Kim B.M., Teffera S., Zeldin M.D.: Characterization of PM2.5 and PM10 in the South Coast Air Basin of Southern California: Part 2-Temporal variations [J]. J. Air Waste Manage. Assoc. 50(12), 2045–2059 (2000b)
Kim H.S., Huh J.B., Hopke P.K., et al.: Characteristics of the major chemical constituents of PM2.5 and smog events in Seoul, Korea in 2003 and 2004[J]. Atmos. Environ. 41(32), 6762–6770 (2007)
Kipen H., Rich D., Huang W., Zhu T., Wang G., Hu M., Lu S., Ohman-Strickland P., Zhu P., Wang Y., Zhang J.: Measurement of inflammation and oxidative stress following drastic changes in air pollution during the Beijing Olympics: a panel study approach. Ann. N. Y. Acad. Sci. 1203, 160–167 (2010)
Lai S., Zou S., Cao J., et al.: Characterizing ionic species in PM2.5 and PM10 in four Pearl River Delta cities, South China [J]. J. Environ. Sci. 19(8), 939–947 (2007)
Li L., Chen C.H., Fu J.S., Huang C., Streets D.G., Huang H.Y., Zhang G.F., Wang Y.J., Jang C.J., Wang H.L., Chen Y.R., Fu J.M.: Air quality and emissions in the Yangtze River Delta, China. Atmos. Chem. Phys. 11, 1621–1639 (2011a). doi:10.5194/acp-11-1621-2011
Li J., Shang J., Zhu T.: Heterogeneous reactions of SO2 on ZnO particle surfaces [J]. SCIENCE CHINA Chem. 54(1), 161–166 (in Chinese) (2011b)
Li X.R., Wang L.L., Wang Y.S., Wen T.X., Yang Y.J., Zhao Y.N., Wang Y.F.: Chemical composition and size distribution of airborne particulate matters in Beijing during the 2008 Olympics. Atmos. Environ. 50, 278–286 (2012)
Liang J, Jacobson M Z. A study of sulfur dioxide oxidation pathways over a range of liquid water contents, pH values, and temperatures [J]. J. Geophys. Res. Atmos. (1984–2012), 1999, 104(D11): 13749–13769.
Lin W., Xu X., Zhang X., et al.: Contributions of pollutants from North China Plain to surface ozone at the Shangdianzi GAW Station[J]. Atmos. Chem. Phys. 8(19), 5889–5898 (2008)
Liu Y., Shao M., Lu S.H., Chang C.C., Wang J.L., Chen G.: Volatile organic Compound (VOC) measurements in the Pearl River Delta (PRD) region, China. Atmos. Chem. Phys. 8, 1531–1545 (2008). doi:10.5194/acp-8-1531-2008
Ma, N., Zhao, C. S., Nowak, A., M¨uller, T., Pfeifer, S., Cheng, Y. F., Deng, Z. Z., Liu, P. F., Xu, W. Y., Ran, L., Yan, P., Göbel, T., Hallbauer, E., Mildenberger, K., Henning, S., Yu, J., Chen, L. L., Zhou, X. J., Stratmann, F., and Wiedensohler, A.: Aerosol optical properties in the North China Plain during HaChi campaign: an in-situ optical closure study, Atmos. Chem. Phys.., 11, 5959–5973, doi:10.5194/acp-11-5959-2011, 2011.
Ma, N., Zhao, C. S., M¨uller, T., Cheng, Y. F., Liu, P. F., Deng, Z. Z., Xu, W. Y., Ran, L., Nekat, B., van Pinxteren, D., Gnauk, T., M¨uller, K., Herrmann, H., Yan, P., Zhou, X. J., and Wiedensohler, A.: A new method to determine the mixing state of light absorbing carbonaceous using the measured aerosol optical properties and number size distributions, Atmos. Chem. Phys.., 12, 2381–2397, doi:10.5194/acp-12-2381-2012, 2012.
Martinez M, Perner D, Hackenthal E M, et al. NO3 at Helgoland during the NORDEX campaign in October 1996[J]. J. Geophys. Res. Atmos. (1984–2012), 2000, 105(D18): 22685–22695.
McLaren R., Salmon R.A., Liggio J., et al.: Nighttime chemistry at a rural site in the lower Fraser Valley[J]. Atmos. Environ. 38(34), 5837–5848 (2004)
McMurry P H, Wilson J C. Droplet phase (heterogeneous) and gas phase (homogeneous) contributions to secondary ambient aerosol formation as functions of relative humidity [J]. J Geophysical Res: Oceans (1978–2012), 1983, 88(C9): 5101–5108.
Meng Z., Seinfeld J.H.: On the source of the submicrometer droplet mode of urban and regional aerosols [J]. Aerosol Sci. Technol. 20(3), 253–265 (1994)
Menon S., Hansen J., Nazarenko L., et al.: Climate effects of black carbon aerosols in China and India[J]. Science. 297(5590), 2250–2253 (2002)
Mentel T.F., Sohn M., Wahner A.: Nitrate effect in the heterogeneous hydrolysis of dinitrogen pentoxide on aqueous aerosols [J]. Phys. Chem. Chem. Phys. 1(24), 5451–5457 (1999)
Michaels R.A., Kleinman M.T.: Incidence and apparent health significance of brief airborne particle excursions. Aerosol Sci. Technol. 32, 93–105 (2000)
NIOSH: Elemental carbon (diesel exhaust). in NIOSH Manual of Analytical Methods. National Institute of Occupational Safety and Health, Cincinnati, OH (1996)
NIOSH: Method 5040 issue 3 (Interim): elemental carbon (diesel exhaust). in NIOSH Manual of Analytical Methods. National Institute of Occupational Safety and Health, Cincinnati, OH (1999)
Novakov T, Menon S, Kirchstetter T W, et al. Aerosol organic carbon to black carbon ratios: Analysis of published data and implications for climate forcing[J]. J. Geophys. Res. Atmos. (1984–2012), 2005, 110(D21).
O’Dowd C.D., Lowe J.A., Smith M.H.: The effect of clouds on aerosol growth in the rural atmosphere [J]. Atmos. Res. 54(4), 201–221 (2000)
Ohta S., Okita T.: A chemical characterization of atmospheric aerosol in Sapporo [J]. Atmos Environ. Part A. General Topics. 24(4), 815–822 (1990)
Park S.S., Jung S.A., Gong B.J., et al.: Characteristics of PM2.5 haze episodes Revealed by Highly time-resolved measurements at an air pollution monitoring Supersite in Korea [J]. Aerosol Air Qual. Res. 13, 957–976 (2013)
Pathak R.K., Yao X., Chan C.K.: Sampling artifacts of acidity and ionic species in PM2.5 [J]. Environ. Sci. Technol. 38(1), 254–259 (2004)
Pathak R.K., Wu W.S., Wang T.: Summertime PM2.5 ionic species in four major cities of China: nitrate formation in an ammonia-deficient atmosphere. Atmos. Chem. Phys. 9,, 1711–1722 (2009). doi:10.5194/acp-9-1711-2009
Pierson W.R., Brachaczek W.W., McKee D.E.: Sulfate emissions from catalyst-equipped automobiles on the highway[J]. J. Air Pollut. Control Assess. 29(3), 255–257 (1979)
Qin Y., Kim E., Hopke P.K.: The concentrations and sources of PM2.5 in metropolitan New York City [J]. Atmos. Environ. 40, 312–332 (2006)
Quan J., Zhang X., Zhang Q., et al.: Importance of sulfate emission to sulfur deposition at urban and rural sites in China[J]. Atmos. Res. 89(3), 283–288 (2008)
Radojević M.: SO2 and NO x oxidation mechanisms in the atmosphere [J]. Atmos Acidity: Sources, Consequences and Abatement. 5, 73 (1992)
Ramanathan V., Crutzen P.J., Kiehl J.T., et al.: Aerosols, climate, and the hydrological cycle [J]. Science. 294(5549), 2119–2124 (2001)
Rose D., Nowak A., Achtert P., et al.: Cloud condensation nuclei in polluted air and biomass burning smoke near the mega-city Guangzhou, China–Part 1: Size-resolved measurements and implications for the modeling of aerosol particle hygroscopicity and CCN activity[J]. Atmos. Chem. Phys. 10(7), 3365–3383 (2010)
Schlünzen K.H., Meyer E.M.I.: Impacts of meteorological situations and chemical reactions on daily dry deposition of nitrogen into the southern North Sea[J]. Atmos. Environ. 41(2), 289–302 (2007)
Schwartz J., Laden F., Zanobetti A.: The concentration response relation between PM2.5 and daily deaths. Environ. Health Perspect. 110, 1025–1029 (2002)
Seinfeld J.H.: Atmospheric Chemistry and Physics of Air Pollution, p. 348 pp. Wiley, New York (1986)
Seisel S., Börensen C., Vogt R., et al.: Kinetics and mechanism of the uptake of N2O5 on mineral dust at 298 K[J]. Atmos. Chem. Phys. 5(12), 3423–3432 (2005)
Shang J., Li J., Zhu T.: Heterogeneous reaction of SO2 on TiO2 particles [J]. SCIENCE CHINA Chem. 53(12), 2637–2643 (in Chinese) (2010)
Shen X.J., Sun J.Y., Zhang Y.M., Wehner B., Nowak A., Tuch T., Zhang X.C., Wang T.T., Zhou H.G., Zhang X.L., Dong F., Birmili W., Wiedensohler A.: First long-term study of particle number size distributions and new particle formation events of regional aerosol in the North China Plain. Atmos. Chem. Phys. 11, 1565–1580 (2011). doi:10.5194/acp-11-1565-2011
Shon Z.H., Kim K.H., Song S.K., et al.: Relationship between water-soluble ions in PM2.5 and their precursor gases in Seoul megacity [J]. Atmos. Environ. 59, 540–550 (2012)
Song Y., Zhang Y.H., Xie S.D., Zeng L.M., Zheng M., Salmon L.G., Shao M., Slanina S.: Source apportionment of PM2.5 in Beijing by positive matrix factorization. Atmos. Environ. 40, 1526–1537 (2006)
Strader R., Lurmann F., Pandis S.N.: Evaluation of secondary organic aerosol formation in winter [J]. Atmos. Environ. 33(29), 4849–4863 (1999)
Sun Y.L., Zhuang G.S., Wang Y., Han L.H., Guo J.H., Dan M., Zhang W.J., Wang Z.F., Hao Z.P.: The air-borne particulate pollution in Beijing-concentration, composition, distribution and sources. Atmos. Environ. 38, 5991–6004 (2004)
Sun Y., Zhuang G., Tang A., et al.: Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in Beijing [J]. Environ. Sci. Technol. 40(10), 3148–3155 (2006)
Sun Y.L., Jiang Q., Wang Z., Fu P., Li J., Yang T., Yin Y.: Investigation of the sources and evolution processes of severe haze pollution in Beijing in January 2013. J. Geophys. Res. 119, 4380–4398 (2014). doi:10.1002/2014JD021641
Sun Y.L., Wang Z.F., Du W., Zhang Q., Wang Q.Q., Fu P.Q., Pan X.L., Li J., Jayne J., Worsnop D.R.: Long-term real-time measurements of aerosol particle composition in Beijing, China: seasonal variations, meteorological effects, and source analysis. Atmos. Chem. Phys. 15, 10149–10165 (2015). doi:10.5194/acp-15-10149-2015
Tan J., Duan J., He K., et al.: Chemical characteristics of PM2.5 during a typical haze episode in Guangzhou [J]. J. Environ. Sci. 21(6), 774–781 (2009)
Tang M.J., Li M.Q., Zhu T.: Heterogeneous reactions of gaseous methane sulfonic acid with calcium carbonate and kaolinite particles [J]. SCIENCE CHINA Chem. 53(12), 2657–2662 (in Chinese) (2010)
Truex T.J., Pierson W.R., McKee D.E.: Sulfate in diesel exhaust [J]. Environ. Sci. Technol. 14(9), 1118–1121 (1980)
Underwood G M, Song C H, Phadnis M, et al. Heterogeneous reactions of NO2 and HNO3 on oxides and mineral dust: A combined laboratory and modeling study [J]. J. Geophys. Res. Atmos. (1984–2012), 2001, 106(D16): 18055–18066.
Wang G., Huang L., Gao S., et al.: Characterization of water-soluble species of PM10 and PM2.5 aerosols in urban area in Nanjing, China[J]. Atmos. Environ. 36(8), 1299–1307 (2002)
Wang Y., Zhuang G., Tang A., et al.: The ion chemistry and the source of PM2.5 aerosol in Beijing [J]. Atmos. Environ. 39(21), 3771–3784 (2005)
Wang W., Wu M.H., Li L., Zhang T., Liu X.D., Feng J.L., Li H.J., Wang Y.J., Sheng G.Y., Claeys M., Fu J.M.: Polar organic tracers in PM2.5 aerosols from forests in eastern China. Atmos. Chem. Phys. 8, 7507–7518 (2008). doi:10.5194/acp-8-7507-2008
Wang Q., Shao M., Zhang Y., Wei Y., Hu M., Guo S.: Source apportionment of fine organic aerosols in Beijing. Atmos. Chem. Phys. 9, 8573–8585 (2009). doi:10.5194/acp-9-8573-2009
Wang L.L., Wang S.L., Wang X.F., Xu Z., Zhou S.Z., Yuan C., Yu Y.C., Wang T., Wang W.X.: Characteristics of particulate matter pollution during August 2009 in Beijing [J]. China Environ. Sci. 31(4), 553–560 (in Chinese) (2011)
Wang Z.S., Li Y.T., Chen T., et al.: Ground-level ozone in urban Beijing over a 1-year period: temporal variations and relationship to atmospheric oxidation[J]. Atmos. Res. 164, 110–117 (2015a)
Wang Z., Pan L., Li Y., et al.: Assessment of air quality benefits from the national pollution control policy of thermal power plants in China: A numerical simulation [J]. Atmos. Environ. 106, 288–304 (2015b)
Watson J.G., Chow J.C., Lurmann F.W., et al.: Ammonium nitrate, nitric acid, and ammonia equilibrium in wintertime Phoenix, Arizona[J]. Air & Waste. 44(4), 405–412 (1994)
Wu Z.J., Hu M., Liu S., Wehner B., Bauer S., Ma ßling A., Wiedensohler A., Pet¨aj¨a T., Dal Maso M., Kulmala M.: New particle formation in Beijing: China Statistical analysis of a 1-year data set. J. Geophys. Res. 112, D09209 (2007). doi:10.1029/2006JD007406
Wu Z.J., Hu M., Lin P., Liu S., Wehner B., Wiedensohler A.: Particle number size distribution in the urban atmosphere of Beijing, China. Atmos. Environ. 42, 7967–7980 (2008)
Wu S.W., Deng F.R., Niu J., Huang Q.S., Liu Y.C., Guo X.B.: Association of Heart Rate Variability in Taxi Drivers with Marked changes in particulate air pollution in Beijing in 2008. Environ. Health Perspect. 118, 87–91 (2010)
Xiao Y.H., Li J., Kuang Y.W., Tong F.C., Xi D., Chen B.F., Shi X., Pei N.C., Huang J.B., Pan Y.J.: Comparison of TSP,PM2.5 and their water-soluble ions from both inside and outside of Dafushan forest park in Guangzhou during rainy season. Acta Ecol. Sin. 33(19), 6209–6217 (in Chinese) (2013)
Xie S.D., Zhang Y.H., Tang X.Y.: Current situation and trend of motor vehicle exhaust pollution in urban areas of China [J]. Res Environ Sci. 13(4), 22–25 (2000)
Xiu G., Zhang D., Chen J., et al.: Characterization of major water-soluble inorganic ions in size-fractionated particulate matters in Shanghai campus ambient air[J]. Atmos. Environ. 38(2), 227–236 (2004)
Xu J., Zhang X.L., Xu X.B., Ding G.A., Yan P., Yu X.L., Cheng H.B., Zhou H.G.: Variations and source identification of chemical compositions in wet deposition at Shangdianzi background station [J]. Acta Sci. Circumst. 28(5), 1001–1006 (in Chinese) (2008)
Xu B.Y., Zhu T., Tang X.Y., et al.: Heterogeneous reaction of formaldehyde on the surface of TiO2 particles[J]. SCIENCE CHINA Chem. 53(12), 2644–2651 (in Chinese) (2010)
Xu J., Ma J.Z., Zhang X.L., et al.: Measurements of ozone and its precursors in Beijing during summertime: impact of urban plumes on ozone pollution in downwind rural areas [J]. Atmos. Chem. Phys. 11(23), 12241–12252 (2011)
Yan P., Tang J., Huang J., Mao J.T., Zhou X.J., Liu Q., Wang Z.F., Zhou H.G.: The measurement of aerosol optical properties at a rural site in Northern China. Atmos. Chem. Phys. 8, 2229–2242 (2008). doi:10.5194/acp-8-2229-2008
Yang F.M., He K.B., Ma Y.L., Zhang Q., Cadle S.H., Chan T., Mulawa P.A.: Characterization of mass balance of PM2.5 chemical speciation in Beijing [J]. Environ. Chem. 23(3), 326–333 (in Chinese) (2004)
Yang F., He K., Ye B., Chen X., Cha L., Cadle S.H., Chan T., Mulawa P.A.: One-year record of organic and elemental carbon in fine particles in downtown Beijing and Shanghai. Atmos. Chem. Phys. 5, 1449–1457 (2005). doi:10.5194/acp-5-1449-2005
Yang M., Howell S.G., Zhuang J., et al.: Attribution of aerosol light absorption to black carbon, brown carbon, and dust in China–interpretations of atmospheric measurements during EAST-AIRE [J]. Atmos. Chem. Phys. 9(6), 2035–2050 (2009)
Yang F., Tan J., Zhao Q., et al.: Characteristics of PM2.5 speciation in representative megacities and across China[J]. Atmos. Chem. Phys. 11(11), 5207–5219 (2011)
Yang Y.Y., Li J.X., Liang Y.P., et al.: Source apportionment of PM2.5 in Beijing by the chemical mass balance [J]. Acta Sci. Circumst. 35(9), 2693–2700 (2015)
Yao X., Chan C.K., Fang M., et al.: The water-soluble ionic composition of PM2.5 in Shanghai and Beijing, China [J]. Atmos. Environ. 36(26), 4223–4234 (2002)
Yao X.H., Lau A.P.S., Fang M., Chan C.K., Hu M.: Size distributions and formation of ionic species in atmospheric particulate pollutants in Beijing, China: 1-inorganic ions. Atmos. Environ. 37, 2991–3000 (2003)
Ye C.X., Li H.J., Zhu T., et al.: Heterogeneous reaction of NO2 with sea salt particles [J]. SCIENCE CHINA Chem. 53(12), 2652–2656 (in Chinese) (2010)
Yue D.L., Hu M., Wu Z.J., Wang Z.B., Guo S., Wehner B., Nowak A., Achtert P., Wiedensohler A., Jung J., Kim Y.J., Liu S.C.: Characteristics of aerosol size distributions and new particle formation in the summer of Beijing. J. Geophys. Res. 114, D00G12 (2009). doi:10.1029/2008JD010894
Yue D.L., Hu M., Zhang R.Y., Wu Z.J., Su H., Wang Z.B., Peng J.F., He L.Y., Huang X.F., Gong Y.G., Wiedensohler A.: Potential contribution of new particle formation to cloud condensation nuclei in Beijing. Atmos. Environ. 45, 6070–6077 (2011)
Zhang D., Iwasaka Y.: Nitrate and sulfate in individual Asian dust-storm particles in Beijing, China in spring of 1995 and 1996[J]. Atmos. Environ. 33(19), 3213–3223 (1999)
Zhang X., Arimoto R., An Z., et al.: Atmospheric trace elements over source regions for Chinese dust: concentrations, sources and atmospheric deposition on the Loess Plateau [J]. Atmos Environ. Part A. Gen Topics. 27(13), 2051–2067 (1993)
Zhang J.L., Song H.Q., Tong S.L., Li L., Liu B.Y., Wang L.H.: Ambient sulfate concentration and chronic disease mortality in Beijing. Sci. Total Environ. 262, 63–71 (2000)
Zhang Y., Shao M., Zhang Y., et al.: Source profiles of particulate organic matters emitted from cereal straw burnings [J]. J. Environ. Sci. 19(2), 167–175 (2007)
Zhang Q., Zhang J., Xue H.: The challenge of improving visibility in Beijing. Atmos. Chem. Phys. Discuss. 10, 6199–6218 (2010). doi:10.5194/acpd-10-6199-2010
Zhang Y.M., Zhang X.Y., Sun J.Y., Lin W.L., Gong S.L., Shen X.J.: Characterization of new particle and secondary aerosol formation during summertime in Beijing. China, Tellus, B. 382-394, (2011)
Zhang W., Capps S.L., Hu Y., et al.: Development of the high-order decoupled direct method in three dimensions for particulate matter: enabling advanced sensitivity analysis in air quality models [J]. Geosci. Model Dev. 5(2), 355–368 (2012)
Zhao P.S., Dong F., He D., et al.: Characteristics of concentrations and chemical compositions for PM2.5 in the region of Beijing, Tianjin, and Hebei, China[J]. Atmos. Chem. Phys. 13(9), 4631–4644 (2013)
Zheng S., Pozzer A., Cao C.X., Lelieveld J.: Long-term (2001-2012) concentrations of fine particulate matter (PM2.5) and the impact on human health in Beijing, China. Atmos. Chem. Phys. 15(10), 5715–5725 (2015)
Zhou M., Chen C.H., Qiao L.P., Lou S.R., Wang H.L., Huang H.Y., Wang Q., Chen M.H., Chen Y.R., Li L., Huang C., Zou L.J., Mu Y.Y., Zhang G.F.: The chemical characteristics of particulate matters in Shanghai during heavy air pollution episode in Central and Eastern China in January 2013[J]. Acta Sci. Circumst. 33(11), 3118–3126 (in Chinese) (2013)
Zhu T., Shang J., Zhao D.F.: The roles of heterogeneous chemical processes in the formation of an air pollution complex and gray haze [J]. SCIENCE CHINA Chem. 54(1), 145–153 (in Chinese) (2011)
Ziegler E.N.: Sulfate-formation mechanism: theoretical and laboratory studies [J]. Adv Environ Sci Eng. 1, 184–194 (1979)
Acknowledgments
This material is based upon work supported by the Science and Technology Commission of the Beijing Municipality Fund Project (No. Z131100006113009), the Commonwealth Project of the Ministry of Environmental Protection (No. 201409005), and the National Key Technology R&D Program (No. 2014BAC23B03). We thank the automatic monitoring department in Beijing Municipal Environmental Monitoring Center for providing data of PM2.5, SO2, NO2 and meteorological parameters. We also thank the analytical laboratory in Beijing Municipal Environmental Monitoring Center for providing data of chemical components of PM2.5. For detailed data, please see website http://zx.bjmemc.com.cn/ or send email to 18701650609@163.com.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Zhang, D., Liu, B. et al. Analysis of chemical characteristics of PM2.5 in Beijing over a 1-year period. J Atmos Chem 73, 407–425 (2016). https://doi.org/10.1007/s10874-016-9334-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-016-9334-8